Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista argentina de cardiología
versión On-line ISSN 1850-3748
Rev. argent. cardiol. vol.83 no.3 Ciudad Autónoma de Buenos Aires jun. 2015
SCIENTIFIC LETTERS
Vernakalant in atrial Fibrillation: 2-Year Experience
VERNAKALANT EN FIBRILACION AURICULAR: 2 AÑOS DE EXPERIENCIA
Treatment of acute atrial fibrillation (AF) includes a series of antiarrhythmic agents such as vernakalant, a new intravenous drug with a novel mechanism of action different from all known agents, whose effec-tiveness has been proved in randomized studies. (1-3)
It was introduced in the Argentine market in 2012 and since then has been available in our center for patients with acute A F.
The purpose of our study is to show the outcomes of the first 2-year-experience with Vernakalant in our institution.
This is an observational, retrospective, single-centered study, conducted at the Emergency Depart-ment of the Instituto Cardiovascular de Buenos Aires (ICBA). From March 1, 2012 to January 30, 2014, 121 patients with acute AF without hemodynamic insta-bility were registered in our center; receiving ver-nakalant therapy for conversion. An initial dose of 3.0 mg/kg was administered during 10-minutes, followed by a 15-minute observation period; and in the absence of conversion, a second 10-minute dose of 2 mg/kg was given.
Inclusion criteria: Patients ≥18 years of age, weigh-ing between 45 and 136 kg, with clear symptom onset over the last 48 hours, systolic blood pressure >90 mmHg and <160 mmHg, and diastolic blood pressure <95 mmHg. Pregnant women and patients with atri-al flutter, sinus node disease, QRS >140 ms without pacemaker, heart failure or recent acute coronary syn-drome were excluded. Patients with severe valve dis-ease, restrictive or obstructive cardiomyopathy, or pa-tients with ejection fraction <35% were also excluded.
Conversion criteria: Conversion time was the pas-sage to sinus rhythm maintained until patient dis-charge. Failure of pharmacological conversion with vernakalant was persistent AF 2 hours after the sec-ond dose, based on international protocol recommen-dations.
Adverse events: Death, sustained hypotension (systolic blood pressure ≤90 mmHg), bradycardia at <40 bpm, QT prolongation >440 ms, ventricular ar-rhythmia (≥ triplets) or any other event requiring or extending patient hospitalization were considered se-vere adverse events. Taste disturbance, cough, nausea and dizziness, or any other event not included in the "severe" category was considered a non-serious event.
Discrete variables were expressed as percentages. Continuous variables were expressed as mean or median depending on distribution, with the correspond-ing standard deviation or interquartile range.
Results were analyzed using SPSS 21 software.
Data from 121 consecutive patients treated with vernakalant were recorded. Mean age was 58.1±13.9 years, and 67.7% were men (Table 1). A total of 46.2% mpatients were hypertensive, and only 1.65% had diabetes.
The episode of AF that motivated consultation was a first event in 28.9% of cases, with an average heart rate of 118.9±27 beats per minute and a median evo-lution time of 4 hours (2-10) (Table 2).
Structural heart disease was present in 13.2% of patients, with mean ejection fraction of 60.2%±6.4% and atrial area of 20.6±4.4 cm2.
A total of 84.3% patients converted to sinus rhythm, with 45.4% requiring the second dose of vernakalant. Electrical cardioversion was successful in 90% of the patients who did not convert with vernakalant, with only 2 patients remaining in atrial fibrillation.
Time to conversion was 9 minutes (6-18) and total hospital stay was 165 minutes (110-210).
Twenty-four percent of patients had adverse events. Severe adverse events were present in only 4 patients, represented by bradycardia at 40 bpm lasting <5 minutes with no need for medication and
table 1. General population characteristics
| Variable | Value |
| age, years | 58.1±13.9 |
| male gender, % | 67.7 |
| Weight, kg | 79.5±14.9 |
| Height, cm | 173.5±12.5 |
| Body surface área, m2 | 1.93±0.23 |
| HTN, % | 46.2 |
| DM, % | 1.65 |
| COPD, % | 1.65 |
HTN: Hypertension. DM: Diabetes mellitus. COPD: Chronic obstructive pulmonary disease.
Table 2. Characteristics and previous treatment of AF
| Variable | Value |
| Heart rate, bpm | 118.9 ± 27 |
| evolution time, hours | 4 (2-10) |
| First AF episode, % | 28.9 |
| Previous treatment | |
| Beta-blockers, % | 34.7 |
| Calcium blockers, % | 1.65 |
| Propafenone/Flecainide, % | 17.4 |
| Amiodarone, % | 11.6 |
| Anticoagulation, % | 31.4 |
| CHADS, % 2 | |
| 0 | 14.8 |
| 1 | 52.9 |
| 2 | 16.5 |
| 3 | 16.5 |
bpm: Beats per minute. AF: Atrial fbrillation.
without hemodynamic instability in 2 patients, hypo-tension requiring volume expansion with saline in 1 patient, and non-sustained ventricular tachycardia in 1 patient. The remaining adverse events (25 patients) were within the group of non-serious events, repre-sented by taste disturbance and, to a lesser extent, paresthesia, cough and nausea, all of them transient and well tolerated.
