Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencia del suelo
versión On-line ISSN 1850-2067
Cienc. suelo v.25 n.1 Buenos Aires ene./jul. 2007
Chloroform fumigation-extraction labile C pool (microbial biomass C "plus") shows high correlation to microbial biomass C in Argentinian and Brazilian soils
Helvécio De-Polli1; Alejandro Costantini2-4; Romina Romaniuk2 & Márcio Sampaio Pimentel3
1Embrapa-Agrobiologia. Seropédica, Rio de Janeiro, Brazil.
2Facultad de Agronomía. Universidad de Buenos Aires. Av. San Martín 4453. CP 1417. Buenos Aires, Argentina.
3Universidade Estadual da Bahia, Brazil.
4Author for correspondence. E-mail: costanti@agro.uba.ar
Recibido: 27/02/07
Aceptado: 26/05/07
ABSTRACT
Chloroform fumigation-incubation and chloroform fumigation-extraction approaches have significantly contributed to assess soil microbial biomass. Some controversy is found in the literature about the suggestion to calculate microbial biomass carbon (MBC) without the subtraction of the un-fumigated control, in opposition to the originally proposed method that requires such subtraction. Some authors consider the non-subtraction proceeding as a more robust method. Nevertheless, values obtained without subtraction of a control include other labile organic fractions of soil carbon besides microbial biomass. Therefore, due to the usefulness of this measurement we consider more appropriate to call it as chloroform-fumigation labile C pool or microbial biomass carbon "plus" (MBC PLUS). We used a vast series of data from soils of Argentina and Brazil under different management situations to verify whether MBC correlates to MBC PLUS. There was a significant statistical correlation between values of MBC obtained by fumigation-extraction method and the corresponding MBC PLUS. The MBC PLUS performed as well as MBC as an indicator to differentiate soil managements and their impact on soil quality.
Key words. Microbial biomass carbon; Labile carbon; Chloroform fumigation-extraction; Soil quality indicator.
Alta correlación entre el "pool" de carbono lábil por fumigación con cloroformo-extracción (carbono de biomasa microbiana plus) y carbono de biomasa microbiana en suelos de Argentina y Brasil
RESUMEN
Los métodos de fumigación-incubación y fumigación extracción han contribuido significativamente a las determinaciones de biomasa microbiana del suelo. En la literatura se encuentran algunas controversias acerca de la sugerencia de calcular el carbono de biomasa microbiana (CBM) sin la sustracción del control no fumigado, en oposición a las metodologías tradicionales que requieren de dicha sustracción. Algunos autores mencionan que el hecho de no realizar la sustracción hace al procedimiento más robusto. Sin embargo, los valores obtenidos sin la sustracción del control incluyen otras fracciones lábiles del carbono, además de la biomasa microbiana. Debido a lo útil que resulta esta medida consideramos adecuado llamarla "pool" de carbono lábil por fumigación con cloroformo o carbono de biomasa microbiana "plus" (CBM PLUS). Usamos una amplia serie de datos de suelos de Argentina y Brasil bajo diferentes situaciones de manejo para verificar si el CBM correlaciona con el CBM PLUS. Hubo una correlación estadísticamente significativa entre los valores de CBM obtenidos por el método de fumigación-extracción y los correspondientes a CBM PLUS. Este se vislumbra, al igual que el CBM, como un indicador para diferenciar situaciones de manejo de suelos y el impacto sobre su calidad.
Palabras clave. Carbono de biomasa microbiana; Carbono lábil; Fumigación con cloroformo - extracción; Indicador de calidad de suelos.
INTRODUCTION
Soil microbial biomass is a living pool containing 1-5% of the soil organic matter (Jenkinson & Ladd, 1981; Sparling, 1992), excluding root, mesoand macro-fauna. Its activity and often fast turnover impact soil characteristics affecting its quality by conduction of biochemical transformation of organic matter being a source or sink of plant nutrients (De-Polli & Guerra, 1999; Franzluebbers, 2002, Haubensaket al., 2002; Hargreaves et al., 2003). Microbial biomass determinations may indicate changes in the soil organic matter before they can be detected by measuring total soil carbon (Jenkinson & Ladd, 1981; Powlson et al., 1987) making possible its use as an indicator of early changes in soil organic matter content (Costantini et al., 1996; Cosentino et al., 1998).
