Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencia del suelo
versión On-line ISSN 1850-2067
Cienc. suelo vol.32 no.1 Ciudad Autónoma de Buenos Aires jun. 2014
Amidase Activity Of Soils Under Potato Cropping With Conventional Management And Under Native Grassland
Jimena Sánchez Nieves1*; Lizeth Manuela Avellaneda-Torres1; Luz Marina Melgarejo1 & Cilia Leonor Fuentes De Piedrahita2
1 Biology Department. Faculty of Science. Universidad Nacional de Colombia, sede Bogotá. Carrera 45 N° 26-85. Colombia.
2 Faculty of Agronomy. Universidad Nacional de Colombia, sede Bogotá. * Author for correspondence. jsanchezn@unal.edu.co
Recibido: 29-05-13
Recibido con revisines: 23-10-13
Aceptado: 11-01-14
ABSTRACT
Amidase enzymatic activity was evaluated in soils under potato cropping with a conventional management with agrochemical application (PCA) and in soils under pasture without agrochemical application (PSA) in farms at Tausa, Villapinzón, and Zipaquirá located in the province of Cundinamarca (Colombia). Correlations between amidase enzymatic activity and other enzymatic activities related to the nitrogen cycle (e.g., urease and protease) and soil physicochemical parameters were also evaluated. The PCA samples showed null or low amidase activity compared to the PSA samples. The difference could be due to the application of agrochemicals and chemical supplements, which possibly affected soil microbiota and consequently the soil enzymatic activity. Similarly, the low amidase activity observed could be an indicator that carbofuran degradation via the amidase pathway did not occur at a significant level in the PCA soil. A direct relationship was observed between amidase, urease, and protease activities and the concentrations of soil microelements such as Mn, Fe, and Cu.
Key words. Edaphic enzyme, Urease, Protease, Nitrogen cycle.
ACTIVIDAD AMIDASA EN SUELOS BAJO CULTIVO DE PAPA CON MANEJO CONVENCIONAL Y BAJO PASTIZAL
RESUMEN
Se evaluó la actividad enzimática amidasa en suelos bajo cultivo de papa con manejo convencional de aplicación de agroinsumos (PCA) y en suelos bajo pastizal sin aplicación de agroinsumos (PSA), en fincas de las localidades de Tausa, Villapinzón y Zipaquirá en Cundinamarca (Colombia). Asimismo, se estableció su relación con la actividad de otras enzimas del ciclo del nitrógeno (ureasa, proteasa) y los parámetros fisico-químicos de dichos suelos. Se encontró que las muestras PCA presentaron nula o baja actividad amidasa con respecto a las PSA. Esto puede estar relacionado con la aplicación de plaguicidas e insumos químicos que afectan la actividad enzimática del suelo, o ser indicativo de que la degradación de carbofuran (plaguicida utilizado en el cultivo de papa) no se está llevando a cabo de manera significativa mediante la enzima amidasa. Se encontró una relación directa entre la actividad amidasa, ureasa, proteasa y micro-elementos del suelo (Mn, Fe y Cu).
Palabras clave. Enzimas edáficas, Ureasa, Proteasa, Ciclo del nitrógeno.
INTRODUCTION
Soil quality is one of the factors that determines agricultural sustainability, environmental quality, and, consequently, plant, animal, and human well-being (Doran et al., 1999). It depends greatly on long- term agricultural practices, which can affect soil biodiversity and fertility (Anderson, 2003; Nielsen & Winding, 2002; Masera et al., 1999). Some parameters are useful as indicators of changes in physical, chemical, and biological soil properties (Nielsen & Winding, 2002), which include the evaluation of soil microbial populations and enzyme activities (Pankhurst & Doube, 1997; Cerón & Melgarejo, 2005), and are used to establish the soil quality conditions of a particular environment.
