Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. v.42 n.3-4 Córdoba ago./dic. 2007
Iodophanus carneus and I. testaceus (Ascomycota-Pezizales): Independent taxonomic identity or synonymy? A study of their morphology and isozymes*
Isabel E. Cinto1,3 Diana A. Dokmetzian1 and María E. Ranalli1,2
*Trabajo publicado en homenaje a la Dra. Irma J. Gamundí en conmemoración de su 80º aniversario.
1Departamento de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires, Ciudad Universitaria, C1428EHA. Ciudad Autónoma de Buenos Aires, Argentina.
2Miembro de la Carrera de Investigador de CONICET
3e-mail: icinto@bg.fcen.uba.ar
Summary: The aim of this study was to delimit two Iodophanus species: I. carneus and I. testaceus , based on morphological characteristics and electrophoretic patterns of their intracellular isozymes. Twenty monosporic strains were used, including five belonging to I. granulipolaris as a control. Fourteen isozyme systems were tested, and the five having the best resolution selected: aspartate aminotransferase, esterases, alkaline phosphatase, glutamate dehydrogenase, and superoxide dismutase. These analyses confirmed the similarity between I. carneus and I. testaceus , since they both produced the same band patterns, which were in turn different from the band pattern of I. granulipolaris. So, as we couldn´t find any character wich permit us to classify the isolated studied during this work in defferent species, we believe that I. testaceus shoul be consider as a synonym of I. carneus .
Key words: Fungi; Lodophanus; Isozymes; Synonymy; Taxonomy.
Resumen: Iodophanus carneus e I. testaceus (ASCOMYCOTA-PEZIZALES): ¿Identidades taxonómicas independientes o sinonimia? Estudio morfológico e isoenzimático. El objetivo del presente trabajo fue la delimitación taxonómica de dos especies del género Iodophanus : I. carneus e I. testaceus a partir de caracteres morfológicos y de los patrones electroforéticos de isoenzimas intracelulares. Para ello se utilizaron veinte cepas monospóricas, cinco de las cuales pertenecientes a I. granulipolaris que fueron utilizadas como control. Se probaron catorce sistemas isoenzimáticos y se eligieron los cinco con mejor resolución: aspartato amino transferasa, esterasa, fosfatasa alcalina, glutamato dehidrogenasa y superóxido dismutasa. El análisis de los patrones isoenzimáticos corroboró la silimitud existente entre I. carneus e I. testaceus , ya que los patrones de bandas obtenidas para estas dos especies fueron iguales y diferentes de I. granulipolaris. Entonces, al no encontrar ningún caracter que nos permita separar a los aislamientos estudiados en este trabajo en dos especies distintas, proponemos a I. testaceus como un sinónimo de I. carneus.
Palabras clave: Hongos; Lodophanus; Taxonomía; Isoenzimas; Sinonimia.
INTRODUCTION
The genus Iodophanus Korf belongs to the family Pezizaceae (Ascomycota-Pezizales). The distinctive features of Iodophanus species are amyloid asci, eight hyaline ascospores per ascus, and exosporium with callose pectic ornamentations. This kind of ornamentation and having Oedocephalum as the conidial state of some species are typical characteristics of the family Pezizaceae. Therefore, Iodophanus species, formerly assigned to the family Ascobolaceae, have been later assigned to the family Pezizaceae.
Iodophanus species have been found on various different substrates, such as herbivore droppings, soil, paper, clothes, cardboard and a wide range of decomposing plant matter. They are probably cosmopolitan, since they have been reported in North America, South America, Europe, southeast Asia, Australia and Africa (Kimbrough et al ., 1969; Gamundí & Ranalli, 1964; Schumacher, 1992; Kimbrough, 1970; Seaver, 1916; Cinto & Dokmetzian, 2006; Thind & Kaushal, 1978; Jeng & Krug, 1977) .
The most useful characters in classical taxonomy of fungi are spore, asci and apothecia dimension, as well as their form and color. In addition to this, Kimbrough et al. (1969) suggest that «the most diagnostic feature are the size, shape and ornamentation of ascospores». One major problem regarding Iodophanus species delimitation is that the spore and asci dimensions of the different «species» overlap. Moreover, measurements made by different authors for the same species often differ, making species determination even more difficult. In addition to this, the enviromental condition, as well as the quantity and quality of light, among other factors, would have considerable influence on ascospore and asci size, therefore these characters by themselves are not enough to establish species boundaries (Diorio et al. , 1995; Cotty & Misaghi, 1985).