Belowground phenomena have been of increasing interest due to its role on agricultural production, soil genesis and nutrient cycling, greenhouse gases mitigation and emissions, bioremediation, biological control of plant pest and diseases, plant growth promoting factors, source of organisms for industrial use. Those aspects are to some extension mediated by the soil microbial biomass.
The basic concept of treating the entire soil microbial population as a single entity (Powlson, 1994) was proposed by Jenkinson (1966). Soil microbiota treated as a living mass in a "black box" is to some extension a holistic approach when it considers the behavior of this large and important pool, but it is reductionist when refers its huge biodiversity to a single mass measurement. However, this biodiversity is not to be neglected because it may account for the large spatial variability of the microbial biomass measurements.
Several methods have been used to measure soil microbial biomass: direct counting (Sonderstrom, 1977; Paul & Johnson, 1977; Grisi & Gray, 1985; Rodriguezet al., 1992), ATP quantification (Jenkinson & Ladd, 1981; Jenkinson, 1988; Contin et al., 2001), a physiological approach like substrate-induced respiration (SIR) (Anderson & Domsch, 1978), and the lysing-cell approaches of chloroform fumigation incubation (CFI) and chloroform fumigation extraction (CFE), briefly described below.
The work of Jenkinson (1966) and Jenkinson & Powlson (1976) may be considered a landmark on the assessment of microbial biomass in soil. They inaugurated the chloroform fumigation era on this area of research. As reported by Powlson (1994), the work of Jenkinson (1966) was a decomposition study not orignally planned to develop a method for measuring the quantity of carbon held in the cells form soil living microorganisms. The basic methodology for estimating soil microbial biomass-C by chloroform fumigation incubation (CFI) procedure was set up later (Jenkinson & Powlson, 1976).
A much faster method called chloroform fumigation extraction (CFE), derived from CFI method, was later proposed by Vanceet al. (1987) and Tateet al. (1988). CFE method requires the same fumigated and unfumigated approaches as CFI method but the carbon released from the lysed cell is estimated by submitting the soil samples to a chemical extraction with K2SO4 0.5 mol/L, ratio soil:extractant equal 1:4 (Vanceet al., 1987) or 1:2,5 (Tate et al., 1988), and detected in the solution. Even thought this method requires shorter times, the amount of chemicals required is considerable for a routine adoption in a laboratory. Proceedings for these methods are also found with some adaptations in Horwath & Paul (1994) and De-Polli & Guerra (1999).
The rationale of the CFI and CFE implies that there is a background of CO2 emission derived from the soil organic matter besides the CO2 emission derived from the living cells killed and lysed during the chloroform fumigation. In this way, the subtraction of the amount of carbon evolved as CO2 in unfumigated samples used as a control (UF) from the amount of carbon evolved as CO2 in fumigated samples (F) is necessary (Jenkinson et al., 2004). The suggestion of Horwath and Paul (1994) about calculating the MBC without the subtraction of the unfumigated control does not follow the original concept of the method and should be avoid (Powlson, 1994) if one wants to refer to MBC.
Nevertheless, in practicing this methodology it is not rare to find an unfumigated control with higher values than the fumigated treatment, giving a negative value for the microbial biomass, what is unrealistic. These negative numbers may be due to stress situations such as disturbance for sampling and sieving, or soil drought. A few days of soil pre-incubation may avoid this extra flux (Powlson, 1994).
Horwath & Paul (1994) suggested, based on Voroney & Paul (1984), that no subtraction of an unfumigated control could be an alternative approach for microbial biomass determination that would overcome the negative value events. Franzluebbers et al. (1999) say that chloroform fumigation incubation without subtraction of a control, unlike that with subtraction of a control, should be considered a more robust method to determine microbial biomass under a wide range of environmental conditions. We suggest to avoid calling the estimation without subtracting a control as soil microbial biomass, and we consider more appropriate to call it as chloroformfumigation labile C pool or soil microbial biomass carbon "plus" (MBC PLUS).
