Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. v.40 n.1-2 Córdoba ene./jul. 2005
Karyotypic studies and morphological analysis of some reproductive features in five species of Conyza (Astereae: Asteraceae) from Northeastern Argentina
Juan D. Urdampilleta1,4, Aníbal G. Amat1 and Claudio J. Bidau2,3
1Departamento de Biología, Catedra de Biología Vegetal, Facultad de Ciencias Exactas, Químicas y Naturales, Universidad Nacional de Misiones.
2Departamento de Génetica, Laboratorio de Genética Evolutiva, Facultad de Ciencias Exactas, Químicas y Naturales, Universidad Nacional de Misiones.
3Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina.
4E-mail: juanurdampilleta@hotmail.com
Summary: The cosmopolitan genus Conyza Less. comprises about 100 species, 22 of which occur in Argentina. Current taxonomic treatments, largely based on exomorphological characters, are insufficient to characterize and circumscribe some of their polymorphic species. Interspecific variations in inflorescences typology and capitula structure, as well as karyotypic aspects, were studied in five species of Conyza that naturally occur in Misiones Province (Argentina): C. blakei (Cabrera) Cabrera, C. bonariensis (L.) Cronquist var. bonariensis, C. glandulitecta Cabrera, C. primulaefolia (Lam.) Cuatrec. & Lourteig, C. sumatrensis (Retz.) E. Walker var. sumatrensis and C. sumatrensis (Retz.) E. Walker var. floribunda (Kunth) J. B. Marshall. Chromosome numbers for C. bonariensis, C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda, all with 2n= 54 and C. primulaefolia (2n= 72), were confirmed; two new counts are reported: C. blakei (2n= 54) and C. glandulitecta (2n= 54). All karyotypes were compared, and related with the variation both in flower number per capitula as well as typology of the inflorescence. The results obtained considering the different sources of evidence, demonstrated a close relationship between C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda, that exhibit characteristics that may be considered primitive for the genus; C. primulaefolia shows the most advanced ones while C. blakei, C. bonariensis and C. glandulitecta occupy an intermediate position.
Key words: Asteraceae; Astereae; Chromosomes; Conyza; Inflorescence; Interspecific variation; Karyotype.
Resumen: Estudios cariotípicos y análisis morfológico de algunos caracteres reproductivos en cinco especies de Conyza (Astereae: Asteraceae) del Noreste de Argentina. El género cosmopolita Conyza Less. comprende alrededor de 100 especies, de las cuales 22 habitan en Argentina. Los tratamientos taxonómicos actuales, largamente basados en caracteres exomorfológicos, son insuficientes para caracterizar y circunscribir algunas de sus especies polimórficas. Variaciones interespecíficas en la tipología de las inflorescencias y estructura del capítulo, así como aspectos cariotípicos, fueron estudiados en cinco especies de Conyza que ocurren naturalmente en la Provincia de Misiones (Argentina): C. blakei (Cabrera) Cabrera, C. bonariensis (L.) Cronquist var. bonariensis, C. glandulitecta Cabrera, C. primulaefolia (Lam.) Cuatrec. & Lourteig, C. sumatrensis (Retz.) E. Walker var. sumatrensis y C. sumatrensis (Retz.) E. Walker var. floribunda (Kunth) J. B. Marshall. Los números cromosómicos para C. bonariensis, C. sumatrensis var. sumatrensis y C. sumatrensis var. floribunda, todos con 2n= 54, y C. primulaefolia (2n= 72), fueron confirmados; dos nuevos recuentos son reportados: C. blakei (2n= 54) y C. glandulitecta (2n= 54). Los cariotipos son analizados comparativamente, y relacionados con variaciones en el número de flores por capítulo y tipología de las inflorescencias. Los resultados obtenidos, considerando las diferentes fuentes de variación, demuestran una estrecha relación entre C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda, las cuales exhiben características que podrían ser consideradas primitivas para el género. Por su parte C. primulaefolia muestra caracteres derivados, en tanto que C. blakei, C. bonariensis y C. glandulitecta presentarían un estado intermediario entre las anteriores.