Enzymatic activities have been evaluated as soil quality indicators associated to the effects of xenobiotics used in industry and agriculture (Tabatabai & Warren, 1993) and have shown that the use of agro-chemicals influence enzymatic activity (Alvear et al., 2006). Furthermore, enzymatic activity could be an indicator of the presence and effects (some harmful) of agrochemicals on soil quality (Burns, 1982; Klose & Ajwa, 2004; Avellaneda-Torres et al., 2012). The amidase enzyme (important in the nitrogen cycle) occurs widely in nature and catalyses amide hydrolysis to produce ammonium and carboxylic acid by acting on C-N bonds (Frankenberger & Tabatabai, 1980a). Amidase activity has also been associated with the degradation of agro-chemicals (Kay-Shoemake et al., 2000), including carbamates. It has been shown that the mcd gene (coding for the carbofuran hydrolase enzyme) is transferred in soil among microbial populations and contributes to the abundance and genetic diversity of N-methylcarbamate carbofuran-degrading bacteria (Desaint et al., 2003; Plangklang & Reungsang, 2012). The N-methylcarbamate bond in carbofuran occurs as ester and amide bonds; therefore, carbamate bond hydrolysis occurs via amidase, esterase, or both, and the hydrolysis product can be identical for both pathways due to the instability of N-methylcarbamate acid (Chapalamadugu & Chaudhry, 1993).
The potato crop (Solanum tuberosum) is of worldwide importance, along with wheat, rice, and corn. Because of its high nutritional value and adaptability to diverse climates and cultivation systems, potato is one of the 10 most commonly cultivated crops in developing countries (Devaux, 2010), given that it offers a possible solution to worldwide problems of food security, malnutrition, and poverty (Bonilla et al., 2008).
The present study evaluated the amidase enzymatic activity in soil used for potato cropping with conventional management and agrochemical application (PCA) and in grassland soil without agrochemical application (PSA) in farms at Tausa, Villapinzón, and Zipaquirá located in the province of Cundinamarca (Colombia). In addition, the relationship between the amidase activity and other enzyme activities of the nitrogen cycle (e.g., urease and protease) and soil physicochemical parameters was evaluated using a principal component analysis.
MATERIALS AND METHODS Experimental scheme and soil sampling
Three farms located in the province of Cundinamarca (Colombia) were evaluated in the following counties: Tausa (township of Páramo Bajo, farm: Páramo Bajo N 03o40'14,9''; W 073º39'51,1'' and N 03º35'50,4''; W 073º28'56,0''), Zipaquirá Z (township of Páramo Guerrero Oriental, farm: Puente de Tierra N 04º05'59,7''; W 072º54'34,6'' and N 03º37'32,9''; W 073º37'39,8''), and Villapinzón V (township of Salitre Alto, farm: Santa Ana N 03º43'43,5''; W 073º42'18,6'' and N 03º43'43,5''; W 073º42'18,6''). Two sample types were collected according to the method described by Avellaneda et al. , (2012): 1) Potato (Solanum tuberosum) mono-cropped soils, in the post-harvest phase, Parda Pastusa variety, with less than 10 years of agricultural use under a conventional agro-chemical application (PCA) management scheme; these samples were designated as TCA, ZCA, and VCA. The management including agrochemicals (Agricon®, Carbotox®, Nudrin®, Roxion®, Larvin®, Lorsban®, Lannate®, Eltra®, Methox®, Carbofed®, Furalimor SC, Alodrin®, Fursem®, Furadan®, among others), fungicides (Magricen 80%, copper oxychloride, Mancozeb, Acrobat®, Forum®, Antracol®, Fitoraz®, Previcur®, Rhodax®, Curzate®, Manzate®, Kasumin®, among others), herbicides (glyphosate, Sencor®, Gramafin®, Afalon®, Fusilade®, Gramoxone®, Reglone®), and N:P:K (10:30:10) chemical fertilisers applied at 200 kg ha-1 with further fertilisation using urea at 40 days (200 kg ha-1); and 2) Soils under grassland (Calamagrostis sp.) with no agrochemical application (PSA), designated as TSA, ZSA, and VSA. Sampling was performed using a zigzag route in a one-hectare plot at each site. Three soil samples were collected from each site, each composed of ten subsamples (collected every 15 steps). Each subsample was collected from a soil depth of 20 cm and weighed 50 g. Finally, the samples were deposited in sterile plastic bags and transported at 4 ºC to the laboratory for subsequent analyses (i.e., determination of the enzymatic activities in moist soil, with values expressed in terms of dry soil).