The objectives of this work were:
1. To verify if there is a correlation between values from MBC obtained by fumigation-extraction method and the corresponding MBC PLUS in which the unfumigated control was not subtracted.
2. To verify if the MBC PLUS has the same diagnostic performance of MBC as soil management indicator to differentiate early changes in soil quality due to different management situations and degradation status.
MATERIAL AND METHODS
We used in this work a data base collected by the authors during many years. Part of these data was used for MBC calculation (not MBC PLUS) in previous publications, which references are cited in this article. All values were obtained using a fumigationextraction method (Vanceet al., 1987). Several Argentinian locations were used for sampling, representing the principal agricultural zones of the country and the major soils such as at Pehuajó (Haplic Phaeozem), at Pergamino (Luvic Phaeozem), at La Plata (LuviVertic Phaeozem), at Marcos Juárez (Luvic Phaeozem), and at Paraná (Haplic Vertisol). Three major great groups of Brazilian soils from the State of Rio de Janeiro were collected in the Municipality of Magé (Dystric Gleysol), Paty do Alferes-Avelar (Hyperdystric Ferralsol) and Seropédica (Hyperdystric Acrisol). These sets of soils are very contrasting both in its pedological characterists and in its agricultural use. Sampling location can be seen in Figure 1.

Figure 1. Soil sampling places. 1) La Plata, 2) Pehuajó, 3) Pergamino, 4) Paraná, 5) Marcos Juárez, 6) Magé, 7) Avelar, 8) Seropédica.
Figura 1. Lugares de muestreo de suelos. 1) La Plata, 2) Pehuajó, 3)Pergamino, 4) Paraná, 5) Marcos Juárez, 6) Magé, 7) Avelar, 8) Seropédica.
Statistical methodology used was correlation analysis between values obtained by the method of fumigation-extraction with subtraction of control (MBC) and values obtained without subtraction of a control (MBC PLUS) that include other labile organic fractions of soil carbon besides microbial biomass. In the case where there was previous publication for MBC data, we cited the corresponding reference, performed an original analysis (ANOVA) for MBC PLUS, and made the comparison for the performance of MBC and MBC PLUS as soil management indicator. In the cases where there was no previous publications we compared MBC and MBC PLUS behavior as soil indicator for management impact and cited the statistical test. We performed the correlation analysis of these two attributes.
RESULTS AND DISCUSSION
As regards Argentinian soils, there was a high significant correlation (P<0.01) between MBC and MBC PLUS (r = 0.99) for soils from Pehuajó, as can be seen in the Figure 2a. These data were obtained from an experiment with different crop rotations and soil tillage, where there was statistical difference among all treatments for MBC data, as described in Costantini et al. (1995). The same statistical differences were found when performing the ANOVA on MBC PLUS data (Table 1).
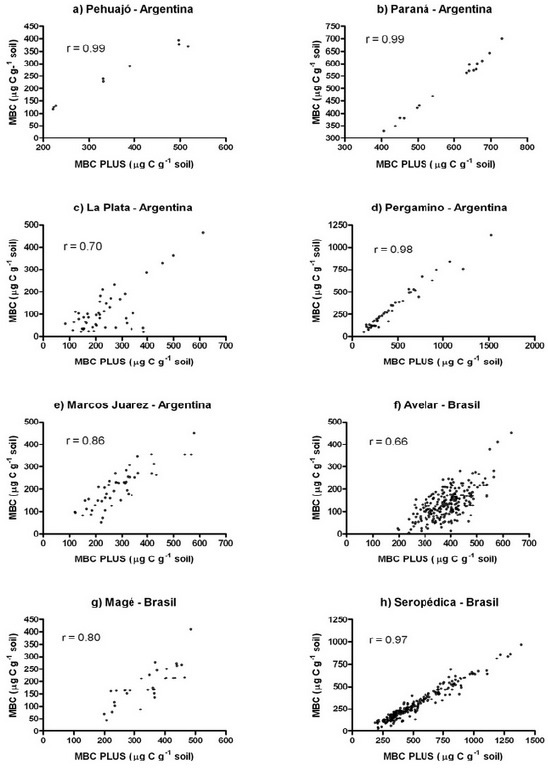
Figure 2. Correlation between microbial biomass carbon (MBC) and microbial biomass carbon "plus" (MBC PLUS) from data obtained at different sampling places. a) Pehuajó, b) Paraná, c) La Plata, d) Pergamino, e) Marcos Juárez, f) Avelar, g) Magé, h) Seropédica.
