Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. v.41 n.3-4 Córdoba ago./dic. 2006
Anatomical changes in Willow Wood Decayed by the brown rot fungus Coriolellus malicola (Basidiomycota)
Mónica A. Murace1 , María L. Luna2,3 , Gabriel D. Keil4 y Natalia N. De Cristófano4
1Protección Forestal, Facultad de Ciencias Agrarias y Forestales, UNLP, CC 31 (1900), La Plata, Argentina. (E-mail: indforest1@ceres.agro.unlp.edu.ar).
2Facultad de Ciencias Naturales y Museo, UNLP, Paseo del Bosque s/n (1900), La Plata, Argentina.
3Comisión de Investigaciones Científicas de la Provincia de Buenos Aires.
4Industrias Forestales I, Facultad de Ciencias Agrarias y Forestales, UNLP, CC 31 (1900), La Plata, Argentina.
Summary: In Argentina, Salix wood is employed mainly in pulp and paper industry. In this country, the brown rotter C oriolellus malicola was found in association with willow plantations. The purpose of this work was to study the anatomical changes caused by C. malicola in willow wood in order to provide information on the effects of brown rot decay in the yield and quality of pulp. Two willow clones were employed: Salix nigra 4 and Salix babylonica x Salix alba cv I 131-25 . Two exposure times were used: 75 and 150 days. The percentages of weight loss produced by this fungus in both clones was ca. 30% at 75 days and ca. 60% at 150 days of decay. C. malicola degraded mainly fibre walls. Microscopically, the loss of cell shape, the presence of transwall fractures and the loss of birefringence were the main anatomical modifications observed. According to our observations decayed Salix wood by C. malicola seems to be inadequate for pulp industry.
Key words: Brown-rot decay; Hardwoods; Anatomy; Salix; Coriolellus malicola .
Resumen: Modificaciones anatómicas en madera de sauce por acción Coriolellus malicola (Aphyllophorales) agente de pudrición castaña. En la República Argentina la madera de Salix es empleada principalmente en la industria papelera. En este país el hongo de pudrición castaña C oriolellus malicola se encontró asociado a plantaciones comerciales de sauce. El objetivo de este trabajo fue estudiar las modificaciones anatómicas causada por C. malicola en la madera de sauce con el fin de aportar información sobre los efectos de la pudrición castaña en los rendimientos y calidad de la pulpa para papel. Se emplearon dos clones: Salix nigra 4 y Salix babylonica x Salix alba cv I. 131-25 . Se trabajó con dos tiempos de exposición: 75 y 150 días. Las pérdidas de peso producidas por esta cepa en ambos clones fueron de ca. del 30% a los 75 días y de ca. del 60% a los 150 días de incubación. C. malicola degradó principalmente las paredes de las fibras. Microscópicamente, las principales modificaciones anatómicas observadas fueron: deformación del tejido, presencia de fracturas transversales en las paredes celulares y pérdida de birrefringencia. De acuerdo con nuestras observaciones la madera de Salix degradada por C. malicola sería inadecuada para la industria del pulpado.
Palabras clave: Pudrición castaña; Madera de latifoliadas; Anatomía; Salix; Coriolellus malicola.
INTRODUCTION
Many microorganisms are likely to degrade wood. The most relevant of them are the xylophagous fungi because they produce important changes on the anatomical, physical and mechanical wood properties (Wilcox, 1978; Highley et al ., 1994).
Wood rotting fungi are known to cause three basic types of degradation: white rot, soft rot and brown rot. Brown rotters remove cellulose and hemicelluloses whereas lignin degradation is limited (Wilcox, 1978; Blanchette, 1995). The modified lignin remaining gives the decayed wood its characteristics colour and consistency (Schwarze, et al ., 2003). Brown rot decay involve the penetration of enzymes into cell wall from hyphae growing in the lumen. These enzymes penetrate the entire secondary walls and produce an extensive degradation of cellulose and hemicelluloses (Schwarze et al ., 2000).
Although brown rot decay is typically associated with softwoods, some brown rotters are reported to attack hardwoods (Wilcox 1968; Schwarze et al . 2000).
Wright et al (1973) and Wright & Deschamps (1976) recorded the brown rot fungus Coriolellus malicola (Berk. & Curt.) Murr. (Basidiomycetes, Aphyllophorales) on willow plantations in Argentina. As Salix wood is employed mainly for paper industry (SAGPyA, 1999) in this country, the study of the decay produced by brown rot fungi is important, because they cause significant reductions in the yield and quality of the pulp (Lindgren, et al . 1961).