Physicochemical analyses of soils
The following physicochemical parameters were evaluated by the Certified Service Unit of the Instituto Geográfico Agustín Codazzi (González & Malagón, 1990): moisture content, pH, organic carbon (OC) by the Walkley-Black method, total nitrogen (TN) by the Kjeldahl method, cation-exchange capacity (CEC) by the ammonium acetate method, the microelements manganese (Mn), iron (Fe), zinc (Zn), and copper (Cu) by Dietilen-Triamin-Penta-Acetic acid extraction, available boron (B) by hot water application, and available phosphorus (P) by the Bray II method.
Determination of amidase activity in soil
Amidase (EC 3.5.1.4) activity measurements were performed according to Frankenberger & Tabatabai (1980a) using formamide as a substrate. The final detection of N-NH4 (ammonium nitrogen) produced by the enzyme's activity was performed by distillation with 25% NaOH and titration with H2SO4 (0.005M). Two grams were used from each soil sample with a particle size of < 2 mm, weighed in triplicate, and the reagent ratio was maintained according to the amount of sample.
Statistical analysis and relationship to other enzymes of the nitrogen cycle
The variance homogeneity and normality assumptions were analysed using Bartlett and Shapiro-Wilk tests, respectively, and by performing an analysis of variance with a factorial design (DCA) by means of Statistix 9.0. A principal component analysis was performed between amidase (EC 3.5.1.4), urease (EC 3.5.1.5), protease (EC 3.4.2.21.24) activity and physicochemical parameters. The results of urease and protease activity for the studied soils were reported by Avellaneda (2008) and Anacona (2008), respectively. The principal component analysis (PCA) was performed using Primer 6 V 6.1.14 and Permanova+ Version 1.0.4.
RESULTS AND DISCUSSION
Physicochemical analyses of soils
Table 1 shows that the soils were acidic (pH less than 5.5) with a high CEC (>20 meq/100 g) and high levels of TN (>0.5%, except ZSA). The total OC content was high for all soil samples (>5%). There were Zn (<3 ppm), Cu (<1.5 ppm), and Mn (<15 ppm) deficiencies but high Fe (>30 ppm) and B (0.6-1.5 ppm) concentrations.
Table 1. Physicochemical parameters of soils evaluated in this study. TSA, VSA, and ZSA: Tausa, Zipaquirá, and Villapinzón without the application of agrochemicals. TCA, VCA, and ZCA: Tausa, Zipaquira and Villapinzón with the application of agrochemicals. H: moisture content, CEC: cation-exchange capacity, OC: organic carbon, TN: total nitrogen.
Tabla 1. Parámetros fisicoquímicos de los suelos evaluados en este estudio. TSA, VSA, ZSA: Tausa, Zipaquirá y Villapinzón sin aplicación de agroquímicos. TCA, VCA y ZCA: Tausa, Zipaquirá y Villapinzón con aplicación de agroquímicos. H: humedad, CEC: Capacidad de intercambio catiónico, OC: carbono orgánico, TN: nitrógeno total.