Figura 2. Correlación de carbono de biomasa microbiana y carbono de biomasa microbiana "plus" a partir de los datos obtenidos en diferentes lugares de muestreo. a) Pehuajó, b) Paraná, c) La Plata, d) Pergamino, e) Marcos Juárez, f) Avelar, g) Magé, h) Seropédica.
Table 1. Microbial biomass carbon (MBC) and microbial biomass carbon "plus" for Pehuajó and Paraná soils.
Tabla 1. Carbono de biomasa microbiana (MBC) y carbono de biomasa microbiana "plus" (MBC PLUS) para los suelos de Pehuajó y Paraná.

For samples taken in Paraná region, the correlation analysis was highly significant (P<0.01) between MBC and MBC PLUS (r = 0.99) as shown in Figure 2b. Four different plot areas representing a gradient of soil degradation as indicated by chemical and mainly morphological characteristics (A horizon depth) were used, as described by Cosentino & Costantini (2000). They showed only a tendency in the MBC values, without statistical significance when analyzed by Cosentino & Costantini (2000), and the same occurred for MBC PLUS (Table 1).
Nearby La Plata City, a large number of samples were taken for this work in an area dedicated to horticultural production with very contrasting farming management (organic horticulture with reduced tillage during 5 years and with conventional tillage during 20 years, conventional horticulture during 5 years and during 20 years) and a control plot. Correlation analyses showed significance (P < 0.01; R=0.70) between MBC and MBC PLUS (Figure 2c).
Data from Pergamino (Argentinian Pampa region) also showed high significance for MBC and MBC PLUS (P<0.01; r = 0.98) as shown on Figure 2d. In this case samples came from different management situations such as soil with intense plowing practice for long period of time, soil under pasture, native prairie and soil that received organic amendment. The native prairie, organic amended soils and cultivated grasslands gave the higher values for both MBC and MBC PLUS. ( 903, 422, 502 µg C g-1 soil for MBC; 1169, 684, 647 µg C g -1 soil for MBC PLUS respectively)
For the last group of soils from Argentina, a large amount of data from several experiments with different crop rotations and tillage systems was obtained in the Experimental Agricultural Station "Marcos Juárez" (Instituto Nacional de Tecnología Agropecuaria-INTA). Crop rotations and tillage systems were: corn monoculture under three tillage systems, corn-wheat/soybean-wheat, under reduced tillage and no-tillage. The MBC analyses were performed by Costantiniet al. (1996); Costantini (1997) and Cosentinoet al. (1998). For the present work we performed the correlation analysis for MBC and MBC PLUS which resulted significant (P<0.01; r=0.86) as shown in Figure 2e.
With respect to soils from the Brazilian Federal Republic, we worked in three different situations. In the first case, a set of data was collected for this work in the Avelar region of Paty do Alferes Municipality, from soils under different management system including coffee (Coffea arabica L.) plantation, horticultural practice, and forest. The MBC and MBC PLUS correlation was not so high as shown before for the treatment of organic amendment and sampling time but still a high significant correlation was obtained (r = 0.66; P<0.01) as shown in Figure 2f.
The second data set was from the Municipality of Magé (costal lowland region of Rio de Janeiro State). Different horticultural farming managements were analyzed in different time of the year with contrasting climatic conditions. The MBC data presented significant differences between sampling time and also for different treatments of soil management (Costantini & Segat, 1994). The ANOVA for MBC PLUS showed the same level of significance obtained before for MBC. Besides, applying correlation analyses to the whole set of data, we obtained high significance for MBC and MBC PLUS (P < 0.01; r=0.80) as shown on Figure 2g.