Palabras clave: Asteraceae; Astereae; Cromosomas; Conyza; Inflorescencia; Variación interespecífica; Cariotipo.
INTRODUCTION
The genus Conyza Less. includes about 100 species in the world (Zardini, 1976). Twenty-two species occur in Argentina of which nine are endemic. Seven species have been reported for Mi siones province: C. blakei (Cabrera) Cabrera, C. bonariensis (L.) Cronquist var. bonariensis, C. glandulitecta Cabrera, C. lorentzii Griseb., C. pampeana (Parodi) Cabrera and C. primulaefolia (Lam.) Cuatrec. & Lourteig (= C. chilensis Spreng.), C. sumatrensis (Retz.) E. Walker var. sumatrensis (= C. albida Willd. ex. Spreng.) y C. sumatrensis (Retz.) E. Walker var. floribunda (Kunth) J. B. Marshall (= C. floribunda Kunth) Walker) (Zuloaga & Morrone, 1999; Sancho & Ariza Espinar, 2003). Conyza species usually grow in disturbed habitats, and because of their colonizing ability they have been considered as weeds or invasive species (Thebaud & Abbott, 1995). Highly related species groups, some of them very polymorphic, constitute this genus. In consequence the taxonomic treatments result insufficient when based only on morphological characters.
The Asteraceae family shows great variation in chromosome number, ranging from n = 2 (Haplopappus) to n = 106 (Werneria), with 78% of the species having gametic numbers between 4 and 18, and 21% of the entities with n = 9. More than 30% of the species have gametic numbers that are multiples of 9, due to which, n= 9 has been suggested as possible ancestral number of the family (Solbrig, 1977). If n = 9 is the ancestral number, then the 63% of the species are derived by eu- or aneuplodization processes. The basic number of the tribe Astereae is x = 9, which occurs in the genera Aster, Erigeron and Conyza (Solbrig, 1964; Nesom, 1978). Chromosome counts in Conyza confirm the ancestral number and show a polyploid series that ranges from n = 9 to n = 36 (Bernardello, 1986; Hunziker et al., 1989; Hunziker, 1990; Wulff, 1998; Carr et al., 1999).
Considering ligule characters and relative number of female and hermafrodite flowers, Cronquist (1943) transferred the section Coenotus from the genus Erigeron L. to Conyza. This author recognized a transition from Erigeron to Conyza through the section Coenotus that possesses a higher number of female flowers and a trend towards a shortening of the ligule. Subsequently, Nesom (1990) redefined the genus after a complete separation of Laennecia Cass. Since delimitation between these genera and their species is conflictive, more intensive biosystematic studies are required.
The purpose of this work was to perform a comparative cytogenetic study of five Conyza species from Misiones widely distributed in South America, in addition to a detailed morphological analysis of their reproductive features.
MATERIALS AND METHODS
This paper is based on the specimens detailed below. The collected specimens were deposited at the herbaria of the Departamento de Farmacia of the Facultad de Ciencias Exactas, Químicas y Naturales, Universidad Nacional de Misiones (MNEF), Instituto de Botánica Darwinion (SI) and Museo de Ciencias Nturales de la La Plata (LP). Various specimens for locality were used in this stady and the general terminology to describe the inflorescences is that suggested by Troll (1964) and Weberling (1985). The following floral characters of the capitulum were quantified: number of total (TF), female (FF), and hermaphroditic flowers (HF), and the relationship HF/FF (sex- ratio, SR).
To obtain chromosome preparations root tips were collected from potted plants and pretreated with 0.0029 mol/L of 8-hydroxyquinoleine for 4-5 h at 4oC. The root tips were fixed and stored in ethanol-lactic acid (5:1) (Fernandez, 1973) at 4oC. They were then hydrolyzed in 1 N HCl for 10 min at 60oC and squashed in 45% acetic acid and 2% propionic haematoxylin.