Intravenous vernakalant is an atrial-selective an-tiarrhythmic drug that prolongs the refractory period with little effect on ventricular repolarization. The ACT and ACT 3 studies supported vernakalant safety with a low rate of hypotension and proarrhythmic com-plications. (1,2) The AVRO study demonstrated safety in patients with moderate structural heart disease, excluding patients with obstructive cardiomyopathy, severe valve disease or recent myocardial infarction, among others. (3) From these results, the 2012 Eu-ropean guidelines recommend the use of vernakalant for conversion of acute A F, excluding patients with EF <35% due to lack of experience, severe valve disease, evolving heart failure or recent coronary event. (5)
In our experience, most patients had absence of structural heart disease, with normal or slightly increased atrial size, which would probably explain the high rate of successful conversion with the medica-tion, higher than that shown in other studies.
Time to conversion was similar to that published in the AVRO study(3), which impacts directly on pa-tient stay in the Emergency Department. This faster conversion time is directly associated with reduced hospital stay compared to previous studies with drugs such as propafenone or flecainide, resulting in health, financial and well-being benefits for patients, who ex-perience less hospitalization stress. (6)
Regarding safety, patients treated with vernaka-lant presented mainly non-serious adverse events, such as dysgeusia, cough and nausea, as in the case of large studies. (1-3, 5) These effects did not have an im-pact on patient health and were very well tolerated. In turn, vernakalant caused no reaction in the adminis-tration area, which is an advantage when compared to drugs such as amiodarone, with a high rate of chemi-cal phlebitis.
These results have generated enough confidence in our center to use vernakalant as the first-line an-tiarrhythmic drug for the treatment of patients with acute A F.
In our experience, vernakalant has been an effec-tive and safe drug for the treatment of A F, enabling a rapid resolution and discharge of these patients.
Juan P. Costabel, Florencia Lambardi, Martín Aragón, Roberto Campos, Alberto GinigerMTSAC, Diego CondeMTSAC
Instituto Cardiovascular de Buenos Aires (ICBA) -Cardiovascular
Emergency Unit
Blanco Encalada 1543 - (1428) CABA
e-mail: jpcostabel@icba.com.ar
REFERENCES
1. Roy D, Rowe BH, Stiell IG, Coutu B, Ip JH, Phaneuf D, et al. A randomized, controlled trial of RSD1235, a novel anti-arrhythmic agent, in the treatment of recent onset atrial fibrillation. J Am Coll Cardiol 2004;21:2355-61. http://doi.org/b39mdx
2. Roy D, Pratt CM, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation 2008;117:1518-25. http://doi.org/bp978f
3. Camm AJ, Capucci A, Hohnloser SH, Torp-Pedersen C, Van Gelder IC, Mangal B, Beatch G; AVRO Investigators. A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J Am Coll Cardiol 2011;57:313-21.http://doi.org/bsf438
4. Savelieva I, Graydon R, Camm AJ. Pharmacological cardioversion of atrial fibrillation with vernakalant: evidence in support of the ESC Guidelines. Europace 2014;16:162-73. http://doi.org/2r3
5. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. http://doi.org/n97
6. Conde D, Costabel J P, Aragon M, Lambardi F, Klein A, Corrales Barbosa A, et al. Propafenone versus vernakalant for conversion of recent-onset atrial fibrillation. Cardiovasc Ther 2013;31:377-80. http://doi.org/2r4
Rev Argent Cardiol 2015;83:246-247 - http://dx.doi.org/10.7775/rac.v83. Í3.5020
thromboangiitis Obliterans in a Young Woman with Dilated Cardiomyopathy
Thromboangiitis obliterans (TAO) is a non-atheroscle-rotic occlusive segmental inflammatory disease affect-ing most commonly small and medium-sized arteries and superficial venules. (1, 2) In 1908, Leo Buerger described this entity in heavy young male smokers with amputated lower limbs, secondary to ischemic lesions. Cases described in women are intermittent. Thromboangiitis obliterans is considered a form of vasculitis (2), with uncommon visceral involvement. (3) Only a few cases with cardiac involvement have been published in the literature. Most of them corre-spond to young patients with acute myocardial infarc-tion and presumptive coronary artery disease. Dilated cardiomyopathy has not been described in the context of this disease.
We report a case of TAO in a 19 year-old woman, occasional smoker, with symptomatic dilated cardio-myopathy.
She presented with dyspnea and a 2-month his-tory of lower limb intermittent claudication. She was an occasional smoker and had no history of diabetes, hypertension, dyslipidemia, or heart disease. Physical examination: normotensive, well-defined ischemic necrosis of hallux, second and third toes of the left foot (Figure 1). Pulses: absence of right radial, posterior tibial, and left pedal pulses; weak left radial and right ulnar pulses. Normal S1 and S2 in 4 areas, without murmurs; no S3. No peripheral edemas. Lab tests: normal complete blood cell count, blood glucose and renal function; ESR 47 mm, normal Quick and KPTT, cholesterol 162 mg/dl, LDL 108 mg/dl, HDL 32 mg/dl, TAG 87 mg/dl. Negative results for C3, C4, c-ANCA, p-ANCA, FAN, anti-DNA antibodies (AB), anti-cen-tromere AB, anti-ScL-70 AB, anti-basal membrane AB, anti-β2-glycoprotein AB, and anticardiolipin AB. Normal TSH. Negative tests for Chagas, CMV, adeno-virus, enterovirus, and HIV.
ECG: sinus rhythm, CLBBB. Doppler echocardiog-raphy / TEE: global hypokinesia, asynchronous septal motion, severe systolic function impairment. Ejection fraction: 35%.
Arterial Doppler ultrasound of the lower limbs: decreased flow of the right (posterior and anterior) tibial artery; no flow of distal posterior tibial artery. Lower extremity arteriography: normal aortography and renal arteries, occlusion in right posterior tibial (third proximal), left anterior tibial (middle third) and ipsilateral peroneal vessels. Collateral vessels in "corkscrew"-shaped pattern. Coronary angiography: normal thoracic aorta and neck vessels. Normal left main coronary artery, right coronary and circumflex arteries. Occlusion between the middle third and dis-tal portions of the anterior descending artery, filled by collateral circulation.
