Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencia del suelo
versión On-line ISSN 1850-2067
Cienc. suelo vol.29 no.1 Ciudad Autónoma de Buenos Aires ene./jul. 2011
TRABAJO
Acidification evidences of no-tilled soils of the central region of argentina
Evidencias de acidificación de suelos de la región central de la argentina bajo siembra directa
Laura Antonela Iturri1,*; Daniel Eduardo Buschiazzo2 & Martín Díaz-Zorita3
1 CONICET (Instituto de Ciencias de la Tierra y Ambientales de La Pampa, INCITAP, CONICET-UNLPam) y Facultad de Agronomía de la Universidad Nacional de La Pampa.
2 INTA Anguil, Facultad de Agronomía de la Universidad Nacional de La Pampa y CONICET (Instituto de Ciencias de la Tierra y Ambientales de La Pampa, INCITAP, CONICET-UNLPam).
3 CONICET, Facultad de Agronomía de la Universidad de Buenos Aires y Merck Crop Bioscience Argentina S.A.
* Autor para correspondencia: antonelaiturri@conicet.gov.ar
Recibido: 07-01-10
Aceptado: 13-04-11
Abstract
Empiric evidences indicate that agricultural soils of Argentina tend to acidify. The objective of this study was to determine the pH values of no-tilled and urea-fertilized-agricultural soils of Argentina during several years. Results indicated that both the actual pH (pHA) and the potential pH (pHP) values were lower in humid than in dry environments. The ratio between «mean annual precipitation:mean annual temperature» of the sites explained between 60 and 80% of the variability in pH values. This suggests that climatic conditions were responsible for current soil pH values. The pHA was 1.14 points higher tan pHP in all studied sites (p<0.01), indicating that a generalized natural acidification process existed. In soils of drier environments, differences between both pHA and pHP were, on average, higher than 1.21, indicating a more intense acidification process. However, pH values were not low enough to affect the normal growth of crops and soil organisms. In soils of humid environments, differences between pHA and pHP were higher than 1.10, being pHA values (6.17 and 5.80) acidic enough to affect the microbial activity and the development of pH sensitive crops. Fertilization with urea decreased pHA between 0.18 and 0.32 points compared to non-fertilized treatments (p<0.05), indicating that fertilization contributed to a decrease in pH values in the studied soils. In conclusion, fertilization with urea slightly increased the natural tendency to soil acidification in most of the studied soils.
Keywords: Soil acidification; Nitrogen fertilizers; Soil degradation.
Resumen
Evidencias empíricas indican que los suelos agrícolas de la Argentina tienden a la acidificación. El objetivo de este estudio fue determinar valores de pH de suelos agrícolas de la Argentina bajo siembra directa de larga duración y fertilización con urea. Los resultados indican que tanto los valores de pH actual (pHA) como de pH potencial (pHP) fueron más bajos en ambientes húmedos que en los más secos. El cociente entre «precipitación media anual : temperatura media anual» de los sitios explicó entre un 60 y un 80% de la variabilidad de los valores de pH. Esto sugiere que las condiciones climáticas fueron responsables de los valores de pH presentes en estos suelos. El pHA fue 1,14 puntos mayor que el pHP en todos los sitios estudiados (p < 0,01) indicando que existió un proceso natural generalizado de acidificación. En suelos de ambientes más secos, las diferencias entre el pHA y el pHP fueron, en promedio, mayores a 1,21. Esto indicaría una acidificación más intensa. Sin embargo, los valores de pH no fueron lo suficientemente bajos como para afectar el normal crecimiento de cultivos y de organismos del suelo. En suelos de ambientes húmedos, las diferencias entre el pHA y el pHP fueron superiores a 1,10, siendo los valores de pHA (6,17 and 5,80) lo suficientemente ácidos como para afectar la actividad microbiana y el desarrollo de cultivos sensibles a bajos pHs del suelo. La fertilización con urea disminuyó el pHA entre 0,18 y 0,32 puntos en relación a los tratamientos no fertilizados (p < 0,05), indicando que la fertilización contribuyó al descenso de los valores de pH en los suelos estudiados. Se concluye que la fertilización con urea incrementa levemente la tendencia natural de los suelos a la acidificación en la mayoría de los sitios estudiados.