For chromosome identification and measurement, five well-spread metaphase plates of each species were analyzed and used for the construction of the idiograms. Absolute chromosome length and the centromeric index (CI= short arm length/ total chromosome length x 100) were used for comparison of the karyotypes. Chromosome morphology was determined according to the nomenclature proposed by Levan et al. (1964). Karyotype asymmetry was determined according to the indexes suggested by Romero Zarco (1986):

where b = length of short arm, B = length of long
arm, n = number of chromosome pairs, X = mean chromosome length, and s = standard desviation of mean chromosome length. Total karyotype length (TKL), was determined for all taxa
Material examined (all from Argentina, Misiones Province)
C. blakei. Dpto. Capital, Miguel Lanús, Urdampilleta 31-I-2000 (LP, SI), Urdampilleta 20-III- 2000 (LP).
C. bonariensis. Dpto. Capital, Miguel Lanús, Urdampilleta 7-XI-1999 (MNEF), Urdampilleta 16-XI-1999 (MNEF); Posadas, Urdampilleta 8-VIII-1999 (LP, SI).
C. glandulitecta. Dpto. Capital, Miguel Lanús, Urdampilleta 7-XI-1999 (MNEF), Urdampilleta 16-XI- 1999 (MNEF), Urdampilleta 30-I-2000 (SI, MNEF), Urdampilleta 31-I-2000 (LP, MNEF); Dpto. L. N. Alem, 2 Arroyos, Urdampilleta 23-I-2000 (LP, SI).
C. primulaefolia. Dpto. Capital, Miguel Lanús, Urdampilleta 7-XI-1999 (LP, SI), Urdampilleta 16-XI- 1999 (MNEF), Urdampilleta 30-I-2000 (MNEF); Dpto. Concepción, Concepción de la Sierra, Urdampilleta 28-II-2000 (MNEF).
C. sumatrensis var. sumatrensis. Dpto. San Ignacio, Corpus, Urdampilleta III-1999 (SI, LP); Dpto. Capital, Miguel Lanús, Urdampilleta 18-X-1999 (MNEF), Urdampilleta 7-XI-1999 (MNEF), Urdampilleta 20-XI-1999 (SI); Posadas, Urdampilleta 30-I-2000 (LP). Dpto. Candelaria, Santa Ana, Amat & Urdampilleta 10-III-2000 (MNEF); Dpto. L. A. Alem, Dos Arroyos, Urdampilleta 23-I-2000 (MNEF); Dpto. Concepción, Concepción de la Sierra, Urdampilleta 28-II-2000 (MNEF).
C. sumatrensis var. floribunda. Dpto. Candelaria, Santa Ana, Amat & Urdampilleta 10-III-2000 (SI, MNEF); Santa Ana, Amat & Urdampilleta IV- 2000 (SI, LP, MNEF).
RESULTS
Inflorescences
All the studied species show monotelic inflorescences with multiple capitula arranged in four basic forms (Fig. 1). The number of flowers varied from 50 to 700 per capitula, with similar SR's (Table 1). Female flowers are always more numerous and are located at the periphery, while hermaphroditic flowers are disposed at the center.

Fig. 1. Four basic forms of inflorescence observed in Conyza. A, leafy pyramidal pseudopanicle; B, leafy cylindrical pseudopanicle; C, leafy ramose infloresence; D, bracteate ramose inflorescence.
Table 1. Female and hermaphrodite flowers numbers.

C. blakei shows a monotelic sinflorescence of the leafy pseudopanicle type with cylindrical shape (Fig. 1B). TF was 83.4 and SR= 0.08 (Table 1, Fig 2).

Fig. 2. Dispersion diagram of hermaphrodite (FH) and female flowers (FF) of C. blakei ![]() C. bonariensis
C. bonariensis ![]() C.glandulitecta
C.glandulitecta ![]() C. primulaefolia
C. primulaefolia ![]() C. sumatrensis var. sumatrensis
C. sumatrensis var. sumatrensis ![]() and C. sumatrensis var. floribunda
and C. sumatrensis var. floribunda ![]()
C. bonariensis presents a monotelic sinflorescence of the leafy pseudopanicle type with pronounced acrotonic development of the inferior paracladia producing a branched inflorescence (Fig. 1C). TF was 226.4 and SR= 0.07 (Table 1, Fig 2).