While in hospital, the patient suffered cardiopul-monary arrest in the context of VT/VF. She responded to advanced CPR, requiring MV and inotropic agents for 5 days. ICD was indicated to treat recurrent VT. Right radial artery biopsy: medium-sized artery with a preserved internal elastic lamina (Figure 2), luminal occlusion by loose fibrosis and neoformed vessels with focal intimal involvement, fibrin remains, and iso-lated polymorphonuclear leukocytes in the muscular layer. Diagnosis: progress of TAO organization.
The patient made good progress and was dis-charged with advice to quit smoking, which she fol-lowed. Ischemic necrosis of the lower limbs was resolved with medical treatment.
The case reported is similar to those described by Winiwarter and Buerguer more than a century ago: limb ischemia in a young smoker. (1) What is remark-able in our patient is her gender, age, the fact that she was not a heavy smoker and the coexistence of severe dilated cardiomyopathy.
Thromboangiitis obliterans should be suspected in young smokers with limb ischemia, in the absence of other vascular conditions (atherosclerosis, diabetes, thrombophilia, rheumatic diseases, etc.). Symptoms are secondary to stenosis and occlusion: limb claudica-tion, pain at rest, necrosis, and ulceration. Up to 40% of patients experience asymmetric Raynard's phe-nomenon and migratory superficial thrombophlebitis. (1, 2, 5) There are only a few publications about mesenteric, cerebral, renal, or coronary alterations. (3, 4)
While smoking determines the occurrence, progres-sion and prognosis of ischemic lesions, pathophysiolo-gy of TAO remains uncertain. A genetic predisposition has been proposed and described in non-smokers. (2) The populations with the highest prevalence of this disease are from Middle and Far East countries rather than from countries in the Western Hemisphere or America. (1, 2)
It is known that the cellular immune responses against type I and III collagen of the vessel wall and anti-endothelial cell antibodies play an important role in this entity. (1, 2, 5) In the acute phase, it is charac-terized by highly cellular and inflammatory thrombi,

Fig. 1. Ischemic necrosis on left foot.

Fig. 2. Preserved internal elastic lamina in small-sized vessel.
including "microabscesses" and giant cells in small-and medium-sized vessels with intact internal elas-tic lamina. This feature of the elastic lamina distin-guishes TAO from other types of systemic vasculitis and from atherosclerotic changes. (2, 4) In subacute and chronic phases, the thrombus is organized and becomes fibrosis.
Although there are no universally accepted diag-nostic criteria for TAO, those proposed by Olin are commonly followed. (1) Diagnosis requires ruling out diabetes, scleroderma, CREST syndrome, vascu-litis, distal embolization, and hypercoagulable states. Erythrocyte sedimentation rate and C-reactive pro-tein values are typically normal in these patients.
Computed tomography angiography and magnetic resonance angiography can show the vascular involve-ment. Angiography is the gold standard, presenting a typical (non-pathognomonic) "corkscrew"-shaped pat-tern and distal collateral vessels with "tree root" or "spiderweb" configuration. (1, 5)
In 1980, the prevalence among women was <2%. In 1986, a Mayo Clinic study (6) presented 12 histo-logically verified cases of TAO in women (11%), seen over a 5-year period in a series of 109 patients. All of them required amputation or revascularization and none presented organic vascular involvement.
Cardiac involvement is described in young men with previous diagnosis of TAO, who present an acute coronary event. (4) We have not found articles describ-ing cardiac involvement in women. In the present pa-tient, other causes of dilated cardiomyopathy were ruled out. Coronary angiography suggested cardiac involvement due to TAO.
The clinical course of TAO is characterized by phases of exacerbations separated by phases of remis-sion that often last for several years. Giving up smoking is indispensable to the prognosis of patients with TAO. (1, 2, 5) Nicotine replacement therapy should be strongly discouraged, as it may perpetuate the inflammatory activity. Local ulcer care together with the use of vasodilators, are also a priority.
Revascularization treatment is rarely effective due to the distal diffuse nature of vascular damage.
Ischemic lesions of the limbs in the absence of systemic disease should be suggestive of TAO in both men and women, regardless of their smoking habit, or even in its absence.
Thromboangiitis obliterans is a rare, aggressive, chronic disease that may present systemic vascular in-volvement. Anatomopathological findings and angio-graphic patterns are the basic diagnostic instruments. In cases of cardiac involvement, other causes of heart disease should be ruled out.
Andrea N. Odzak, Florencia Arcondo,
Leandro Bono, Jorge Estrada,
Marcelo Amante, Marcelo Zylberman.
Hospital General de Agudos "Dr. CosmeArgerich" e-mail: andreaodzak@gmail.com
REFERENCES
1. Olin J W. Thromboangiitis obliterans(Buerger'sdisease).N Engl J Med 2000;343:864-9. http://doi.org/fcfdnk
2. Puéchal X, Fiessinger JN. Thromboangiitis obliterans or Buerg-er's disease: challenges for the rheumatologist. Rheumatology 2007;46:192-9. http://doi.org/cmx557
3. Calgüneri M, Oztürk MA, Ay H, Arsava EM, Altinok D, Ertenli I, et al. Buerger's disease with multisystem involvement. A case report and a review of the literature.Angiology 2004;55:325-8.