| SAMPLE | pH | Pppm | H% | OC% | TN% | CECmeq/100 mg | Mnppm | Feppm | Znppm | Cuppm | Bppm |
| TSA | 5.1a | 18.8a | 50.4a | 9.5a | 1.10a | 65.1a | 0.64a | 116a | 3.20a | 0.08a | 0.52a |
| TCA | 4.7a | 67.3b | 47.1a | 18.6b | 1.20a | 76.4a | 0.88a | 81.2b | 1.30a | 0.18a | 1.50b |
| VSA | 3.9b | 70.1b | 32.6b | 8.1c | 0.57b | 42.1b | 3.40b | 368a | 2.00a | 0.16a | 0.86a |
| VCA | 3.9b | 198c | 33.7b | 9.3a | 0.56b | 46.4b | 1.80c | 233a | 0.52b | 0.22a | 0.66a |
| ZSA | 4.1b | 18.8a | 33.6b | 5.6d | 0.42b | 30.4c | 1.70c | 314a | 1.10a | 0.40a | 0.51a |
| ZCA | 4.0b | 76.4b | 30.1b | 7.4c | 0.50b | 41.0b | 2.70c | 305a | 0.48b | 0.26a | 0.82a |
Furthermore, the PCA samples had higher phosphorus contents than did the PSA sample (statistically significant), which is consistent with the application of synthetic phosphorus through the addition of N:P:K fertilisers. Likewise, the OC content was greater in the PCA samples than in the PSA samples (statistically significant); this difference can be attributed to movements of the soil during potato cropping in the PCA samples, which cause a shift in the soil layers and a possible increase in the clay contents on the surface, as well as a decrease in organic matter; meanwhile, in the PSA samples, more organic matter can be accumulated due to the absence ofthe mechanic activities on the soil. . The soil moisture content was higher in the PSA samples, possibly due to the sunlight protection provided by the grassland, in contrast to PCA samples that are subject to a constant turnover by farmers and can lose water through evaporation due to direct solar radiation.
Amidase activity and its relationship to urease and protease activities
The PSA soil samples showed a greater amidase activity than did the PCA samples. These differences are statistically significant between treatments with and without a record of potato cropping and agrochemical application, and also between sites (Fig. 1). In the case of the TCA and VCA samples, no amidase activity was detected, which can be associated with hydrolysis products of the chemical supplements applied (i.e., those that modify soil pH), the type and concentration of chemical substances present in the soil, and a greater biological activity related to a greater organic matter content in the PSA samples, as mentioned in the previous section. According to Ochoa et al. (2007), both microbial activity as well as microbial enzyme production can be adversely affected in the presence of inorganic fertilisers used in conventional agricultural systems. Moreover, the obtained results indicate a possible decrease in the biological activity of soils under cultivation and agrochemical application. Klose and Ajwa (2004) reported that the presence of agrochemicals can affect soil quality. In addition, Avellaneda et al. (2012) reported different extracellular enzymatic activities (urease, protease, acid phosphatise and alkaline phosphodiesterase, ß-glucosidase, arylsulfatase) in the bacterial consortia (Bacillus subtilis, Brevundimonas diminuta, Flavimonas oryzihabitants) from the same soils used in this study (PCA and PSA) from Tausa, Villapinzón, and Zipaquirá counties . The type of land use and the location affected the enzymatic activities, observing higher protease, alkaline phosphatase, and arylsulphatase activities in the bacterial consortia for the PSA samples compared to the PCA samples (50, 83, and 58% increases, respectively) in Villapinzón. The same behaviour was reported at Zipaquirá for protease, alkaline phosphatase, and arylsulphatase activities (63, 67, and 21% increase, respectively), and at Tausa for urease and arylsulphatase (70 and 81% increase, respectively), indicating a possible inhibitory effect due to fertilisation, agrochemical use, and conventional tillage practices.

Figure 1. Amidase activity of soils evaluated in this study (ugN-NH4/g dry soil). TSA, VSA, and ZSA: Tausa, Zipaquirá, and Villapinzón without agrochemical application. TCA, VCA, and ZCA: Tausa, Zipaquirá, and Villapinzón with agrochemical application.
Figura 1. Actividad amidasa de los suelos evaluados en este estudio (ugN-NH4/g de suelo seco). TSA, VSA y ZSA: Tausa, Zipaquirá y Villapinzón sin aplicación de agroquímicos. TCA, VCA y ZCA: Tausa, Zipaquirá y Villapinzón con aplicación de agroquímicos.
The pesticide carbofuran is widely used as an insecticide, acaricide, and nematicide throughout the world (Chelinho et al., 2011) and in Colombia (Fedepapa, 2005). There have been reports of carbamate hydrolase enzyme participation in methylcarbamate degradation (Tomasek & Karns, 1989) in bacterial-mediated processes (Parekh et al., 1994) as well as in soils used for the cultivation of a unique potato variety in the municipality of Silos (department of Norte de Santander, Colombia) with different temporal history of plaguicide application than in the sites studied here Castellanos et al., 2013). Carbofuran can be hydrolysed by amidases or esterases (Chapalamadugu & Chaudhry, 1993).