C. glandulitecta has a monotelic sinflorescence of the leafy pseudopanicle type with pyramidal shape (Fig. 1A). TF = 231.5 and SR = 0.09 (Table 1, Fig 2).
C. primulaefolia possesses a bracteate monotelic sinflorescence with acrotonic development of all paracladia, producing a branched sinflorescence with few capitula (Fig. 1D). It presents the highest TF (548) and SR value (0.06) (Table 1, Fig. 2).
C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda possess monotelic sinflorescences of the leafy pseudopanicle type with a pyramidal shape (Fig. 1A). For C. sumatrensis var. sumatrensis, the mean number of total flowers per capitulum (TF) was 142 and SR = 0.11 (Table 1, Fig 2), however in C. sumatrensis var. floribunda shows the lowest mean number of total flowers per capitulum (57.4) and the highest SR value (0.13) (Table 1, Fig 2).
Karyotypes
Four species were hexaploid (2n= 6x= 54) and one octoploid (2n= 8x= 72) (Table 2, Fig. 3). The six taxa show relatively symmetric karyotypes, with metacentric and submetacentric chromosomes. The size of the chromosomes ranged between 1.07 and 2.93 1m

Fig. 3. Metaphase plates of, A, C. sumatrensis var. sumatrensis; B, C. blakei; C, C. bonariensis; D, C. sumatrensis var. floribunda; E, C. glandulitecta; F, C. primulaefolia. Scale bar = 5µm.
The somatic chromosome number in C. blakei is 2n= 54, with 21 m and 6 sm pairs of chromosomes. The chromosome length ranges between 1.25 and 2.75 1m (Table 2). Chromosome pair n° 23 bears a satellite in the short arm (Fig. 4A).
Table 2. Karyotypic analys of six studies species
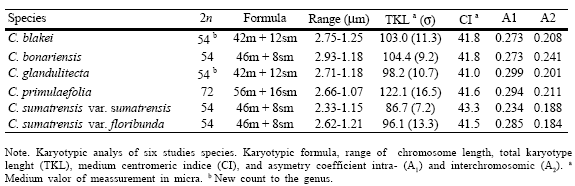

Fig. 4. Idiograms of, A, C. blakei; B, C. bonariensis; C, C. glandulitecta; D, C. primulaefolia; E, C. sumatrensis var. sumatrensis; F, C. sumatrensis var. floribunda. Scale bar = 3µm.
Conyza bonariensis shows 2n= 54, with 23 pairs of m and 4 pairs of sm chromosomes. The chromosome length varies from 1.18 to 2.93 1m. It presents the highest TKL of the hexaploid species and the highest interchromosomal assimetry coefficient (A2) (Table 2, Fig. 5). Secondary constrictions were observed in the short arms of pairs 3, 24 and 26 (Fig. 4B).
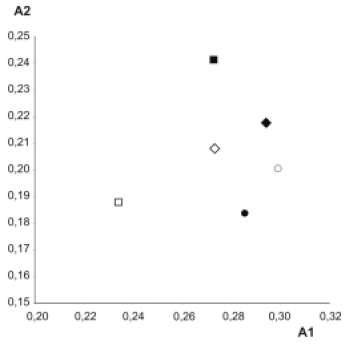
Fig. 5. Dispersion graphic of asymmetry coefficient, A1 and A2, of C. blakei ![]() C. bonariensis
C. bonariensis ![]() C. glandulitecta
C. glandulitecta![]() C. primulaefolia
C. primulaefolia ![]() C. sumatrensis var. sumatrensis
C. sumatrensis var. sumatrensis ![]() and C. sumatrensis var. floribunda
and C. sumatrensis var. floribunda ![]()
Conyza glandulitecta shows 2n= 54, having 21 m and 6 sm pairs of chromosomes; chromosome length varied between 1.18 and 2.71 1m. This species shows the highest A1 index (Table 2, Fig. 5). Secondary constrictions occur in the short arms of chromosome pairs 22 and 23 (Fig. 4C).