4. Becit N, Unlu Y, Kocak Hl, Ceviz M. Involvement of the coronary artery in a patient with thromboangiitis obliterans. A case report. Heart Vessels 2002;16:201-3. http://doi.org/d8457g
5. Piazza G, Kreager M. Thromboangiitis obliterans. Circulation 2010;121:1858-61. http://doi.org/cf3gmz
6. Lie J T. Thromboangiitis obliterans(Buerger's disease) inwomen. Medicine 1987;66:65-72. http://doi.org/bxq6n5
Rev Argent Cardiol 2015;83:247-249 - http://dx.doi.org/10.7775/rac.v83. 13.5071
Cardiac and renal amyloidosis: Combined trans-plantation as therapeutic Option
Amyloidosis defines a group of diseases characterized by extracellular deposition of fibrillar proteins. It can be acquired or hereditary. They are differentiated by their pathogenesis, clinical manifestation and prognosis. AL amyloidosis (primary systemic amyloidosis) is the most frequent type.
While uncommon, the incidence and prevalence of this disease have been difficult to estimate. The aver-age age for diagnosis is about 65 years and only 10% of patients are less than 50 years old when diagnosed.
Monoclonal light-chain deposition occurs in AL amyloidosis, usually associated with plasma cell dys-crasia, and rarely multiple myeloma. It is the most severe form of amyloidosis due to its systemic involve-ment, with chain deposition occurring in multiple or-gans causing their progressive failure. All the organs can be affected.
The kidney is the most commonly affected organ, and renal amyloidosis is found in 2/3 of patients at diagnosis. It is usually manifested with proteinuria, renal failure and nephrotic syndrome.
Myocardial infiltration occurs in 90% of patients, clinically expressed as restrictive cardiomyopathy with diastolic heart failure in 50% of diagnosed cases. This is one of the most significant prognostic factors. Once heart failure has supervened, median patient survival is 4-6 months. (1) Patients die due to ad-vanced heart failure as a result of poor response to treatment, or due to ventricular arrhythmias or atrio-ventricular blocks secondary to amyloid deposition in the conduction system.
The cornerstone in the treatment of systemic AL amyloidosis is chemotherapy with or without bone marrow transplantation. There are various oncohema-tological schedules; however, mortality rates are high and clinical response is observed after a year. (1, 2)
For that reason, solid organ (heart, kidney) transplantation arises as complementary/alternative treat-ment. It remains controversial due to multiple rea-sons: organ shortage, recurrence of amyloidosis in the graft, and progression of systemic deposits in the remaining involved organs. (2, 3) However, encourag-ing results have been obtained in cardiac transplan-tation preceded or followed by immunosuppressive regimens, or in tandem transplants (heart-kidney, kidney-heart).
We report the case of a 67 year-old male patient, with no significant comorbidities or cardiovascular history, who was referred to our center for evaluation of progressive renal failure secondary to renal amy-loidosis, despite having been treated with rituximab.
He was evaluated by the Department of Cardiol-ogy as he presented signs of congestive heart failure. Physical examination revealed a patient in good general condition, and in anasarca: edema in the lower limbs up to the thigh roots, edema in sacral region, ascites, pleural effusion and circumorbital edema.
Lab tests revealed mild anemia and renal dysfunc-tion, with hemoglobin 11.4 g/dl, and creatinine 7.37 g/dl with renal clearance (by the MDRD method) of 6 ml/min/1.73 m2. Pro-BNP was >35,000 pg/ml and high-sensitivity troponin was 222 pg/ml. Liver func-tion test was normal, except for ALP of 141 UI/L (cut-off point of 100). Complete urinalysis: density 1,015, pH 5, proteins +++, with presence of granular hya-line casts. 24-hour urine protein was 8.34 g/24 h, or its equivalent 4.39 g/L. A monoclonal band in beta-globulin was obtained by protein analysis.
Bilateral pleural effusion was targeted in the chest x-ray, and the ECG showed sinus rhythm, with right QRS axis deviation (+120°) and complete right bundle branch block with micro-voltage (Figure 1).
Transthoracic color Doppler echocardiography identified features consistent with amyloidosis: marked wall thickening with mild deterioration of global systolic function, restrictive filling pattern, bi-atrial enlargement, and myocardial hyperrefringence with reduced tissue velocities (Figure 2; see video on the website).
After negative fluid balance of 3 liters with intra-venous diuretics, cardiac evaluation was completed with right heart catheterization, presenting features of fluid overload: right atrium: 20 mm Hg; pulmonary artery: SP 52, DP 20, MAP 35 mmHg; wedge pres-sure: 22 mmHg; cardiac output: 4.3 L/min, cardiac in-dex 2.5 L/min/m2, and Wood units: 3.6.
Due to poor response to intravenous furosemide, hemodialysis and ultrafiltration were performed, with a 10-kg weight loss. In this scenario, the case was discussed in a multidisciplinary panel including the Departments of Cardiology, Hematology, Nephrology, and the heart and kidney transplant teams. It was de-cided to delay the oncohematological treatment and begin the evaluation process for combined heart and kidney transplant, but the patient rapidly progressed to cardiogenic shock refractory to medical therapy, and finally died.
AL amyloidosis patients face a condition with poor prognosis if there is cardiac and/or renal involvement for which no well-defined treatment is available in ad-vanced stages.
Despite cardiac involvement is a poor prognostic factor, only a few heart transplants are performed with that indication. (3-5) As mentioned above, there are limitations, such as organ shortage, recurrence of amyloidosis in the graft, and disease progression in the remaining tissues.