The results obtained for the PCA soils with no amidase activity (TCA and VCA) and low amidase activity (ZCA) suggest that the application of various agrochemicals is preventing the establishment of an adequate enzymatic stability and activity, and therefore, agrochemical degradation via amidase is not a significant process in the potato crop. Currently, there is quantitative evidence of carbofuran bacterial degradation in the studied soils Burns and Wallenstein (2010) reported that soil enzyme stability represents a potential reservoir because enzymes represent the first catalytic response towards changes in substrate availability in soil. Furthermore, this give rise to the production of signalling molecules for substrate sensing by microbial communities as well as population growth sensing, in which gene function is related to cellular density. As a consequence, enzymes are only synthesized when cell numbers are sufficiently high.
Avellaneda (2008) reported urease activity values (μg N g-1 dry soil 2 h-1) for the studies soils without agrochemical application (TSA: 4.1; VSA: 4.8; ZSA: 22.7) and with agrochemical application - PCA - (TCA: 2.8; VCA: 2.1; ZCA: 20.7). In addition, Anacona (2008) reported protease activity values (μg Tir g-1 dry soil 2 h-1) for the same soils without agrochemical application - PSA - (TSA: 133; VSA: 199; ZSA: 437) and with agrochemical application - PCA - (TCA: 258; VCA: 168; ZCA: 273). The urease and protease activities showed the same tendency as the amidase activity, with the exception of the protease enzyme in TCA. The PSA samples showed greater enzymatic activity than PCA samples. It is likely that the contrasting protease activity values obtained for the TSA and TCA soils could be associated with variations in specific physicochemical and environmental soil characteristics unique to each siteand well as variations in the temperature, nutrient availability, and litter fall, as observed for dehydrogenase, urease, phosphatase, and arylsulphatase activities in a forest soil in Korea (Kang et al., 2009). In addition, Ahmad et al. (2001) reported that microorganisms and plant roots are the greatest contributors to soil enzyme sources; however, enzyme production is unstable due to factors such as seasonal variations, low enzyme levels in plant tissues, the influence of the soil status and the fractions with which these enzymes can associate, the clay mineral content, organic matter levels, and the aqueous soil phase (Burns, 1982).
The principal components analysis (PCA) obtained for the various studied parameters is illustrated in Fig. 2. These results show an accumulated variance of 76.8% for axes 1 and 2, which explains a high percentage of the data variance. These demonstrate that the PSA samples (TSA, VSA, ZSA) had an overall greater activity of enzymes associated with the nitrogen cycle compared to the PCA samples. The activities of three enzymes showed a direct relationship, as indicated by the location of the vectors. This correlation suggests a synergistic relationship among these enzymes without antagonistic or competitive effects with respect to soil biochemistry. Therefore, this result is consistent with amidase catalysis of amide hydrolysis resulting in the production of ammonium and carboxylic acid as well as ammonium nitrogen and CO2 resulting from urea-type compounds produced by urease; these findings are also consistent with soil protein hydrolysis and degradation by proteases. Likewise, the PCA results indicated a direct relationship between these enzymes and soil microelement levels (Mn, Fe, and Cu), which have been suggested as possible cofactors of these enzymes associated with the nitrogen cycle. In contrast, Frankerberger and Tabatabai (1981) reported an inhibitory effect of trace elements on amidase activity based on assays indicating a 3% inhibition due to the effects of Cu (I), Ba (II), Cu (II), Fe (II), Ni (II), Al (III), Fe (III), Ti (III), V (IV), As (V), Mo (VI), W (II). Likewise, Frankerberger and Tabatabai (1985) indicated that the soil components notably influence the reactions catalyzed by this enzyme, since, according to comparative studies of the characteristics a soil amidase and an amidase obtained from a soil bacterial isolate, they reported that the bacterial amidase had a 25% inhibitory percentage in the presence of different elements such as Ag (I), Cd (II), Cu (II), Hg (II), Ni (II), Pb (II), Zn (II), Al (III), Se (IV). In contrast, Frankerberger and Tabatabai (1980a) indicated that amidase activity requires Mg2+ and/ or Ca2+ ions. Using dialysis and carbamate hydrolase enzyme inactivation assays, Alam (2009) indicated that amidase activity was recovered by the addition of Mn (II) and Co (II) as cofactors; furthermore, Karns and Tomasek (1991) reported the requirement of Mn (II) for adequate enzymatic activity of the carbofuran hydrolase of Achromobacter sp. strain WM II.