Finally we analyzed a large number of data obtained at the Agroecological Farm of the Embrapa Agrobiology Center in Seropédica Municipality, Rio de Janeiro State, from a horticultural experiment with lettuce (Lactuca sativa L.) and carrot (Daucus carota L.) amended with four farm yard manure doses (0, 12, 24 and 48 Mg ha-1) where MBC and MBC PLUS were measured four times during the crop season (1, 6, 57 and 101 days after planting). Results showed high correlation between MBC and MBC PLUS (correlation coefficients of 0.96; 0.98; 0.99 and 0.91; P < 0.01, for sampling time of 1, 6, 57 and 101 days after planting, respectively). Fig 2h shows the correlation analysis from the combined data (r = 0.97; P < 0.01). ANOVA and Tukey's test showed almost the same separation for MBC and MBC PLUS, both for the organic amendment treatments and sampling times (Table 2).
Table 2. Microbial biomass carbon (MBC) and microbial biomass carbon "plus" (MBC PLUS) for four sampling dates and four farm yard manure doses in soils of Seropédica, Rio de Janeiro, Brazil.
Tabla 2. Carbono de biomasa microbiana (MBC) y carbono de biomasa microbiana "plus" (MBC PLUS) para los cuatro momentos de muestreo y cuatro dosis de abono en suelos de Seropédica, Rio de Janeiro, Brasil.

CONCLUSIONS
1. There was a high significant statistical correla-tion between values of MBC obtained by fumigationextraction method and the corresponding MBC PLUS, where the non fumigated control was not subtracted.
2. The MBC PLUS performed as well as MBC as soil management indicator to differentiate situations of soil management and the impact on its quality. MBC PLUS determination constitutes an advantage with respect to MBC, since it is less time consuming and saves chemicals, facilitating its adoption in the routine of soil test laboratory.
ACKNOWLEDGEMENTS
The authors want to acknowledge the State of Rio de Janeiro Science Foundation (FAPERJ), the Brazilian National Research Council (CNPq) and Associated Centers Project (CAPES - SPU) for partial financial support for this work, and Dr. Mabel Susana Pazos for soil classification.
REFERENCES
1. Anderson JPE & KH Domsh. 1978. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 10: 215-221. [ Links ]
2. Contin, M; A Todd & PC Brookes. 2001. The ATP concentration in the soil microbial biomass. Soil Biol Biochem 33: 701-704. [ Links ]
3. Cosentino, D; A Costantini; A Segat & M Fertig. 1998. Variations in organic carbon, microbial biomass carbon and its relationship with some physical properties of an argentine pampa soil under three different tillage systems. Pesquisa Agropecuaria Brasileira 33: 981-986. [ Links ]
4. Cosentino, D & A Costantini. 2000. Evaluación de algunas formas de carbono como indicadores de degradación on Argiudoles vérticos de Entre Rios, Argentina. Revista de la Facultad de Agronomía 20: 31-34. [ Links ]
5. Costantini, A. 1997. Carbono de biomasa microbiana y cociente metabólico qCO2. Algunos factores que los afectan e inferencias a partir de los mismos. Tesis de Magister Scientiae. Escuela para Graduados, Facultad de Agronomía. UBA. [ Links ]
6. Costantini, A & A Segat. 1994. Seasonal changes in soil microbial biomass of brazilian soils with different organic matter contents. Commun Soil Sci Plant Anal 25: 3057-3068. [ Links ]
7. Costantini, A; A Segat & D Cosentino. 1995. The effect of different soil management procedures on carbon cycle components in an Entic Hapludoll. Commun Soil Sci Plant Anal 26: 2761-2767. [ Links ]
8. Costantini, A; D Cosentino & A Segat. 1996. Influence of tillage systems in biological properties of a typic Argiudoll soil under continuous maize in Central Argentina.Soil Till Res 38: 265-271. [ Links ]