Palabras clave: Acidificación de suelos; Fertilizantes nitrogenados; Degradación de suelos.
Introduction
Acidification is a frequent chemical degradation process of many soils (Mayer, 1998; Borùvka et al., 2007). The main natural cause of this process is the leaching of exchangeable bases by infiltration water (Dubiková et al., 2002) while the use of fertilizers (Haynes & Mokolobate, 2001), the extraction of bases by crops (Zhang et al., 2009) and acid rain (Kelly & Stricklan, 1986; Lee et al., 2006; Ward, 2009) are the main anthropogenic causes.
Acidification affects soil properties and plant growth (Malhi et al., 1998). Acid soils are deficient in exchangeable bases for crop development (Darusman et al.,1991; Dubiková et al., 2002) and have higher concentrations of phytotoxic substances in the soil solution, mainly active compounds of aluminum (Al) (Borùvka et al., 2005; Drábek et al., 2005), iron (Fe) (Hell & Stephan, 2003; Rust Neves et al., 2009), and manganese (Mn) (Watmough et al., 2007).
The use of fertilizers in agricultural systems, mainly under no-till (NT) farming, has drastically increased in Argentina in the last years (Montoya et al., 1999; Díaz- Zorita, 2005). The effect of nitrogenous fertilizers, especially urea, on soil acidification has been scarcely analyzed in this country. Vazquez (2005) demonstrated that ammonium fertilizers acidify the soil. Also, Fabrizzi et al. (1998) found that urea fertilization reduced the pH of a Typic Argiudoll by 0.39 units. Urricarriet et al. (1999) observed that after seven years of urea fertilization, pH values of a Typic Argiudoll from Argentina decreased from 6.40 to 5.60. Other authors found that liming increased crop production in soils with pH under 5.00 in the Humid Pampas (Gambaudo, 1998; García et al., 2002). This practice can improve some physical soil properties.
No-till farming is used for crop production along a broad climatic and edaphic gradient in Argentina. Climatic conditions vary from subtropical to temperate; agricultural soils are composed by different Subgroups (US Soil Taxonomy) of Haplustolls, Hapludolls and Argiudolls (Moscatelli, 1990). Considering this variability, it can be assumed that the more developed soils, with higher organic matter, clay contents, cation exchange capacity and base saturation will present a lower acidity than the less developed soils. The objective of this study was to test this assumption by analyzing the pH values of no-tilled agricultural soils, with and without urea fertilization history in the central region of Argentina.
Materials and methods
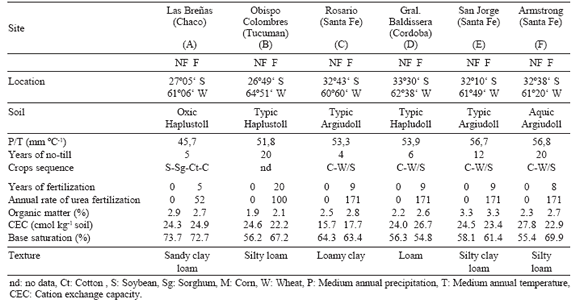
Soils of six sites of Argentina under varying climatic and intrinsic properties were sampled from field plots of a 5 year long no-till experiments. Three soil samples were taken randomly from the topsoil (0-20 cm) of every 100 m2 area of fertilized (urea) and non-fertilized plots at each site. Table 1 shows the main characteristics of soils and management history at each site.
Table 1. Main characteristics of the studied soils (F = Fertilized and NF = Non- Fertilized soils).
Tabla 1. Principales características de los suelos estudiados ( F = Fertilizados y NF = No Fertilizados).
Soil samples were air-dried, ground and sieved through a 2 mm mesh to determine actual pH (pHA, soil:water 1:2.5) and potential pH (pHP, soil:KCl 1 eq dm-3 1:2.5) (Vazquez, 2005). Exchangeable cations were extracted with ammonium acetate 1 eq dm-3 buffered at pH 7, and subsequently determined by atomic absorption spectrometry (Page et al., 1982). Soil cation exchange capacity (CEC) and the percent of base saturation (BS) were then calculated. Soil organic matter content was determined by the Walkey & Black method (1934). Soil particle size distribution was determined by the Robinson's pipette method (Klute, 1982).