According to Wilcox (1993) microscopical examination remains the most accurate dependable means of diagnosing and evaluating wood decay. Many authors consider that the first signs of brown rot decay are the presence of hyphae in the cell lumen and the loss of birefringence in the cell walls (Wilcox 1968, 1993; Anagnost 1998, Schwarze et al ., 2000).
The purpose of this work was to study the anatomical changes caused by C. malicola in willow wood in order to provide information on the effects of brown rot decay in the yield and quality of pulp.
MATERIALS AND METHODS
Sample preparation: Sound sapwoods of Salix nigra 4 and S. babylonica x S. alba cv. I 131-25 from Paraná River Delta plantations, Buenos Aires Province, Argentina (34° 05´ LS; 58° 38´ LW) were used. Both clones are the most widely planted in this area, where climatic and soil conditions are optimal for this species (Blanco, 1977; Alonso, 1980). Five trees of each clone were randomly selected. A 2-meter-long log immediately above the stump was extracted from each specimen. Logs were sawn into 5 x 5 cm pieces and air-dried until constant weight was reached. Once dry, small cubic blocks (2 cm x 2 cm x 2 cm) to be submitted to decay in vitro were cut.
Decay test experiment: Coriolellus malicola (Berk. & Curt.) Murr. (BAFC Strain 472 ) was grown for two weeks in Petri plates with malt extract-agarose medium.
ASTM D-2017 (78) standard with some modifications based on IRAM 9518 (1962) was employed. Twenty glass bottles (cylindrical, capacity nominal 450 cm 3 ) were filled with 43 cc distilled water and 200 g of a substrate (sand 160g, soil 40 g) previously oven dried (105± 2 ºC) and sifted. Then two feeder strips were introduced in each bottle. Culture bottles were sterilized for 30 minutes at 120 ºC and inoculated with C. malicola. After 20 days blocks were placed over feeder strips.
Twenty cubic blocks (2 cm side) of each clone were used for decay test. Blocks were oven dried (70 ºC) and weighed to determine the initial weight (IW); then moistened by immersion in distilled water and sterilized for 20 minutes at 100º C.
The assay was incubated for 75 and 150 days at 27 ±1ºC and 70% RH. After each incubation period 10 blocks per clone were withdrawn from the bottles, cleaned to remove mycelia, and oven dried at 70ºC for three days until constant weight, considered as final weight (FW). Percentages of weight loss (WL %) due to decay were then calculated as:
![]()
Twenty uninoculated blocks (10 of each clone) were introduced in the culture bottles, as was previously described, and employed as controls.
Anatomy of decay: Wood samples at different stages of decay were selected. For light microscopy studies (LM) of cell wall degradation and hyphal growth, pieces of decayed material were fixed in formaldehyde-acetic acid-alcohol and embedded in paraffin (D'Ambrogio de Argüeso, 1986). Sections (8-12 µm) were double stained with safranin-fast green.
To detect cellulolysis some sections were left unstained and viewed between crossed polarizing filters. Loss of birefringence was considered as an indicator of cellulose depletion and, as a consequence, of brown rot decay (Wilcox, 1968, 1993; Schwarze et al. 2003).
Blocks were also examined in a Jeol JSMT-100 scanning electron microscope (SEM). Specimens were mounted on stubs without pretreatment and covered with gold-palladium. Sound wood was used as control.
RESULTS AND DISCUSSION
Weight loss due to decay: The percentages of weight loss (% WL) after each incubation period are given in table 1. No significant differences of % WL were found between Salix clones at both incubation times.
Anatomy of decay: Coriolellus malicola caused the same pattern of decay in both clones. Macroscopically decayed material showed a brown discoloration. Blocks with higher weight losses (ca. 60% WL) showed in addition shrinkage and a cubical pattern of cracks. The characteristic shrinkage of brown rotted wood is attributed to the extensive removal of carbohydrates. Thus, when the wood is dried, the lignin residue and carbohydrate depolymerization products lack sufficient strength to maintain the integrity of the cells (Wilcox, 1968).
Microscopically sound Salix wood showed the typical shape and cell wall thickness of vessels and fibres (Fig. 1 A-B).