Amyloid recurrence in the heart has been observed in average at 11 months. (2) This progression is ob-served in patients undergoing cardiac transplantation but with no additional treatment. (6) Therefore, af-ter heart transplantation, autologous stem cell trans-plant (1, 3, 4) or chemotherapy treatment has been suggested, with positive outcomes. (1, 2, 6)
Regarding noncardiac involvement, transplanta-tion is usually performed in patients with minimal involvement, which is rare in AL amyloidosis. Com-bined transplants could be an option to overcome that disadvantage.

Fig. 1. 12-lead ECG revealing sinus rhythm with axis in +120°, incomplete right bundle branch block, and micro-voltage.

Fig. 2. Apical window in transthoracic echocardiography shows marked wall thickening with mild deterioration of global sys-tolic function, restrictive flling pattern, biatrial enlargement, and myocardial hyperrefringence.
Only two cases of heart-kidney transplant in sys-temic amyloidosis have been described in the litera-ture. (5, 7) Both patients were under 50 years of age. In the Brazilian case, the procedure was performed sequentially, first the heart transplant, and the kid-ney transplant a year later, with no prior or posterior oncohematological treatment. In the French case, however, heart-kidney transplant was performed si-multaneously after failed chemotherapy in a patient with cardiac, renal and gastrointestinal involvement. Although our patient was older and had more system-ic involvement, the decision was to attempt the simultaneous transplantation of both organs, with no prior chemotherapy treatment.
In conclusion, the purpose of presenting this clini-cal case report is to pose the need of considering heart-kidney transplant as a therapeutic option for patients with this systemic disease when there is cardiac and renal involvement, either alone or combined with che-motherapy treatment or bone marrow transplant, and to contemplate the adoption of aggressive therapeutic decisions quickly, given the poor short-term prognosis of this disease when both organs are involved.
María Noël Brögger, Anibal Arias MTSAC, Rodolfo Pizarro MTSAC, César BelzitiMTSAC, Ricardo Marenchino, Guillermo Rosa Diez
Department of Cardiology, Hospital Italiano de Buenos Aires E-mail: maria.brogger@hospitalitaliano.org.ar
REFERENCES
1. Sattianayagam PT, Gibbs SD, Pinney JH, Wechalekar AD, Lach-mann HJ, Whelan CJ, et al. Solid organ transplantation in AL amy-loidosis. Am J Transplant 2010;10:2124-31. http://doi.org/dw6rb8
2. Mignot A, Bridoux F, Thierry A, Varnous S, Pujo M, Delcourt A, et al. Successful heart transplantation following melphalan plus dexamethasone therapy in systemic AL amyloidosis. Haematologica 2008;93:e32-5. http://doi.org/d65j6z
3. Gillmore JD, Goodman HJ, Lachmann HJ, Offer M, Wechalekar AD, Joshi J, et al. Sequential heart and autologous stem cell trans-plantation for systemic AL amyloidosis. Blood 2006;107:1227-9. http://doi.org/bxnc35
4. Belziti C, Bagnati R, Torres Bianqui C, Arbelbide J, Nucifora E, Domenech A y cols. Trasplante cardíaco y de médula ósea en un paciente con amiloidosis AL e insuficiencia cardíaca refractaria. Rev Argent Cardiol 2009;77:309-11.
5. Baumgratz J F, Vila JH, Guilhen CJ, Fonseca L, Leite W F, D'Andretta C, et al. Heart transplantation in primary amyloidosis. Rev Bras Cir Cardiovasc 2009;24:409-12. http://doi.org/dzsg75
6. Dubrey SW, Burke MM, Hawkins PN, Banner NR. Cardiac trans-plantation for amyloid heart disease: The United Kingdom experi-ence. J Heart Lung Transplant 2004;23:1142-53. http://doi.org/ d8grgm
7. Audard V, Matignon M, Weiss L, Remy P, Pardon A, Haioun C, et al. Successful long term outcome of the first combined heart and kidney transplant in a patient with systemic AL amyloidosis. Am J Transpl 2009;9:236-40. http://doi.org/dksq9f
arrhythmogenic right ventricular dysplasia: unmasking the epsilon wave
Arrhythmogenic right ventricular (RV) dysplasia (ARVD) described by Fontaine et al. (1) is a progres-sive cardiomyopathy of unknown etiology and genetic predisposition. It mainly affects the right ventricle replacing the normal myocardium by adipose or fibro-fatty tissue. The disease may run in families and in approximately 30% of patients different genetic disor-ders can be identified. (2)
The case presented here corresponds to a 19-year old female diagnosed with ARVD based on different complementary tests, emphasizing the usefulness of the epsilon wave in the electrocardiogram.
The patient consults at the emergency depart-ment for long-standing palpitations irradiating to the neck, associated with functional class II-III dyspnea and peripheral cyanosis. She denies personal or fam-ily history of relevant diseases. On admittance she presented a brevilineal biotype, was alert, conscious, eupneic and normotensive, with filiform peripheral pulses and no jugular distension. On auscultation she exhibited rhythmic, hypophonic cardiac sounds and a 2/6 protomesosystolic murmur in the tricuspid re-gion. The rest of the physical exam was normal. Blood tests did not show significant abnormalities. Chest X ray revealed increased cardio-thoracic ratio with ef-facement of the grooves identifying the different car-diac structures. The electrocardiogram showed sinus rhythm with 90° axis, increased P wave amplitude (3 mV) indented in L1 and from V1 to V3, and negative T waves from V1 to V5 (Figure 1a) The echocardio-gram evidenced severe dilatation of the chambers on the right side of the heart with tricuspid ring dila-tation, RV inflow tract and apical aneurysms with wall thinning (2 mm), severe dilatation of the right atrium (area 40 cm2) (Figure 2A), left ventricle with normal diameters and wall thickening, paradoxi-cal interventricular septal motion and moderate left ventricular (LV) systolic function impairment. Mag-netic resonance imaging showed severe enlargement of the chambers in the right side of the heart, with RV free wall thinning in all its extension (Figure 2B), akinesia in the middle third and apical portions and systolic and diastolic protrusion of localized areas, se-vere RV outflow tract dilatation and severe tricuspid regurgitation.
