INTRODUCTION
Although influenza is primarily considered a viral infection usually limited to the respiratory system, several cardiovascular complications have been described. 1 Cardiovascular disease (CVD) and influenza have been associated for a long time due to an overlap in the peak incidence of each disease in the winter months. 2 Epidemiological studies observed an increase in cardiovascular (CV) mortality during influenza outbreaks, indicating that CV complications of influenza, including exacerbation of heart failure (HF), acute ischemic heart disease, and, less often, other CV manifestations (stroke, cardiac arrhythmias, venous thromboembolism, or myocarditis), are important contributors to morbidity and mortality during influenza virus infection. 3
The connection between heart disease and influenza is complex: it can occur via the inflammation-thrombosis pathway, direct effects of the virus on the myocardium, or exacerbation of pre-existing CV disease. 4 The mechanisms postulated to explain the increased risk of vascular events include precipitating plaque rupture, endothelial dysfunction, triggering of other latent infections contributing to plaque rupture, triggering of the procoagulant pathway, tachycardia and vasodilation associated with fever, and infection-related metabolic disorders, including elevated triglyceride and blood glucose levels. 5,6
Influenza vaccination (IV) is a well-established strategy for reducing influenza-related morbidity and mortality patients with CVD. 7,8 Based on observational studies and randomized clinical trials, vaccination has been associated with significant reductions in all-cause mortality and major adverse cardiovascular events. 9-12
Currently, the World Health Organization, the Centers for Disease Control and Prevention, the American Heart Association/American College of Cardiology, and the European Society of Cardiology recommend annual influenza vaccination for patients with established CVD. 13-15 In 2021, the Inter- American Society of Cardiology published a consensus statement on IV and CVD, 16 citing the most recent meta-analysis with 4 randomized clinical trials that showed that IV was associated with a reduction in cardiovascular events, 17 and another meta-analysis based on observational data from HF patients, which had consistent findings. 18)
Because two new clinical trials have been recently published in the last two years, we decided to perform an updated meta-analysis of randomized clinical trials on the impact of IV on CV mortality and outcomes in patients with CVD.
OBJECTIVE
Our primary objective was to perform a systematic review and meta-analysis of randomized clinical trials to evaluate the effect of IV on mortality in patients with CVD. The secondary objective was to evaluate the effect of IV on CV mortality, myocardial infarction, and major adverse cardiovascular events (MACE) in patients with HF and coronary artery disease (CAD).
METHODS
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist was used to perform and report this systematic review. 19
Search method for identification of studies
A systematic search was conducted to identify articles pub lished up to April 2022 in MEDLINE (PubMed database), the Cochrane Library, and the International Clinical Trials Registry Platform. We searched the following keywords or MESH terms in the title or abstract: "influenza," "influenza vaccine," "vaccine," "myocardial infarction," "coronary artery disease," "acute coronary syndrome," "heart failure," and "congestive heart disease".
We performed manual searches checking the reference list of all the relevant publications to ensure complete collec tion of relevant articles, and we also reviewed recent presentations at international cardiovascular congresses.
Selection of relevant studies for inclusion
Two reviewers (LMB, EJZ) independently screened titles and abstracts to identify potentially relevant articles. Any discrepancy in the data collected was resolved via discussion. Full-text articles were included in this review if they met all the following inclusion criteria: (1) randomized clinical trials comparing influenza vaccination with placebo or no intervention when data on one of the outcomes were reported; (2) articles providing data on the effectiveness of influenza vaccination in patients with HF or CAD compared with an unvaccinated control group; (3) influenza vaccination was administered within one year after study enrollment; (4) articles published in English or Spanish language.
We excluded duplicates, studies that included patients with different doses of influenza vaccination but without an unvaccinated arm, and all nonrandomized controlled trials.
Type of participants
Participants >18 years with established CVD; HF or (CAD stable or unstable angina and ST-segment elevation or non- ST-elevation myocardial infarction) were included in the study.
Outcome measures
The primary outcome measure was all-cause mortality, while the secondary outcome measure was CV mortality, myocardial infarction, and major adverse cardiovascular events (MACE) among vaccinated and unvaccinated patients with HF and CAD.
Data collection and management
Two reviewers independently extracted data and all disagreements were resolved by discussion or arbitration. The following data were systematically extracted:
− Trial characteristics: design, duration, region, scope, year of publication.
− Intervention: type and method of vaccination, control intervention.
− Participants: number of participants, inclusion and exclusion criteria, total number and number in comparison groups, baseline characteristics (age, sex, cardiovascular risk factors, cardiovascular medication).
− Results: primary and secondary outcomes according to trial, myocardial infarction or reinfarction, unstable angina, cardiovascular death, and related outcomes.
Any discrepancy in data extraction was resolved via discussion with another author (ASL).