Soil pH values were correlated by simple linear regression analysis with the Lang's climatic index (Lang, 1920), expressed by the ratio between the mean annual precipitation (mm) and the mean annual temperature (ºC) (P/T) at each site, in order to evaluate the effect of climatic conditions.
The Fisher test was used to compare variances of pH values and the Student t-test to compare mean values. A covariance analysis was performed to compare regression lines. A principal component analysis (PCA) was performed to analyze the association between variables. All statistical and mathematical analyses were done with the Microsoft Excel and the InfoStat/ Professional version 1.1. (Di Rienzo et al., 2002) programs.
Results and discussion
Soil pHA values ranged from 5.62 to 7.07 and soil pHP ranged from 4.64 to 6.01 (Table 2). Both pHA and pHP values were highly correlated (linearly and negatively) with the P/T ratio of each site in both fertilization treatments (Fig. 1). This ratio explained between 60 and 80% of pH variances. These results indicate that pH values are highly dependent on climatic conditions, as a probable result of higher losses of exchangeable bases by leaching (Dubiková et al., 2002) in more humid environments, as well as the higher exchangeable base extraction by more productive crops (Vázquez et al., 2000; Gelati & Vázquez, 2008; Zhang et al., 2009).
Table 2. Actual (pHA) and potential pH (pHp) of no-tilled soils, fertilized (F) and nonfertilized (NF) with urea.
Tabla 2. pH actual (pHA) y potencial (pHp) de suelos bajo siembra directa, fertilizados (F) y no fertilizados (NF) con urea.


Figure 1. Values of pHA and pHp as a function of the ratio «mean annual precipitation: mean annual temperature» (P/T) of a) non-fertilized and b) urea-fertilized soils.
Figura 1. Valores de pHA y pHp en función del cociente «precipitación media anual: temperatura media anual» (P/T) de a) suelos no fertilizados y b) suelos fertilizados con urea.
Linear regression slope was significantly (p < 0.01) higher in the pHA - P/T than in the pHP - P/T relationship, for both fertilization treatments. These results show that differences between pHA and pHP were higher in dry than in humid environments. In environments with a P/T quotient between 45 and 52, differences between actual and potential pH values averaged 1.21 points, while in environments with P/T quotient between 53 and 57, the differences averaged 1.10. This would mean that, even though absolute pH values were lower in humid environments, the acidification magnitude in the driest environments is higher. On the other hand, such results can be also be affected by measurement methods, as mentioned by Thomas (1996).
Values of soil pHA were significantly (p < 0.01) higher than those of soil pHP in all studied sites (Table 2). Soil pHA values averaged 6.33 for the non-fertilized treatments and 6.15 for the fertilized ones, whereas pHP values averaged 5.13 and 5.08, respectively. The mean pHA - pHP difference was 1.20 and 1.08 in non-fertilized and fertilized treatments, respectively. A difference between pHA and pHP higher than 1.00 indicates that soil acidification existed, as this pH difference would potentially produce an increase of H+ by 10 times in the soil solution if the dissociation of all adsorbed H+ occurred.
Both, pHA and pHp values were lower in fertilized than in non-fertilized soils (Table 2). The non-fertilized soil of the site with the lowest P/T ratio (site A from Chaco province) had an average pHA value of 7.07 and an average pHP of 5.97; the difference was 1.10. In turn, the soil of the site with the highest P/T ratio (site F from Santa Fe province) had a pHA of 6.11 and a pHp of 4.98, with a difference of 1.30. The fertilized soils of site A presented a pHA of 6.92 and a pHP of 6.01; with a difference of 0.91. The soil of site F presented a pHA of 5.79 and a pHp of 4.81, being their difference 0.98. These results indicate that the observed pH decreases are due to an acidification of environmental origin rather than being anthropogenic (Cnossen et al., 2008; Noyes et al., 2009). Urea fertilization increased this general acidification trend linked to a medium to low base saturation (72.7 for site A and 69.9 for site F) and to low organic matter contents (2.7 in both sites).