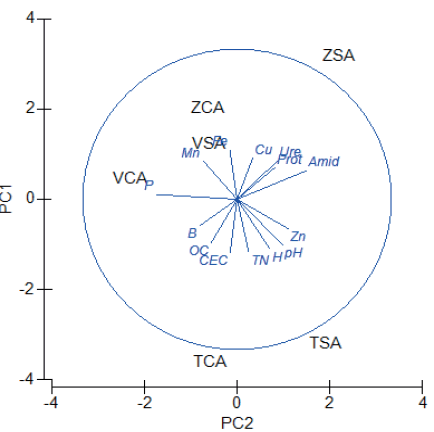
Figure 2. Principal component analysis of enzyme activities and physicochemical parameters. TSA, VSA, and ZSA: Tausa, Zipaquirá, and Villapinzón without agrochemical application. TCA, VCA, and ZCA: Tausa, Zipaquirá, and Villapinzón with agrochemical application. Amid: amidase, Ure: urease, Prot: protease, H: humidity, CEC: cation-exchange capacity, OC: organic carbon, TN: total nitrogen, Cu: copper, Fe: iron, Mn: manganese, P: phosphorus, B: boron, Zn: zinc.
Figura 2. Análisis de componentes principales de actividades enzimáticas y parámetros fisicoquímicos. TSA, VSA y ZSA: Tausa, Zipaquirá y Villapinzón sin aplicación de agroquímicos. TCA, VCA y ZCA: Tausa, Zipaquirá y Villapinzón con aplicación de agroquímicos. Amid: amidasa, Ure: ureasa, Prot: proteasa, H: humedad, CEC: capacidad de intercambio catiónico, OC: carbono orgánico, TN: nitrógeno total, Cu: cobre, Fe: hierro, Mn: manganeso, P: fósforo, B: boro, Zn: zinc.
In summary, PCA soil samples (TCA, VCA, ZCA) showed a null or low amidase activity compared to PSA samples (TSA, VSA, ZSA). This difference indicates a possible inhibitory effect of agrochemicals on the amidase activity and suggests that the degradation of carbofuran (an agrochemical used in potato cropping) via amidase activity is not significant. In addition, there is a direct relationship among amidase, urease, and protease activities as well as the levels of soil microelements (Mn, Fe, and Cu).
ACKNOWLEDGMENTS
We acknowledge the financial support provided by the Division de Investigación sede Bogotá of the Universidad Nacional de Colombia. We also acknowledge the farm owners who allowed us to collect the samples, FEDEPAPA for logistic support, C. Narváez for contributions to this study, M. Melgarejo for collaboration with the analytical assays and S. Campos for the statistical analysis.
REFERENCES
- Ahmad, Z; M Ateeq & M Arshad. 2001. Soil enzymes research: a review. OnLine J. Biol.Sci. 1(5): 299-307. [ Links ]
- Alam-Naqvi, S. 2009. Biodegradation of carbamates by soil bacteria and characterization of the methyl carbamates degradation hydrolase «med» heterologously expressed in Escherichia coli. Thesis doctoral, Department of Microbiology. Quaid-i-Azam University. Islamabad, Pakistan. 154 p.
- Alef, K & P Nannipieri (eds). 1995. Methods in applied soil microbiology and biochemistry. Academic Press. London, England. 575 p.