9. De-Polli, H & JGM Guerra. 1999. C, N e P na biomassa microbiana do solo. In: GA Santos & FAO Camargo(eds). Fundamentos da matéria orgânica do solo: ecossistemas tropicais e subtropicais, 389-411. Gênesis. Porto Alegre, Brazil. [ Links ]
10. Franzluebbers, AJ. 2002. Soil organic matter stratification ratio as an indicator of soil quality. Soil Till Res 66: 95-106. [ Links ]
11. Franzluebbers, AJ; RL Haney & FM Hons. 1999. Relationships of chloroform fumigation-incubation to soil organic matter pools. Soil Biol Biochem. 31: 395-405. [ Links ]
12. Grisi, BM & TRB Gray. 1985. Biomassa microbiana do solo estimada do biovolume com o uso da microscopia de fluorescência. Rev Bras Ci Solo 9: 131-138. [ Links ]
13. Hargreaves, PR; PC Brookes; GJS Ross & PR Poulton. 2003. Evaluating soil microbial biomass carbon as an indicator of longterm environmental change. Soil Biol Biochem. 35: 401-407. [ Links ]
14. Haubensak, KA; SC Hart&JMStark. 2002. Influences of chloroform exposure time and soil water content on C and N release in forest soils. Soil Biol Biochem. 34: 1549-1562. [ Links ]
15. Horwath,WR & EA Paul. 1994. Microbial biomass. In: RWWeaver; S Angle; P Bottomley; D Bezdicek; S Smith; A Tabatabai & A Wollum(eds.). Methods of soil analysis, 753-773. Madison, SSSA. Part 2. (Soil Science Society of America Series, 5). [ Links ]
16. Jenkinson, DS. 1966. Studies on the decomposition of plant material in soil. II. Partial sterilization of soil and the soil biomass. J. Soil Sci. 17: 280-302. [ Links ]
17. Jenkinson, DS. 1988. Determination of microbial biomass carbon and nitrogen in soil. In: JR Wilson(ed.).Advances in nitrogen cycling in agricultural ecosystems, 368-386. C.A.B International, Wallingford. U.K. [ Links ]
18. Jenkinson, DS & DS Powlson. 1976. The effects of biocidal treatments on metabolism in soil. V. A method for measuring soil biomass. Soil Biol. Biochem. 8: 209-213. [ Links ]
19. Jenkinson, DS & JN Ladd. 1981. Microbial biomass in soil: measurement and turnover. In: Soil Biochemistry, eds. EA Paul & JN Ladd. 415-471. Marcel Dekker: New York. [ Links ]
20. Jenkinson, DS; PC Brookes; & DS Powlson. 2004. Measuring soil microbial biomass. Soil Biol Biochem. 36: 5-7. [ Links ]
21. Paul, EA &RL Johnson. 1977. Microscopic counting and adenosine 5-triphosphate measurement in determining microbial growth in soils. Appl Environ Microbiol 34: 263-269. [ Links ]
22. Powlson, DS. 1994. The soil microbial biomass: Before, beyond and back. In: K Ritz; J Dighton & KE Giller (eds.). Beyond the biomass; composition and functional analysis of soil microbial communities. 3-20. John Wiley. Chichester [ Links ]
23. Powlson, DS; PC Brookes; & BT Christensen. 1987. Measurement of microbial biomass provides an early indication of changes in total soil organic matter due to the straw incorporation. Soil Biol Biochem. 19: 159-164. [ Links ]
24. Rodriguez, GG; D Phipps; K. Ishiguro & HF Ridgway. 1992. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol 58: 1801-1808. [ Links ]
25. Sonderstrom, BE. 1977. Vital staining of fungi in pure cultures and in soil with fluorescein diacetate. Soil Biol Biochem. 9: 59-63. [ Links ]
26. Sparling, G. 1992. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Austr J Soil Res 30: 195-197. [ Links ]
27. Tate, KR; DJ Ross & CW Feltham. 1988. A direct extraction method to estimate soil microbial C: effects of experimental variables and some different calibration procedures.Soil Biol Biochem. 20: 329-335. [ Links ]
28. Vance, ED; PC Brookes & DS Jenkinson. 1987. An extraction method for measuring soil microbial biomass. Soil Biol Biochem. 19: 703-707. [ Links ]