Urea fertilization caused significant decreases in soil pHA(p < 0.05). The decreases were greater in the more humid sites (C, E, F and D) than in the drier sites (A and B). In site A (Chaco province), pHA and pHP values varied between 6.00 and 7.00 across all fertilization treatments. Such values should not cause detrimental effects on pH sensitive crops and soil organisms (Borùvka et al., 2005; Drábek et al., 2005; Watmough et al., 2007; Rust Neves et al., 2009). However, pHP was significantly lower than pHA (p < 0.05) in both fertilization treatments (differences between 1.10 and 0.91, respectively), suggesting that a weak acidification process is in progress. Such process is probably not linked to fertilization, as the fertilization rate of urea was low in this site (Table 1).
The soil of site B (Tucumán province) showed no evidences of acidification due to fertilization, even though the fertilization rate was twofold and the fertilization events quadruplicated that of site A. In site B, soil pHA was 1.50 points higher than pHP in the non-fertilized soil and 1.31 points in the fertilized one. This indicates that acidification was not produced by the use of fertilizers but by another process. In site B, the intense industrial activity may be the source of protons added to the soil. Even though acidification is evident in this site, pHA values are within the optimum range for the growth of most crops (Porta et al., 1999; Drábek et al., 2005; Stevens et al., 2009). However, soil pHP values are below 5.56, which can be considered critical for the growth of most crops. The acidification sources in this region must be detected in order to avoid future adverse effects on soils and crops.
Both soil pHA and pHP values were significantly (p< 0.05) lower in fertilized soils than in non-fertilized soils of more humid environments (sites C, D and F) (Table 2). Differences between pHA and pHP were 0.97 and 1.15 in average, respectively. In the C, D and F sites, pHA values ranged from 6.25 to 5.70 in the non-fertilized soils, and 5.90 to 5.77 in the fertilized soils. The corresponding pHP values ranged from 4.90 to 4.64 in the non-fertilized soils and 4.91 to 4.62 in the fertilized soils. These pH values indicate that, despite of their higher buffer capacity linked to their higher organic matter contents and CECs (Table 1), the application of urea was responsible for the decreased pH values of these soils. All these values were low enough to produce Ca and Mg deficiencies (Dubiková et al., 2002), reduction of the microbial activity (Porta et al., 1999; Drábek et al., 2005; Stevens et al., 2009) and the solubilization of Fe, Mn or Al (Drábek et al., 2005; Watmough et al., 2007 y Rust Neves et al., 2009).
A principal component analysis (PCA) was applied as a data reduction or structure detection method (Fig. 3). The average values of the studied variables are represented in the center of the biplot. At that point, nonfertilized and fertilized treatments presented the following values: pHA 6.33 and 6.15, pHP 5.13 and 5.08, CEC 23.48 and 23.00 cmol kg-1, percent of base saturation 60.68 and 64.94% and OM 2.51 and 2.70%. Both pH values were negatively correlated with OM contents (r = -0.35 and -0.18, p < 0.05), and positively with CEC (r = 0.34 and r = 0.32, p < 0.05) and BS (r = 0.41 and 0.59, p < 0.05), as reflected by both the flat and acute angles between variables in the biplot (Fig. 2).

Figure 3. Principal components analysis. Biplot in the plane of the two first principal components (PCs).
Figure 3. Análisis de componentes principales. Biplot en el plano de las dos primeras componentes principales (PCs).
This PCA confirmed the results from the simple regression analysis, which determined three soil populations (Fig. 2): a) soils of sites A and B characterized by their pH values near neutrality, medium to high exchangeable base saturation and medium to high CEC values and OM contents; b) soils of sites D, E and F, with pH lower than the average of all the studied sites, medium to high BS and medium to low CEC and OM content; and c) soils of site C with the lowest pH values, high BS, and low CEC and OM contents of all analyzed sites (Table 1).
Conclusions
Differences between pHA and pHP higher than 1.00 were observed in all studied soils, indicating a generalized acidification process. Nevertheless, less developed soils showed higher pH differences than the more developed ones. The existence of higher acidification in less developed soils must be further analyzed.
The pH values of the less developed soils were always above the critical threshold for crop and microorganism growth.
Soil acidification was slightly promoted by urea fertilization in most of the studied sites.
The «mean annual precipitation: mean annual temperature» ratio was negatively related to both the actual and the potential pH, and explained 60- 80% of the variability.