At 30 % WL decayed wood exhibited a slight loss of cell shape. Transwall fractures were frequent in fibres and vessels (Fig. 2 A).
Separation among fibres was occasionally observed in some specimens (Fig. 2 B). Cell separation occurred between the S1 layer and the compound middle lamella (ML), which appeared intact at this stage of decay. Wilcox (1968) observed separation among cells in brown rotted Liquidambar styraciflua . However, in a later paper, the author considered that this feature was not diagnostic of brown rot decay because its appearance was not regular (Wilcox, 1993). As in Salix wood separation among cells was rarely observed, we don't consider this as diagnostic feature of brown rot decay, in agreement with Wilcox (1993).
In longitudinal sections ray parenchyma cells appeared unaltered (Fig. 2 C), as occurred in sound wood (Fig. 1 C). Wilcox (1968) and Worrall et al. (1997) observed the same feature in brown rot decayed Liquidambar styraciflua and Betula alleghaniensis at similar weight losses (near 30 % WL). Moreover, Anagnost (1998) mentioned that brown rot fungi do not attack rays in hardwoods.
In decayed Salix wood bore holes were rarely found. In contrast, hyphal penetration through pits was frequently observed (Fig. 2 D). Shwarze et al . (2000) also noted that in Robinia sp., the brown rotter Laetiporus sulphureus advanced to neighboring cells via pit connections. Cell wall erosion and mycelia were no registered in sound Salix wood (Fig. 1 D).
Examination under polarized illumination revealed loss of birefringence in fibre walls in some portions of the samples (Fig. 2 E). By contrast, vessels appeared relatively intact and birefringent during decay. Comparable patterns were observed by Wilcox (1968) and Schwarze et al . (2000) in brown rotted Liquidambar styraciflua and Robinia pseudoacacia woods, respectively. In sound wood samples both, fibres and vessels, appeared birefringent (Fig. 1 E).
Longitudinal sections of sound willow wood showed birefringence in vessel, fibre and ray parenchyma cell walls (Fig. 1 F). However, a weak birefringence was observed in ray parenchyma cells at 30% WL (Fig. 2 F). Schwarze et al . (2003) observed in brown rotted Quercus robur, Acer pseudoplatanus, Betula pendula and Robinia pseudoacacia woods that rays could be seen bright at lower weight losses (5.51-12.11 % WL). They related the absence of radial fibril orientations in the secondary walls of vessels and parenchyma cell walls with a presumably increase in the resistance to degradation by these fungi. However, at higher weight losses (30%) they could probably also be degraded, as occurred in willow wood.
At ca. 60% WL the diagnostic features of brown rot decay were more pronounced. Loss of cell shape and cell wall thinning were evident (Fig. 3 A-B). In some samples fibres appeared completely collapsed (Fig. 3 B). As a result of shrinkage, vessels and rays acquired a sinuous run (Fig. 3 C). Cracks into cubical fragments were also registered (Fig. 3 D-E). Under polarized light, mainly vessel elements displayed birefringence (Fig. 3 F).
Cellulolysis by brown rot fungi involve the radial penetration of enzymes into cell wall from hyphae growing in the lumen. The S2 layer of the secondary wall is the first to be attacked and decomposed towards the middle lamella, which remains unaltered together with the S3 layer (Schwarze et al . 1997). In the present study the ML was resistant to attack and appeared birefringent during decay.
From a pulp and papermaker's point of view wood characteristics such as basic density, fibre length, chemical composition and whiteness are of importance for the paper industry (Senisterra et al . 2000). Discolored decayed wood tend to darken pulps and to increase the consumption of chemical. In addition, the pulp from decayed wood contains many short fibres, with low tensile strength, low tear and low bursting strength (Shema, 1955; Lindgren et al ., 1961).
In the present study brown rotted willow wood showed discoloration, cell wall fractures and loss of birefringence indicating cellulose depolymerization. These characteristics produce negative effects on the yield and quality of pulp.

Fig. 1. Sound willow wood. A-D, SEM micrographs. A , Transverse section showing the characteristic cell shape of sound vessels and fibres. Note absence of mycelia and fractures. B, Typical fibre walls in detail. C-D, Longitudinal sections. C, Typical aspect of sound rays. D, Typical aspect of sound fibre and vessel walls. Note absence of mycelia and fractures. E-F, Polarized light micrographs. E, Transverse section showing brightness of vessel and fibre walls. F, Longitudinal section showing birefringent ray, fibre and vessel walls. References: v: vessel; fi: fibre; r: parenchyma ray. Bars: A, E-F = 50 µm; B = 10 µm; C-D = 100 µm.