Subgroup analysis
A subgroup analysis was performed to compare the effects of vaccination on mortality in patients with HF and CAD.
Bias assessment
Bias was independently assessed by two investigators. We assessed evidence of bias of randomized controlled trials with the Cochrane risk of bias tool, 20,21 with evaluation of the following criteria: random sequence generation (adequate method), allocation concealment, blinding of participants and personnel, management of incomplete outcome data, loss to follow-up or withdrawal from the study, intention-to-treat analysis, selective reporting, similarity in baseline characteristics, any other observed biases.
Measures of treatment effect
All outcome measures were dichotomous results and were presented as risk ratios (RR) at the last follow-up reported.
Heterogeneity assessment
Heterogeneity between trials was quantified with the I2 statistics, which is independent of the number of studies in a meta-analysis, and with the chi-square test, with significance levels set at a value of p = 0.1. An I2 value > 50% meant significant heterogeneity between studies. 22
Data synthesis
Based on heterogeneity test, the pooled RR was calculated using fixed effects model when there was no heterogeneity and random effects model in case of heterogeneity.
Statistical analysis
All outcome measures were dichotomous results and were presented as risk ratios (RR) and 95% confidence intervals (95% CI) at the last follow-up reported.
Two-tailed p value < 0.05 was considered statistically significant.
Publication bias was estimated in case there were more than 10 studies by visual assessment in the funnel plot. Egger's regression test was used to examine the asymmetry of the funnel plot. 23
The selection process was carried out using the Reference Manager Rayyan QCRI. (24) All data extracted from the included studies were entered into Review Manager (RevMan 5.3).
RESULTS
Search results
A total of 957 studies were identified through litera ture search; 527 studies were selected and 486 were excluded after an initial screening of titles and abstracts. The remaining 41 publications were reviewed in full text and evaluated according to the inclusion criteria. Finally, 6 trials were selected for the quantitative analysis. 25-30
The search and selection process is represented in a PRISMA flow diagram (Figure 1).
Characteristics the studies included
A total of six clinical trials comprising 9316 patients were analyzed. Five trials included CAD patients (FLUVACS, FLUCAD, IVCAD, IAMI and Phrommintikul et al.), and the IVVE trial included HF patients. Mean follow-up was 16 ± 9.7 months. Table 1 summarizes the main general characteristics of the trials. A description of each study included in the meta-analysis can be found in Table 1 of the supplementary material.
Risk of bias in included studies
Risk of bias across studies is shown in Figure 1 of the supplementary material, and risk of bias within stud ies is shown in Figure 2 of the supplementary material.
Effects of influenza vaccination
Primary outcome measure: All-cause mortality.
Influenza vaccine was associated with lower mortality compared to control: RR 0.67, 95% CI 0.47-0.95; p = 0.03; I2 = 53% (Figure 2).
Secondary outcome measure: Cardiovascular death, myocar dial infarction and MACE
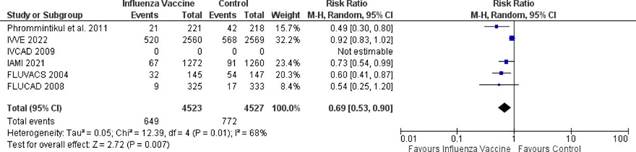
Influenza vaccine was associated with lower cardiovascular death compared to control: RR 0.64, 95% CI 0.44-0.94; p = 0.02; I2 = 54% (Figure 3), and with reduction of MACE: RR 0.69, 95% CI 0-53-0.90; p = 0.007; I2 = 68% (Figure 4). There was a non-statistically significant reduction in myocardial infarction compared to control: RR 0.82, 95% CI 0.60-1.12; p = 0.57; I2 = 0% (Figure 5).
Subgroup analysis
A subanalysis of overall mortality was performed comparing IV vs. control, stratified by history of CAD and HF. This effect was not consistent between the two study populations: in HF, RR 0.91 (95% CI 0.80-1.02; p = 0.1) and in CAD RR 0.56 (95% CI 0.41-0.76; p = 0.0002), p for interaction = 0.004. (Figure 3 of the supplementary material).
DISCUSSION
In this updated meta-analysis of controlled clinical tri als including 9316 patients with CAD or HF, IV was associated with a significant reduction in all-cause mortality, cardiovascular death, and MACE. Vaccinated patients presented a non-significant reduction in the incidence of acute myocardial infarction.
The information about the association of influenza and CVD is conclusive, but its mechanisms are still under study. Yet, the inflammation-thrombosis model seems to be the most widely accepted one. Other factors, such as increased metabolic demand due to the adrenergic surges and hyperdynamic CV response, and hypoxia secondary to pulmonary infection also seem to play an important role. 31,32
Since the pioneering study conducted in Argen tina by Gurfinkel et al. was published in 2004 25 and then incorporated as the main and only evidence in the CDC guidelines in the United States in 2009, 33 IV has been gradually established as a prevention strategy for CV events.