Acknowledgments
The authors wish to thank Agr. M. Boxler, MSc. A. Sanzano and Dr. O. Ingaramo for sampling the studied soils. This study was financed by the National Council for Research and Technology of Argentina (CONICET), the Agencia Nacional de Promoción Científica y Técnica, Argentina (ANPCyT) and Facultad de Agronomía, Universidad Nacional de La Pampa, Argentina.
References
1. Borùvka, L; L Mládková y O. Drábek. 2005. Factors controlling spatial distribution of soil acidification and Al forms in forest soils. Journal of inorganic biochemistry 99: 1796-1806. [ Links ]
2. Cnossen, I.; MJ Harris; NF Arnold & E Yiðit. 2008. Modelled effect of changes in the CO2 concentration on the middle and upper atmosphere: Sensitivity to gravity wave parameterization. Journal of atmospheric and solar-terrestrial physics. In press.
3. Darusman, LR; DA Stone; KA Whitney & JH Long. 1991. Soil properties after twenty years of fertilization with different nitrogen sources. Soil Sci. Soc. Am. J. 55: 1097-1100. [ Links ]
4. Drábek, O; L Mládková; L Borùvka ; J Skakova; A Nikodem & K Nemecek. 2005. Comparison of water-soluble and exchangeable forms of Al in acid forest soils. Journal of inorganic biochemistry 99: 1788-1795. [ Links ]
5. Díaz Zorita, M. 2005. Cambios en el uso de pesticidas y fertilizantes. Ciencia hoy. 28-29. [ Links ]
6. Di Rienzo, JA; M Balzarini; F Casanoves; L González; M Tablada & CW Robledo. 2002. Infostat/Professional version 1.1.
7. Fabrizzi, K; L Picone; A Berardo & F García. 1998. Efecto de la Fertilización Nitrogenada y Fosfatada en las Propiedades Químicas de un Argiudol Típico. Ciencia del Suelo 16: 71-76. [ Links ]
8. Gambaudo, S. 1998. Encalado de suelos ácidos para la producción de alfalfa. Fertilizar Nº. Especial pasturas. EEA. INTA Pergamino. Buenos Aires, Argentina.
9. García, F; F Micucci; G Rubio; M Ruffo & I Daverede. 2002. Fertilización de forrajes en la región pampeana. Una revisión de los avances de la fertilización de pasturas, pastizales y verdeos. Instituto de la Potasa y el Fósforo - INPOFOS Cono Sur; Potash and Phosphate Institute (PPI); Potash and Phosphate Institute of Canada (PPIC). [ Links ]
10. Gelati, P & ME Vázquez. 2008. Extracción agrícola de bases en el N de la provincial de Buenos Aires, Argentina: costo de su remediación e implicancias económicas. Revista de la Red Iberoamericana de Economía Ecológica (Rebivec) 7: 117-129. [ Links ]
11. Haynes RJ & MS Mokolobate. 2001. Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: A critical review of the phenomenon and the mechanism involved. Nutrient Cycling in Agroecosystems 59: 47-63. [ Links ]
12. Hell, R & UV Stephan. 2003. Iron uptake, trafficking and homeostasis in plants. Planta 216: 541-551. [ Links ]
13. Kelly, JM & RC Stricklan. 1986. Through fall and plant nutrient concentration response to simulated acid rain treatment. Water Air Soil Pollut. 29: 219-231. [ Links ]
14. Gee GW & JW Bauder. 1982. Particle size analysis. P. 383-404. In A Klute (ed.) Methods of soil analysis, Part 1, Physical and mineralogical methods. Soil Science Society of America- Agronomy Society of America, Madison, WI. 1188. [ Links ]
15. Lang, R. 1920. Verwitterung und Bodenbildung als Einführung in die Bodenkunde. Schweitzerbart'sche Verlag. Cited by Buol, SW; Hole, FD; McCracken, RJ 1983. Génesis y clasificación de suelos. Ed. Trillas. Stuttgart. [ Links ]
16. Lee Y; J Park; K Im; K Kim; J Lee & K Lee. et al. 2006. Arabidopsis leaf necrosis caused by simulated acid rain is related to the salicylic acid signaling pathway. Plan Physiol. Biochem. 29: 33-38. [ Links ]