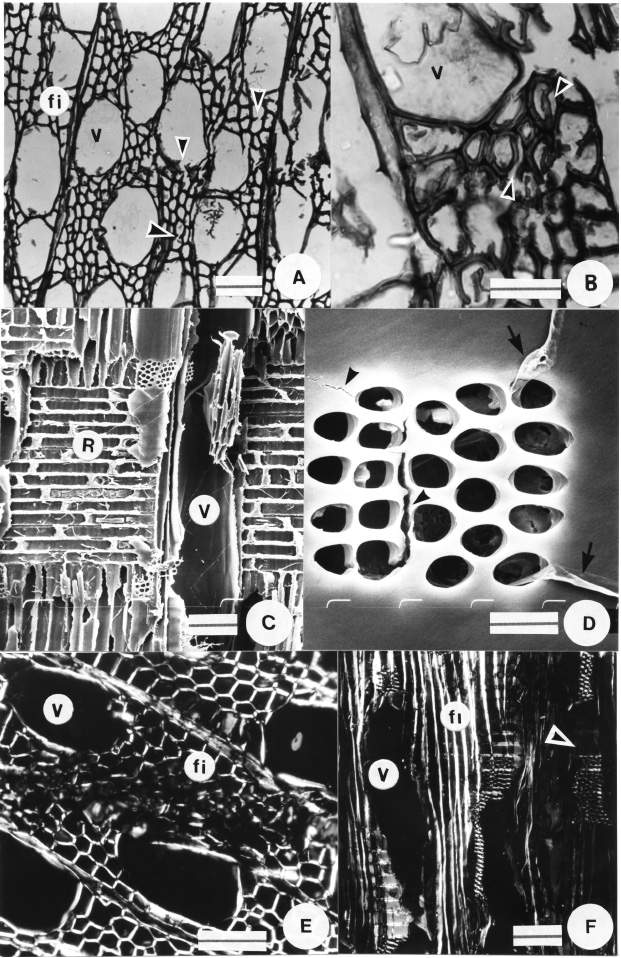
Fig. 2. Anatomical features of brown rot decay in willow wood at ca. 30 % WL. A-B, LM micrographs. A, Slight loss of cell shape and transwall fractures in vessels and fibres (arrowheads). B, Separation among fibres (arrowheads). C-D, SEM micrographs. C, Unaltered ray parenchyma cells. D, Cracks (arrowheads) and hyphal penetration via pits (arrows). E-F, Polarized light micrographs. E, Transverse section showing loss of birefringence in fibres. F, Longitudinal section. Loss of birefringence in ray parenchyma cells in the right half of the micrograph where degradation occurred (arrowhead). References: v: vessel; fi: fibre; r: parenchyma ray. Bars: A, E-F = 50 µm; B = 25 µm; C = 100 µm; D = 10 µm.

Fig. 3. Anatomical features of brown rot decay in willow wood at ca. 60 % WL. A-C, LM micrographs. A, Marked loss of cell shape and cell wall thinning. B, Portion of the sample showing collapse of fibre walls (arrowheads). C, Sinuous run of vessel walls (arrowheads). D-E, SEM micrographs. D, Cracks extended throughout the tissue (arrowheads). E, Cubical pattern of cracks (arrowheads). F, Polarized light micrograph showing birefringence mainly in vessels. References: v: vessel; fi: fibre; r: parenchyma ray. Bars: A-B, D-F = 50 µm; C = 100 µm.
Table 1 . Average percent weight loss (WL) during each incubation time.

* Different letters represent statistical differences with Tukey at p<0.05. Coefficient of variation (CV %) is given between brackets.
CONCLUSION
The anatomical changes observed in brown rotted Salix wood were: transwall fractures, loss of birefringence mainly in fibre walls, loss of cell shape, cell wall thinning and a typical brown discoloration. C. malicola attacked predominantly fibre walls, which are employed in paper industry. According to our observations Salix wood decayed by C. malicola is not appropriate for pulp and paper industry.