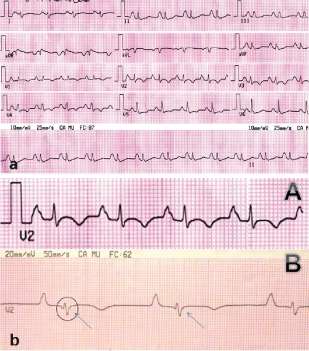
Fig. 1. a. Patient electrocardiogram on admission. b. Standard V2 lead (A). V2 lead modifed according to Fontaine. Epsilon wave (arrows) (B)

Fig. 2. a. Echocardiogram showing severe dilatation of the chambers on the right side of the heart. Right ventricular in-fow tract and apical (arrows) aneurysms with wall thinning. b. Cardiac magnetic resonance imaging. Signifcantly enlarged chambers on the right side of the heart, with right ventricular free wall thinning in all its extension. RV: Right ventricle. RA: Right atrium.
Based on these studies, a presumptive diagnosis of ARVD was made, and a new electrocardiogram was performed using a 40 Hz high-pass filter which in-creases gain to 20 mV/mm and recording velocity to 50 mm/s. A modification in the position of the limb leads (right arm lead placed on the sternal manubrium, left arm lead on the xiphoid process and the left leg lead on a rib between the normal V4 and V5 positions) im-proved sensitivity, (3) showing the presence of epsilon waves (Figure 1b, B), which together with imaging findings constitute major criteria for definitive ARVD diagnosis.
The unspecific character of most clinical signs and the absence of a unique diagnostic test encum-ber ARVD diagnosis. The International Task Force guideline postulated standardized diagnostic criteria based on the identification of structural, histological, electrocardiographic, arrhythmic and familial charac-teristics, that were then subdivided, according to the perceived specificity, into major criteria (severe RV dilatation and reduced ejection fraction not affecting the left ventricle, localized RV aneurysms, fibrofatty replacement of myocardial tissue, epsilon wave or QRS prolongation of >110 ms in right precordial leads, and confirmed familial disease) and minor criteria (mild, segmented RV dilatation, RV regional hypokinesia, in-verted T waves in right precordial leads in the absence of right bundle branch block, late potentials in signal-averaged electrocardiogram, ventricular tachycardia with LV block pattern, and history of sudden death in family member <35 years with suspected ARVD). (4) Diagnostic confirmation was the detection of two ma-jor criteria or one major and two minor criteria, or four minor criteria of different diagnostic categories. (5)
The epsilon wave, named by Fontaine (1) as a postexcitation wave, contrary to preexcitation (it was called epsilon as it was the next letter after delta in the Greek alphabet and a mathematical symbol of small-ness), is generated in areas of RV delayed activation as a consequence of fibrous or fibrofatty replacement of RV myocardial tissue and can be found in up to 30% of cases. (6) Use of leads facilitating the identification of epsilon waves in cases of difficult diagnosis, as in the case of this patient, is a tool that should be taken in consideration.
Daniel Cornejo, Mario Fitz MauriceMTSAC,
Fernando Di Tommaso, Susana Taboada,
Eduardo Fernández Rostello, Enrique Domine
Cardiology Department, Hospital General de Agudos Bernardino
Rivadavia, Buenos Aires e-mail: dgcornejo@gmail.com
REFERENCES
1. Fontaine GH, Guiraudon G, Frank R. Stimulation studies and epicardial mapping in ventricular tachycardia: study of mechanisms and selection for surgery. En: Kulbertus HE, editor. Re-entrant ar-rhythmias: mechanisms and treatment. Baltimore: University Park Press; 1977. p. 334-50.
2. Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 1996;335:1190-6. http://doi.org/c9vv36
3. Quarta G, Elliott PM. Diagnostic criteria for arrhythmogenic right ventricular cardiomyopathy. Rev Esp Cardiol 2012;65:599-605. http://doi.org/f2fkwj
4. McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 1994;71:215-8. http://doi.org/fvbvq8
5. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomy-opathy/dysplasia: proposed modification of the Task Force criteria. Eur Heart J 2010;31:806-14. http://doi.org/bjc6bg
6. Gemayel C, Pelliccia A, Thompson PD. Arrhythmogenic right ven-tricular cardiomyopathy. J Am Coll Cardiol2001;38:1773-81. http:// doi.org/fmj35g
Rev Argent Cardiol 2015;83:251-253 - http://dx.doi.org/10.7775/rac.v83. Í3.5161
Flash acute pulmonary edema as atypical presenta-tion of type a aortic dissection
Flash acute pulmonary edema (flash APE) is a form of sudden heart failure precipitated, among other causes, by ischemia, hypertension, and hypervolemic and valvular conditions. Flash APE as a manifestation of type A aortic dissection is a rare presentation.
A case is described with this etiological mechanism.
An 85-year old female patient, hypertensive, dyslipidemic, with history of infarction at 48 years, with unknown arteries affected and treatment performed, presented at the emergency department due to mini-mal effort dyspnea, orthopnea and paroxysmal nocturnal dyspnea of 4 days duration. She denies angina, chest pain or other associated symptoms. On physical examination she presents with normal blood pressure (130/80 mmHg), tachycardia (110 beats per minute), normal oxygen saturation, and with signs of heart fail-ure (bibasilar crackles and 2/3 jugular venous disten-sion) and no signs of low cardiac output. On auscul-tation she presents aortic diastolic murmur, difficult to characterize owing to tachycardia. The electrocar-diogram shows sinus tachycardia without evidence of acute ischemia. Lab tests have no special characteris-tics and cardiac enzymes are negative.