Conyza primulaefolia is octoploid with 2n = 72. The karyotype comprises 28 m and 8 sm pairs of chromosomes; chromosome length ranges between 1.07 and 2.66 1m. TKL is the highest of all studied species (Table 2). A single secondary constriction was observed in pair 5 (Fig. 4D).
Conyza sumatrensis var. sumatrensis and C. sumatrensis var. floribunda presented 2n= 54, with 23 m and 4 sm chromosome pairs. In C. sumatrensis var. sumatrensis chromosome length varies from 1.15 to 2.33 1m, showing the lowest total karyotypic length (TKL) and intrachromosomal assimetry coefficient (A1) (Table 2, Fig. 5). A secondary constriction was observed in the short arm of the chromosome pair 3 (Fig. 4E). On the other hand, C. sumatrensis var. floribunda chromosome length varies from 1.21 to 2.62 1m (Table 2) and a secondary constriction was observed in the short arm of pair 2 (Fig. 4F).
DISCUSSION
The studied species of Conyza show differentiation in the typology of the inflorescence. They have monotelic sinflorescences, with predominance of leafy pseudopanicle, with the exception of C. burkartii that presents only terminal capitula (Amat, 1991). The absolute predominance of the monotelic sinflorescence, in addition to its leafy type and pyramidal shape, reinforce the idea of Troll (1964, 1969) and Weberling (1985), who consider this inflorescence type as primitive in Angiospermae.
Conyza sumatrensis var. sumatrensis, C. sumatrensis var. floribunda and C. glandulitecta have monotelic sinflorescences of the leafy pseudopanicle type with pyramidal appearance, and zone of innovation with capacity of nonsileptic (perennation) and sileptic development (caused by alterations or injury). Conyza sumatrensis var. sumatrensis presents dense pubescence on the stem and leaves while C. sumatrensis var. floribunda is almost glabrous with few rigid hairs on the stem and numerous small capitula and C. glandulitecta is completely covered by glandular hairs. On the other hand, C. blakei possesses a monotelic sinflorescence of cylindrical shape, characterized by its sympodial structure and almost glabrous leaves with rigid hairs on the margins and inflorescence of cylindrical shape.
Conyza bonariensis is distinguishable for possessing a branched inflorescence and smaller size. C. bonariensis presents a modification with respect to the formerly mentioned species: the inferior paracladia show a pronounced acrotonic development, in which each stem rises a small panicle to a higher level, producing a branched inflorescence.
Conyza primulaefolia is the most easily distinguishable species of the analysed group because of its scapiform crown, rosulate, large capitula and the bracted inflorescence with few capitula. C. primulaefolia possesses a branched monotelic sinflorescence in which all the paracladia present acrotonic development. According to Troll (1964, 1969) and Weberling (1985), the acrotonic development of the paracladia is a derivation of the pyramidal form, due to which the inflorescence of C. primulaefolia would have originated in successive processes of homogeneization and reduction.
The capitula show differences in flower numbers among species. Female flowers are located at the periphery of the capitulum while the hermafrodite flowers occupy its center; the size of the capitula is a consequence of the total number of flowers. A transition in total number of flowers was observed throughout the species, from C. primulaefolia with almost 600 flowers to C. sumatrensis var. floribunda with only 50 flowers. The transitional secuence is: C. sumatrensis var. floribunda- C. blakei- C. sumatrensis var. sumatrensis- C. glandulitecta- C. bonariensis- C. primulaefolia. This increase in the number of flowers is reflected in the size of the capitula. All the species present gynomonoecy; an adaptative feature in Asteraceae (Bertin and Kerwin, 1998) related with the reduction of the sex-ratio. C. primulaefolia is the species with the lowest value for this character and the highest utilization of the reproductive resource.
Our cytogenetical study confirmed the chromosome numbers of C. bonariensis, C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda (Bernardello, 1986; Hunziker, 1990; Solbrig, 1964), which are all hexaploid with 2n = 6x = 54. On the other hand, C. primulaefolia presents 2n = 8x = 72, confirming the octoploid nature of this species (Bernardello, 1986; Solbrig, 1964) and that its ploidy level variation is associated with morphological features characteristic of this species. Two new chromosomal counts were added to the genus: C. blakei and C. glandulitecta both with 2n = 6x = 54, confirming x = 9 as the basic number of Conyza, shared whith Erigeron and Aster (Solbrig, 1964). These genera possesses a polyploid series of 2n = 18, 36, 54 and 72, highlighting the important role of polyploidy in evolution of this group. Polyploidy is one of the more widespread mechanisms of speciation in Angiospermae and some authors suggest that this event is associated with colonization of extreme environments (Davis & Heywood, 1967).