Some recognized limitations to broaden the use of IV include uncertainty about external validity, reproducibility in different regions, climates, and high and low resource countries, and the safe use in the setting of an acute event or its efficacy in subjects with HF. These factors led to the development of the new clinical trials evaluated here.
In addition, although the recommendation for IV is not new, it is still sub-optimally accepted. In the Unit ed States, only 50% of patients with CAD received IV, with important disparities according to socioeconomic determinants. 34 Similarly, one third of those hospitalized for HF did not receive IV. 35
We compared the results of our meta-analysis with those of previous publications. A Cochrane review published in 2015 of four secondary prevention trials included 1682 patients with CVD and reported reduc tion in CV mortality (RR 0.45; 95% CI, 0.26- 0.76), but not in AMI. 36 More recently, in 2021 a meta-analysis of these four randomized trials and 12 observational studies including more than 237 000 patients with CVD, reported that influenza vaccination was associated with significant reductions in the risk of all-cause mortality, CV mortality, and MACE at a median follow-up of 20 months. 17
We believe that the main findings of this meta-analysis are confirming that the benefit of reducing all-cause mortality and cardiovascular death and the trend towards a reduction in the risk of myocardial infarction remains after including new randomized clinical trials involving more than 7000 subjects. The benefit in reducing events is also maintained when two populations that were not previously evaluated are included in the meta-analysis: subjects with a recent coronary event (IAMI trial) and subjects with heart failure (IVVE trial).
As for the IAMI study, 29 the indication of IV in subjects with coronary artery disease was supported by different clinical trials and previous meta-analyses; however, the authors proposed the strategy of indicat ing IV during hospitalization due to an acute coronary event, demonstrating the safety and efficacy of this average age was 57 years, and the patients came from low- and middle-income countries in Asia and Africa. The authors of the IVVE study reported a reduction in outcomes as all-cause mortality, cardiovascular death, and MACE during periods of peak circulation of influenza, and a trend towards a reduction in events throughout the duration of the study. Rather than a neutral result, this finding reinforces the pathophysiological association and strengthens the indication for IV in this population. When the overall results of this study were included in our meta-analysis, the benefits in reducing events had the same direction and magnitude of effect.
The characteristics mentioned above could explain the differences found in the stratified analysis of sub groups according to baseline CV disease. However, through this meta-analysis we cannot distinguish how many of the subjects recruited for CAD had con comitant HF and vice versa, or whether the benefit is greater or not in subjects with both clinical conditions.
To become aware of the magnitude of the findings on the effectiveness of IV in reducing events in patients with CVD, the results can be compared with those of traditional pharmacological treatments such as statins, beta-blockers and angiotensin-converting enzyme inhibitors (ACE inhibitors). 40 Cardiovascular death decreased with each treatment in the meta-analyses of the main trials: RR was 24% for statins, 41 23% for beta-blockers, 42 and 16% for ACE in hibitors. 43 Similarly, smoking cessation reduces the risk of CVD by 39%. 44
The strengths of this review are the extensive sys tematic review of the literature performed and the inclusion of only randomized clinical trials with low risk of bias. We found low heterogeneity in the pri mary and secondary outcomes analyzed, probably due to the similar trial design, population included, and definition of outcomes.
However, this review has limitations. We could not assess publication bias due to the low number of stud ies included in the meta-analysis. The COVID-19 pandemic had an impact on recruitment, follow-up, and influenza circulation, which affected the last two large randomized clinical trials; however, the benefit in reducing major events was sustained. Few clinical trials have been included, and there is great variability in the number of subjects included and in their baseline characteristics. Nevertheless, the reduction in events was in the same direction, although with a significant difference in the magnitude of the effect. Finally, the data from the IVVE study have not been published yet in an indexed journal at the time this analysis was completed and come from the presentation at a scientific congress; therefore, the results could be modi fied or have a different interpretation than the one we have used for this analysis.
CONCLUSION
In this updated meta-analysis of six randomized controlled clinical trials, influenza vaccination was asso ciated with a 33% and 36% relative risk reduction of all-cause mortality and cardiovascular death, respectively, in patients with CVD.
Over the past few decades, considerable evidence has accumulated about the cardioprotective effects of influenza vaccination in patients with established cardiovascular disease.
We sought to promote consensus based on the highest level of evidence about the persistent benefits of influenza vaccination in patients with CVD by including two new clinical trials in CAD and HF, confirming the association of vaccination with risk reduction in subjects with CVD. The present meta-analysis may help health care workers to strongly recommend influenza vaccination for secondary prevention of cardiovascular events.