AKNOWLEDGMENTS
The authors wish to thank Ana María Bucsinszky, Instituto Spegazzini, Facultad de Ciencias Naturales y Museo (UNLP-CONICET) for providing the fungal strain, and Bruno Pianzola and Xavier Kriscautzky for their technical assistance with photography.
BIBLIOGRAPHY
ALONSO, A.E. 1980. Salix nigra: Un interesante productor de madera y de pasta celulósica. Asociación Forestal Argentina 34: 53-62. [ Links ]
BLANCO, J. 1977. Susceptibilidad y resistencia al ataque de roya en los alamares cultivados del Delta del Paraná. IFONA. Folleto Técnico Forestal Nº 50, 10 p. [ Links ]
ANAGNOST, S.E. 1998. Light microscopic diagnosis of wood decay. IAWA J. 19: 141-167. [ Links ]
ASTM D-2017. Society for testing and materials. 1978. Standard method accelerated laboratory test of natural decay resistance of wood. In Annual Book. [ Links ]
BLANCHETTE, R.A. 1995. Degradation of the lignocellulose complex in wood. Can. J. Bot . 73 (Suppl. 1): 999-1010. [ Links ]
D'AMBROGIO DE ARGÜESO, A. 1986. Manual de técnicas en histología vegetal. Ed. Hemisferio Sur. Buenos Aires. [ Links ]
HIGHLEY, T.L., C. A. CLAUSEN, S.C. CROAN, F. GREEN, B. L. ILLMAN & J.A. MICALES. 1994. Research on biodeterioration of wood, 1987-1992. I. Decay mechanisms and biocontrol. USDA Forest Service, Research Paper FPL-RP-529. [ Links ]
IRAM 9518. 1962. Toxicidad, permanencia y eficacia de preservadores de madera. Métodos de laboratorio. Instituto Argentino de Racionalización de Materiales, Buenos Aires : 1-12. [ Links ]
LINDGREN, R. & W. ESLYN.. 1961. Biological deterioration of pulpwood and pulp chips during storage. Tappi 44:419-429. [ Links ]
SAGPyA. 1999. Argentina: oportunidades de inversión en bosques cultivados. Publicación de la Secretaría de Agricultura, Ganadería, Pesca y Alimentación, Buenos Aires : 127-136. [ Links ]
SCHWARZE, F.W.M.R.; D. LONSDALE & S. FINK. 1997. An overview of wood degradation patterns and their implications for tree hazard assessment. Arboricultural Journal 21: 1-32. [ Links ]
SCHWARZE, F.W.M.R, J. ENGELS & C. MATTHECK. 2000. Fungal Strategies of wood decay in trees . Springer Verlag, Berlin. [ Links ]
SCHWARZE, F.W.M.R, S. FINK & G. DEFLORIO. 2003. Resistance of parenchyma cells in wood to degradation by brown rot fungi. Mycological Progress 2: 267-274. [ Links ]
SENISTERRA, G; S. MONTEOLIVA; J. MARQUINA; R. MARLATS Y G. CIOCCHINI. 2000. Propiedades del leño en clones del género Salíx, utilizados en programas de mejoramiento genético con aplicación a la industria papelera. Rev. Forestal YVYRARETA, diciembre (2000): 93-95. [ Links ]
SHEMA, B.. 1955. Introduction to microbiology. The microbiology of pulpwood. Cap. II.28-52. [ Links ]
WILCOX, W.W. 1968. Changes in wood microstructure through progressive stages of decay. USDA For. Serv. Res. Paper FPL 70: 1-46. [ Links ]
WILCOX, W.W.1978. Review of literature on the effects of early stages of decay on wood strength. Wood and Fiber 9: 252-257. [ Links ]
WILCOX, W.W. 1993. Comparative morphology of early stages of brown-rot wood decay. IAWA J .14: 127-138. [ Links ]
WORRALL, J.J., S.E. ANAGNOST & R.A. ZABEL. 1997. Comparison of wood decay among diverse lignicolous fungi. Mycologia 89: 199-219. [ Links ]
WRIGHT, J.E., J. DESCHAMPS & G. ROVETTA. 1973. Basidiomycetes xilófilos de la región mesopotamica. I. Poliporos trametoides. Rev. Investig. Agrop . INTA, ser. 5, Pat. Veg. 10: 117-227. [ Links ] [ Links ]
Recibido el 21 de Febrero de 2006
Aceptado el 17 de Julio de 2006.