Some species of Conyza present intraspecific variations in their chromosome numbers. For C. bonariensis, our results agree whith those of Solbrig (1964) and Bernardello (1986), showing this species as a hexaploid, with 2n = 6x = 54. However, Turner et al. (1979) reported n = 18 in populations of C. bonariensis from Jujuy (NW Argentina) and C. aff. bonariensis karyotype whith 2n = 18 were described by Wulff (1998) in populations from Neuquén (Argentina). Similarly, C. sumatrensis var. floribunda (= C. floribunda) show intraspecific variations, described by Gill (1978) with n = 9 in populations from Tanzania, and by Hunziker et al. (1990) that registered n = ca. 27-28 for populations from Neuquén (Argentina).
Karyotypical characteristics were described for the first time in C. blakei, C. glandulitecta, C. primulaefolia, C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda in this study. For C. aff. bonariensis, Wulff (1998) described a diploid karyotype with 2n = 18 characterized by metacentric chromosomes. However, in this paper, a C. bonariensis karyotype of 2n = 54, having chromosomes of similar shape and size to those described before by Wulff (1998), is reported for the first time.
The morphological karyotypic analysis of all studied taxa showed relatively symmetric karyotypes, with only metacentric and submetacentric chromosomes of 1 to 3 µm in length. Conyza sumatrensis var. sumatrensis presents the most symmetric karyotype, which is reflected in its highest mean centromeric index and lowest intrachromosomal asymmetry coefficient (A1); both characters clearly differentiate this species from the remaining taxa. Conyza bonariensis shows the highest interchromosomal asymmetry coefficient (A2), which reflects the amplitude of its chromosome size. With respect to the total karyotype length (TKL), C. primulaefolia presents the highest value, which is correlated with its ploidy level. Conyza sumatrensis var. sumatrensis presents the lowest TKL within the hexaploid species and this value is not associated with variations in chromosome number.
According to Stebbins (1971), the Angiospermae show a tendency towards the increase of the asymmetry of the karyotype, which has been observed in Asteraceae genera such as Aster, Crepis and Haplopappus. In agreement with this criterion, Conyza sumatrensis var. sumatrensis is the species with the highest number of ancestral characters, while C. bonariensis, according to the A2 index, presents the most derived karyotype. Both the increase in ploidy level and structural chromosomal rearrangements, accompanied the differentiation and speciation of this group.
The study of the inflorescence in the genus Conyza, reaffirms some evolutionary aspects of the inflorescences, as the homogeneization and reduction process, which make this group an interesting model for the study of this subject. With this approach to the evolution of the inflorescence, this character acquires further relevance in systematic studies. The karyotypic studies associated to the inflorescences characterization demonstrate that C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda are closely related, akin to C. blakei, C. bonariensis and C. glandulitecta. Within the studied group, C. sumatrensis var. sumatrensis and C. sumatrensis var. floribunda present primitive characters, while C. bonariensis shows modifications in both morphological and cytogenetic characters, and C. primulaefolia is the more differentiated species of the group. The specializatión and reduction associated to an increase of the ploidy level seems to have played a special role in the evolution of the genus Conyza.