She is admitted in the Coronary Care Unit starting treatment with diuretics and vasodilators. The heart failure episode is resolved within 2 hours of admission, but tachycardia persists.
Six hours after admission she presents with flash APE, so the previously established treatment is inten-sified, with the addition of non-invasive ventilation. The electrocardiogram during this event shows no changes with respect to that on admission. Transthoracic echocardiogram is performed, revealing aneurysmal dilatation of the ascending aorta (52 mm in the sinus portion, 54 mm in the tubular portion, and sinus tubular junction effacement), with dissected internal mobile layer reaching the abdominal aorta, severe ec-centric aortic regurgitation secondary to annular dila-tation and dissected layer prolapse, left ventricular hypertrophy, and moderate ventricular dysfunction (Figures 1 and 2).

Fig. 1. Short-axis transthoracic echocardiography, showing the tubular portion of the ascending aorta with aneurysmal dilata-tion and internaldissection layer.
Fig. 2. Apical 5-chamber view transthoracic echocardiography, showing eccentric aortic regurgitation jet directed to the anterior mitral valve.
The discussion about treatment options arises in the context of an elderly patient, with good general condition and acute valvular involvement possibly secondary to aortic dissection. In agreement with the patient and her family, a multidisciplinary decision is taken to perform emergency surgery. The procedure reveals aortic dissection of the media and intima layers invaginated into the aortic arch and the proximal por-tion of the dissection generating a prolapse through the valve interfering with its function (Figure 3).
Replacement of the ascending aorta is performed using 30 mm Dacron prosthesis with aortic valve preservation (Tirone David technique). Cardiopulmonary bypass time was 84 minutes, aortic clamping 63 minutes and circulatory arrest 17 minutes. She had a favorable postoperative course and was discharged 7 days after surgery.

Fig. 3. Intraoperative fndings. Dissection of the aortic media and intima layers invaginated into the aortic arch, and the proximal portion of the dissection generating a prolapse through the valve interfering with its function.
Acute aortic syndrome is a serious entity, with an annual incidence estimated at 20-40 cases/million in-habitants/year, and 80% of them are dissections. (1)
Chest pain is the most common presenting symp-tom of aortic dissection; however, 6% of cases occur without it, making diagnosis difficult. (2) The natural outcome of dissections has a high mortality rate in the first 24 hours (estimated in 1% to 2% per hour). (2) Emergency surgical resolution presents elevated mor-tality of 37.8%. (1) In patients over 85 years it reaches 58.3%. (3)
Severe aortic failure occurs in 40% of dissection cases, being less frequent in patients older than 70 years (28.7%). (2-4) Pathophysiological mechanisms involved in aortic regurgitation comprise aortic valve prolapse, leaflet alignment distortion, and poor leaf-let cooptation due to aortic root and ring dilatation. In our patient's case a mechanism participates which, although rare, is also described: intima prolapse
through the valve which interferes with its function. The increase in left ventricular end diastolic pressure caused by acute valve failure generates flash APE. (5) The presentation of this case seeks to demonstrate two concepts: firstly, to consider persistent tachycardia despite optimal treatment as a cardinal sign of alarm in patients in cardiac critical care; and secondly, to con-template aortic dissection among the differential diag-noses of flash APE, even if no chest pain, pulse asym-metry or other classic symptoms are present.
María L. Ayerdi, José C. Santucci, Sergio BarattaMTSAC, Jorge Bilbao, Horacio Fernández,
Guillermo VaccarinoMTSAC
Hospital Universitario Austral e-mail: lauraayerdi@hotmail.com
REFERENCES
1. Higa C, Guetta J, Borracci R, Meribilhaa R, Marturano M, Marenchino R y cols. Registro multicéntrico de disección aórtica aguda. Estudio RADAR. Rev Argent Cardiol 2009;77:354-60.
2. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. International Registry of Acute Aortic Dissec-tion (IRAD): new insights from an old disease. JAMA 2000;283:897-903. http://doi.org/c3g229
3. Mehta R, O'Gara P, Bossone E, Nienaber C, Myrmel T, Cooper J, et al. Acute type A aortic dissection in the elderly: clinical character-istics, management, and outcomes in the current era. J Am CollCar-diol 2002;40:685-92. http://doi.org/dtfgvg
4. Braverman AC. Review: Aortic dissection: prompt diagnosis and emergency treatment are critical. CME 2011;78:685-96.
5. Braverman AC, Thompson R, Sanchez L. Diseases of the aorta. En: Bonow RO, Mann DL, Zipes D P, Libby P. Braunwald's Heart Dis-ease. 9thed. Philadelphia: Elsevier; 2011.
biventricular support with two HeartWare continu-ous-fow left ventricular assist devices
Left ventricular assist devices (LVAD) represent a well-known therapeutic option for the treatment of end-stage heart failure and their use and acceptance has increased in the last years. (1)
Unfortunately, an important number of patients present postoperative complications that preclude the result of the procedure, among which severe refracto-ry right ventricular dysfunction has limited long-term therapeutic options.
We present the case of refractory right heart fail-ure resolved with implantation in the right ventricle of another left ventricular assist device.