17. Malhi, SS; M Nyborg & JT Harapiak. 1998. Effect of long-term N fertilizer - induced acidification and liming on micronutrients in soil and bromegrass hay. Soil & Tillage Research 48: 91-101. [ Links ]
18. Mayer, R. 1998. Soil acidification and cycling of metal elements: cause-effect relationships with regard to forestry practices and climatic changes. Agriculture ecosystems and environment 67: 145-152. [ Links ]
19. Montoya, JC.; AA Bono; A Súarez; NA Darwich & FJ Babinec. 1999. Cambios en el contenido de fósforo asimilable en suelos del este de la provincia de La Pampa, Argentina. Ciencia del Suelo 17: 45-58. [ Links ]
20. Moscatelli, G. 1990. Atlas de Suelos de la República Argentina. Escala 1:500000 y 1:1000000. Tomo I y II. Secretaría de Agricultura, Ganadería y Pesca. INTA. Centro de Investigaciones de Recursos Naturales. Buenos Aires. [ Links ]
21. Noyes, PD; MK McElwee; HD Miller; BW Clark; LA Van Tiem; KC Walcott; KN Erwin & ED Levin. 2009. The toxicology of climate change: Environmental contaminants in a warming world. Environment international 35: 971-986. [ Links ]
22. Page, AL; RH Millar & DR Keeney. 1982. Methods of soil analysis. Part 2. Chemical and microbiologycal properties. 2nd edition. USA. American Society of Agronomy. S.S.S. of America. [ Links ]
23. Porta Casanellas, J.; M López-Acevedo Requerín & C Roquero De Laburu. 1999. Edafología para la agricultura y el medio ambiente. España. Ed. Mundi-Prensa. 849. [ Links ]
24. Rust Neves, N; MA Oliva; D da Cruz Centeno; AC Costa; R Ferreira Rivas & E Gusmão Pereira. 2009. Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron one dust deposition: Potential use in environmental risk assessment. Science of the total environment 407: 3740-3745. [ Links ]
25. Stevens, CJ; NB Dise & DJ Gowing. 2009. Regional trends in soil acidification and exchangeable metal concentrations in relation to acid deposition rates. Environmental Pollution 157: 313-319. [ Links ]
26. Thomas, GW. 1996. Soil pH and soil acidity. p. 475-490. In: DL Sparks (ed.) Methods of soil analysis, Part 3, Chemical methods. Soil Science Society of America - Agronomy Society of America, Madison, WI. Torri, SJ & RS Lavado, 2002. Distribución y disponibilidad de elementos tóxicos en suelos representativos de la provincia de Buenos Aires enmendados con biosólidos. Ciencia del Suelo 20(2): 98-109. [ Links ]
27. Urricariet, S & RS Lavado. 1999. Indicadores de deterioro en suelos de La Pampa ondulada. Ciencia del Suelo 17(1): 37-44. [ Links ]
28. Vázquez, ME; E Baridon; J Lanfranco & G Malagrina. 2000. Evaluación de la potencialidad de la problemática de acidez en la región norte de la provincia de Buenos Aires. Actas XVII Congreso Argentino de la Ciencia del Suelo, 11-14 Abril, Mar del Plata, Argentina. [ Links ]
29. Vázquez, ME. 2005. Calcio y Magnesio. Acidez y alcalinidad de los suelos. Cap. 8: 161-188. En: H. Echeverría & F. García (eds.): Fertilidad de los suelos y fertilización de cultivos. Balcarce: Ediciones INTA. 525 p. [ Links ]
30. Vázquez, ME. 2005. Acidez del suelo. p. 71-88. En: Tecnologías en análisis de suelos. L. Marbán & SE Ratto (eds.). Buenos Aires. Asociación Argentina de la Ciencia del Suelo. 215 p. [ Links ]
31. Walkley, A. & IA Black. 1934. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil science 37: 29-38. [ Links ]
32. Ward, PL. 2009. Sulfur dioxide initiates global change in four ways. Thin solid films 517: 3188-3203. [ Links ]
33. Watmough, SA; MC Eimers & PJ Dillon. 2007. Manganese cycling in central Ontario forest: Response to soil acidification. Applied geochemistry 22: 1241-1247. [ Links ]
34. Zhang, HM; BR Wang; MG Xu & TL Fan. 2009. Crop yield and soil response to long-term fertilization on a red soil in southern China. Pedosphere 19: 199-207. [ Links ]