BIBLIOGRAPHY
AMAT, A. G. 1991. Revisión y enfoque quimiosistemático de las especies argentinas del genero Conyza Less., con especial referencia al grupo C. albida -C. bonariensis - C. floribunda (Asteraceae: Astereae). Tesis Doctoral. Universidad Nacional de La Plata. Facultad de Ciencias Naturales y Museo. La Plata, Buenos Aires. [ Links ]
BERNARDELLO, L. M. 1986. Números cromosómicos en Asteraceae de Córdoba. Darwiniana 27: 169-178. [ Links ]
BERTIN R. & M. A. KERWIN. 1998. Floral sex ratio and gynomonoecy in Aster (Asteraceae). Amer. J. Bot. 85: 235-244. [ Links ]
CARR, G. D.; R. M. KING, M. POWELL & H. ROBINSON. 1999. Chromosome numbers in Compositae. XVIII. Amer. J. Bot. 86: 103-1013. [ Links ]
CRONQUIST, A. 1943. The separation of Erigeron from Conyza. Bull. Torrey Bot. Club 70: 629-632. [ Links ]
DAVIS, P. H. & V. H. HEYWOOD. 1967. Principles of Angiosperm Taxonomy. D. Van Nostrand Co., New York. [ Links ]
FERNANDEZ, A. 1973. El ácido láctico como fijador cromosómico. Bol. Soc. Argent. Bot. 15: 287-290. [ Links ]
GILL, L. S. 1978. IOPB chromosome number reports LX. Taxon 27: 223-231. [ Links ]
HUNZIKER, J. H., A. F. WULFF, C. C. XIFREDA, A. ESCOBAR. 1989. Estudios cariológicos en Compositae V. Darwiniana 29: 25-39. [ Links ]
HUNZIKER, J. H. 1990. Estudios cariológicos en Compositae VI. Darwiniana 30: 115-121. [ Links ]
LEVAN, A., K. FREDGA & A. A. SANDBERG. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52: 201-220. [ Links ]
NESOM, G. L. 1978. Chromosome number in Erigeron and Conyza (Compositae). Sida 7: 375-381. [ Links ]
NESOM, G. L. 1990. Further definition of Conyza (Asteraceae: Astereae). Phytologia 68: 229-233. [ Links ]
ROMERO ZARCO, C. 1986. A new method for estimating karyotype asymetry. Taxon 35: 526-530. [ Links ]
SANCHO G. & L. ARIZA ESPINAR. 2003. Asteraceae. En: A. T. HUNZIKER (ed), Fl. Fanerogámica Argentina 81: 14-27. CONICET, Córdoba. [ Links ]
SOLBRIG, O. T. 1964. Chromosome number in compositae V. Astereae II. Amer. J. Bot. 51: 513-519. [ Links ]
SOLBRIG, O. T. 1977. Chromosomal cytology and evolution in the family Compositae. In: HEYWOOD, V.H., J. HARBORNE, & B. L. TURNER (eds.), The biology and chemistry of the Compositae, pp. 267- 281. Academic Press, New York. [ Links ]
STEBBINS, G. L. 1971. Chromosomal evolution in higher plants. Edward Arnold, London. [ Links ]
THEBAUD, C. & R. J. ABBOTT. 1995. Characterization of invasive Conyza species (Asteraceae) in Europe: Quantitative trait and isozime analysis. Amer. J. Bot. 82: 360-368. [ Links ]
TROLL, W. 1964/69. Die Infloreszenzen, Typologie und Stellung in Aufbau des Vegetationskörpers I u. II/1. G. Fischer, Stuttgart. [ Links ]
TURNER, B. L., J. BACON, L. URBATSCH & B. SIMPSON. 1979. Chromosome numbers in South American Compositae. Amer. J. Bot. 66: 173-178. [ Links ]
WEBERLING, F. 1985. Morfología de las inflorescencias. Bol. Soc. Argent. Bot. 24: 1-28. [ Links ]
WULFF, A. F. 1998. Estudios cariológicos en Asteraceae VIII. Darwiniana 35: 37-43. [ Links ]
ZARDINI, E. M. 1976. Contribuciones para una monografía del género Conyza Less. I. Bol. Soc. Argent. Bot. 17: 31-46. [ Links ]
ZULOAGA, F. O. & O. MORRONE (eds.). 1999. Catálogo de las Plantas Vasculares de la República Argentina II. Monogr. Syst. Bot. Missouri Bot. Garden 74: 154-158. [ Links ]
Recibido el 17 de Agosto de 2004
Aceptado el 29 de Abril de 2005