A 23-year-old male patient with idiopathic dilated cardiomyopathy and 15% ejection fraction was evaluated and programmed to receive HeartWare LVAD implantation (HVAD, HeartWare, Framingham, MA). After surgery, he developed severe right ventricular failure, successively requiring delayed thoracic clo-sure and use of epoprostenol (prostaglandin I2), milri-none, adrenaline and nitric oxide (Figure 1).
Refractory right heart failure led to temporary right ventricular support with a Centrimag device (Levitronix Centrimag, LevitronixGMbH; Zurich, Switzerland). After ten days of support with persis-tent echocardiographic and hemodynamic parameters of right ventricular failure, the decision was taken to initiate definitive support adapting the same type of HeartWare device used for the left ventricle (Figure 2). With this support, the patient was weaned from vasoactive drugs and after 12 days in critical care was transferred to a general ward and discharged 54 days after the original implantation.
Right ventricular dysfunction represents a severe postoperative complication of LVAD implantation that can seriously affect the intervention outcome. Its physiopathology is complex, and can be associated with ventricular interaction, abnormal septal motility, preexistent right ventricular dysfunction, changes in pulmonary afterload and other disorders that indicate the inconsistency of the different risk factors usually considered for its prediction.
Right ventricular dysfunction occurs in 20% to 50% of procedures, implicating an increase in postopera-tive mortality, which in some series may reach up to 70%. This condition usually requires use of inotropic drugs and/or pulmonary vasodilators and, occasion-ally, either temporal or definitive, right ventricular support. This necessity has the difficulty of more lim-ited and poorer long-term or definitive right heart me-chanical support, either isolated or as part of a biven-tricular assist device, compared with left ventricular support alone. (2) HeartWare is considered the first third-generation LVAD designed for intrapericardial use, with axial or centrifugal continuous-flow rotary pumps, which to date has more than 3,000 devices im-planted. In November 2012 it was approved as bridge to transplantation by the Food and Drug Administra-tion. It presents several advantages compared to its predecessor, the axial continuous-flow HeartMateII device, as its lower size and weight (160 grams) and a suspended and magnetically-activated rotor, which significantly reduces friction and risk of mechanical failures. (3)
In the multicenter Canadian registry including 71 consecutive patients with HeartWare implantation, right ventricular dysfunction was the most frequent severe complication, affecting 25.4% of the population. (4)
Previous experiences in patients developing refractory right ventricular failure have posed the possibil-ity of adapting the HeartWare LVAD for use as right ventricular support with satisfactory outcome. Bernhardt et al. reported their experience in 8 patients with right ventricular failure implanted with Heart-Ware assist devices in the right ventricle with 30-day survival of 4 patients (50%). Among these 4 patients, one died at 44 days for multiple organ dysfunction, two were transplanted and the other developed functional recovery leading to device explant. (5) Similarly to our patient, Stulak et al. report the use of a HeartWare device in the right ventricle as support for refractory right ventricular dysfunction during the postoperative period of a HeartMateII device implantation. (6) In the present case, evaluation of the HeartWare LVAD characteristics, as preload dependence and afterload sensitivity, led to reduction of the flow cannula length and diameter, implicating a decrease of the normally low right ventricular afterload to avoid an extremely elevated right ventricular flow and the possibility of pulmonary edema. Device implantation allowed wean-ing from vasoactive drugs, suggesting the use of this type of devices, initially designed and used only as left ventricular support, as biventricular support. Further experience of this novel use will establish the useful-ness of the approach.
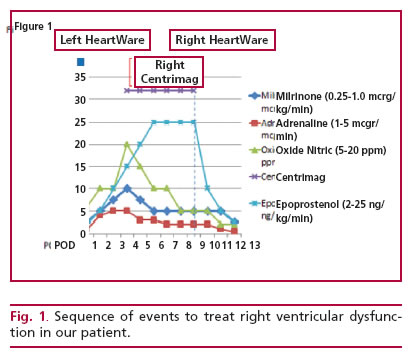

Fig. 1. Sequence of events to treat right ventricular dysfuncvices.
Ricardo LevinMTSAC, Marcela DegrangeMTSAC, Jorge Balaguer, Rafael PorcileMTSAC
Vanderbilt University Medical Center, Nashville, TN, USA y
Universidad Abierta Interamericana, Buenos Aires, Argentina
Dr. Ricardo Levin - Portela 2975 - (1426) CABA
e-mail: rllevin@gmail.com
REFERENCES
1. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte J V, Feld-man D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. http:// doi.org/fsdvpp
2. Mangi AA. Right ventricular dysfunction in patients undergoing left ventricular assist device implantation: predictors, management, and device utilization. Cardiol Clin 2011;29:629-37. http://doi.org/ cp978f
3. Copeland J. HeartWare Ventricular Assist System for bridge to transplant: The new kid on the block. J Heart Lung Transplant 2013;32:671-2. http://doi.org/35r
4. Bashir J, Legare J F, Freed DH, Cheung A, Rao V, Toma M. Mul-ticentre Canadian experience with the HeartWareventricular assist device: concerns about adverse neurological outcomes. Can J Cardiol 2014;30:1662-7. http://doi.org/35s
5. Bernhardt AM, De Buy TM, Reichenspurner H, Deuse T. Isolated permanent right ventricular assist device implantation with the HeartWare continuous-flow ventricular assist device: first results from the European Registry for Patients with Mechanical Circula-tory Support. Eur J Cardiothorac Surg 2014 (in press). http://doi. org/35t
6. Stulak JM, Griffith KE, Nicklas JM, Pagani FD.The use of the HeartWare HVAD for long-term right ventricular support after im-plantation of the HeartMate II device. J Thorac Cardiovasc Surg 2011;142:e140-2. http://doi.org/dwp3qj