- Alvear, Z; B Pino; C Castillo; C Trasar & F Gil. 2006. Efecto de la cero labranza sobre algunas actividades biológicas en un alfisol del sur de Chile. J. Soil Sci. Plant Nutr. 6(2): 38-53. [ Links ]
- Anacona, A. 2008. Efecto del manejo agrícola sobre la composición microbiana y actividad enzimática de suelos provenientes de agroecosistemas de papa (Solanum sp). Tesis magister. Instituto de Biotecnología. Universidad Nacional de Colombia sede Bogotá. Bogotá, Colombia. 107 p.
- Anderson, T. 2003. Microbial eco-physiological indicators to asses soil quality. Agric. Ecosyst. Environ. 98: 285-293.
- Avellaneda, L; LM Melgarejo; C Narváez & J Sánchez. 2012. Actividades enzimáticas en consorcios bacterianos de suelos bajo cultivo de papa con manejo convencional y bajo pastizal. Rev. Fac. Nac. Agron. Medellín. 65(1): 6349-6360. [ Links ]
- Avellaneda, L. 2008. Actividades enzimáticas en suelos con y sin historia de uso agrícola y manejo convencional y de sus consorcios bacterianos. Tesis Magister. Departamento de Química. Facultad de Ciencias. Universidad Nacional de Colombia sede Bogotá. Bogotá, Colombia. 208 p.
- Bonilla, MH; F Cardozo & A Morales. 2009. Agenda prospectiva de investigación y desarrollo tecnológico para la cadena productiva de la papa en Colombia con énfasis en papa criolla. Ministerio de Agricultura y Desarrollo Rural, Universidad Nacional de Colombia, Corporación Colombiana de Investigación Agropecuaria-Corpoica (eds). Bogotá, Colombia.
- Burns, R & W Wallenstein. 2010. Microbial extracellular enzymes and natural and synthetic polymer degradation in soil: current research and future prospects. In: Gilkes R & N Prakongkep (eds). 2010. 19Th world congress of soil science, soil solutions for a changing world. Vol.1. Society of Soil Science Incorporated. Brisbane, Australia. Available online: http://www.iuss.org/19th%20WCSS/Symposium/pdf/0549.pdf. Last visited on May 2013.
- Burns, R. 1982. Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol. Biochem. 14: 423-427. [ Links ]
- Castellanos, J; J Sánchez; D Uribe; L Moreno & LM Melgarejo. 2013. Characterization of carbofuran degrading bacteria obtained from potato cultivated soils with different pesticide application records. Rev. Fac. Nac. Agron. Medellín. 66(1): 6899-6908.
- Cerón, L & LM Melgarejo. 2005. Enzimas del suelo: indicadores de salud y calidad. Acta. Biol. Colomb. 10: 5-18. [ Links ]
- Chapalamadugu, S & G Chaudhry. 1993. Isolation of a constitutively expressed enzyme for hydrolysis of carbaryl in Pseudomonas aeruginosa. J. Bacteriol. 175: 6711-6716. [ Links ]
- Chelinho, S; KD Sautter; A Cachada; I Abrantes; G Brown; DA Costa & JP Sousa. 2011. Carbofuran effects in soil nematode communities: Using trait and taxonomic based approaches. Ecotoxic. Environ. Safe. 74(7): 2002-2012.
- Desaint, S; S Arrault; S Siblot & JC Fournier. 2003. Genetic transfer of the mcd gene in soil. J. Appl. Microbiol. 95(1): 102-108.
- Devaux, A. 2010. El sector papa en la región andina: experiencias recientes y perspectivas. En: XXIV Congreso de la asociación latinoamericana de la papa ALAP. (Cusco, Perú). Asociación Latinoamericana de la Papa ALAP. Dirección de investigación agraria INIA-Perú (ed). 443 p. Available online: http://www.papaslatinas.org/descargar.php?file=alap2010/ Memorias%20XXIV%20Congreso%20ALAP%20-%202010.pdf Last visited on May 2013.
- Doran, WJ; AJ Jones; MA Arshad & JE Gilley. 1999. Determinants of soil quality and health. In: Rattan, L. (ed). Soil quality and soil erosion. CRC Press, Florida, EUA.
- Fedepapa (ed). 2005. Guía para el cultivo de la papa. Panamericana Formas e Impresos S.A. ISSN: 1794-3027. Bogotá, Colombia. 229 p.
- Frankenberger, WTJr & MA Tabatabai. 1980a. Amidase activity in soils: I. Method of assay. Soil Sci. Soc. Am. J. 44: 282-287. [ Links ] In: Alef, K & P Nannipieri. 1995. Methods in applied soil microbiology and biochemistry. Academic Press. Elsevier Ltda. 576 p.
- Frankenberger, WT & MA Tabatabai. 1981. Amidase Activity in Soils: IV. Effects of Trace Elements and Pesticides. Soil Sci. Soc. Am. J. 45: 1120-1124. [ Links ]
- Frankenberger, WT & MA Tabatabai. 1985. Characteristics of an amidase isolated from a soil bacterium. Soil Biol. Biochem. 17: 303- 308. [ Links ]
- González, F A & D Malagon (eds). 1990. Métodos analíticos del laboratorio de suelos. Instituto Geográfico Agustín Codazzi: Bogotá. Colombia. 147 p.
- Kang, H; S Kang & D Lee. 2009. Variations of soil enzyme activities in a temperate forest soil. Ecol. Res. 24: 1137-1143. [ Links ]
- Karns, J & P Tomasek. 1991. Carbofuran hydrolase-purification and properties. J. Agric Food Chem. 39: 1004-1008. [ Links ]
- Kay-Shoemake, J; M Watwood; R Sojka & R Lentz. 2000. Soil amidase activity in polyacrylamide-treated soils and potential activity toward common amide-containing pesticides. Biol. Fertil. Soils. 31: 183-186. [ Links ]
- Klose, S & H Ajwa. 2004. Enzyme activities in agricultural soils fumigated with methyl bromide alternatives. Soil Biol. Biochem. 36: 1625-1635.
- Ladd, JN & JHA Butler. 1972. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 4(1): 19-30. [ Links ]
- Masera, O; M Astier & R López. 1999. Sustentabilidad y manejo de recursos naturales: el marco de evaluación MESMIS. Mundi Pren-sa-GIRA-UNAM (eds). México. 109 p.
- Nielsen, M & A Winding. 2002. Microorganisms as indicators of soil health. National Environmental Research Institute. Denmarck. Technical Report N° 388. 84 p.
- Ochoa, V; B Hinojosa; B Gómez-Muñóz & R García-Ruíz. 2007. Actividades enzimáticas como indicadores de calidad del suelo en agroecosistemas ecológicos. Rev. Ini. Inv. 2: rl. 10 p. Available online: http://revistaselectronicas.ujaen.es/index.php/ininv/article/view/ 251/233 Last visited on May 2013.
- Pankhurst, C & BM Doube. 1997. Biological Indicators of Soil Health. CAB International Washington, USA. 451p.
- Parekh, NR; DL Suett; SJ Roberts; T McKeon; ED Shaw & AA Jukes. 1994. Carbofuran degrading bacteria from previously treated field soils. J. Appl. Bacteriol. 76: 559-567. [ Links ]
- Plangklang, P & A Reungsang. 2012. Isolation and characterization of carbofuran degrading Burkholderia sp. PCL3 from carbofuran phytoremediated rhizosphere soil. Chem. Ecol. 28(3): 253-266. [ Links ]
- Tabatabai, MA & AD Warren. 1993. Significance and potential uses of soil enzyms. In: Blaine, MF.(ed.). Soil Microbial Ecology. Marcel Decker Inc. New York USA. 95-127 p.
- Tomasek, PH & JS Karns. 1989. Cloning of a carbofuran hydrolase gene from Achromobacter sp strain WM 111 and its expression in gram negative bacteria. J. Bacteriol. 171: 4038-4044. [ Links ]
- Zapata, R. 2004. Química de la acidez del suelo. Cali, Colombia. 208 p.