According to Harrington & Rizzo (1999), who tried to arrive at a workable definition for fungal species, «it is important that the individuals comprised in a certain species are derived from a common ancestor and that these individuals are reproductively isolated from sympatric populations of related species. The phenotypic characters most valuable as delimiting characters would be those associated with the ecological adaptations that circumscribe the niche of the species in question.» Molecular markers (e.g. RFLPs, RAPDs, AFLPs) often correlate well with the species-delimiting phenotypic characters, thus providing excellent tools for identification, but they are not the final word in species delimitation (Anderson et al ., 1997; Bruns et al ., 1991; Kohn, 1992).
Isozymes are phenotypic characters, and as such they can reflect fixed differences for delimiting fungal species. Differences in electrophoretic mobility in vitro may differentiate morphologically similar populations and often are congruent with ecological adaptations to specific climatic conditions or pH of substrate. Isozyme analysis has also proved to be very useful for distinguishing asexual species that differ very little morphologically (Zambino & Harrington, 1989).
Kimbrough et al. (1969) separate I. carneus and I. testaceus mainly according to their natural habitat. They sustain that all the I. carneus collections found to date on substrates other than dung (e.g. paper, soil, cardboard and other decomposing cellulose substrates) should be considered as I. testaceus. In addition to habitat, they find other morphological characteristics to separate these species: I. carneus apothecia are quite a lot smaller than I. testaceus apothecia , while the latter contain more carotenoid pigments; I. carneus asci are clearly claviform and 40-50 m m shorter, its ascospores are 4 m m shorter, and their tips less rounded than in I. testaceus ; and the fact that they have never found Oedocephalum as the conidial state for I. carneus. But others authors as Dennis (1968), Thind & Waraitch (1971) and Doveri (2004) do not find enough justification in separating the two species on the basis of substratum alone.
The aim of this study was to delimit two Iodophanus species: I. carneus and I. testaceus , based on morphological characteristics and electrophoretic patterns of their intracellular isozymes.
MATERIALS and METHODS
Strains
Twenty monosporic strains obtained from the germination of spores from polysporic isolates were used. The different strains were routinely maintained on PF medium (Gamundí & Ranalli, 1964) at 5ºC . Table 1 shows the location, substrate, collection date and strain number (BAFC) of the different isolates.
Table 1. List of strains with their geographical location, substrate and BAFC number. ER: Entre Ríos Province; CABA: Ciudad Autónoma de Buenos Aires; Arg: Argentina; BAFC #: Buenos Aires, Facultad de Ciencias Exactas y Naturales.

Liquid culture medium
Synthetic GA (glucose-asparagine) medium: SO4Mg.7H2O, 0.5 g ; PO4H2K, 0.5 g ; PO4H2K , 0.6 g ; SO4Cu.5H2O, 0.4 mg; Cl2Mn.4H2O, 0.09 mg; BO3 H3, 0.07 mg; MoO2 Na.2H2O, 0.02 mg; Cl3Fe, 1 mg; Cl2Zn, 10 mg; biotin, 5 m m; HCl-thiamine, 0.1 mg; glucose, 3 g ; L-asparagine, 0.2 g ; bidistilled water to 1000 mL.
The culture medium was sterilized at 121ºC and 1.2 atm, for 20 minutes.
Culture conditions
Cultures were grown in 125 mL flasks containing 50 mL of liquid medium and inoculated with 9 mm 3 cubes taken from a 3-4 day old colony growing on water- agar in the dark.
The flasks were incubated in a New Brunswick Psicrotherm G-27 culture chamber, at 23ºC , at constant upper ilumination with four 20W fluorescent tubes, and in constant agitation at 125 rpm.
For morphological studies, the fungus was grown on TD culture medium (Tyndallized dung), incubated at 23ºC, at constant upper ilumination.
Gross morphological obsevation were made with a stereocopic microscope, noting such characters as color, size and shape of apothecia. Asci, ascospore, paraphyses and excipular elements were examinaded in crush mount. Blueing of asci were checked with Melzer´s reagent.
The key proposed by Kimbrough et al. (1969) was used for the identification of the species.
Sampling
Mycelium grown in liquid GA medium was harvested by filtering it through a Buchner funnel at reduced pressure, on 2 days before they reached each species' maximum growth day, according to its growth curve which was prevoulsy charter using dry weight as growing stimator. In this particulary case all the species tested shown the same maximun growth day (day 12). Later on, micelium was washed three times with bidistilled water, dried on filter paper and stored at -70ºC until it was processed (Dessauer et al., 1984). The filtered mycelium was freezed with liquid nitrogen and ground in a mortar and cell homogenates was removed with extraction buffer (Soltis et al., 1983). Finally it was divided into aliquots and frozen at -70ºC until it was used.
Electrophoretic conditions and gel preparation
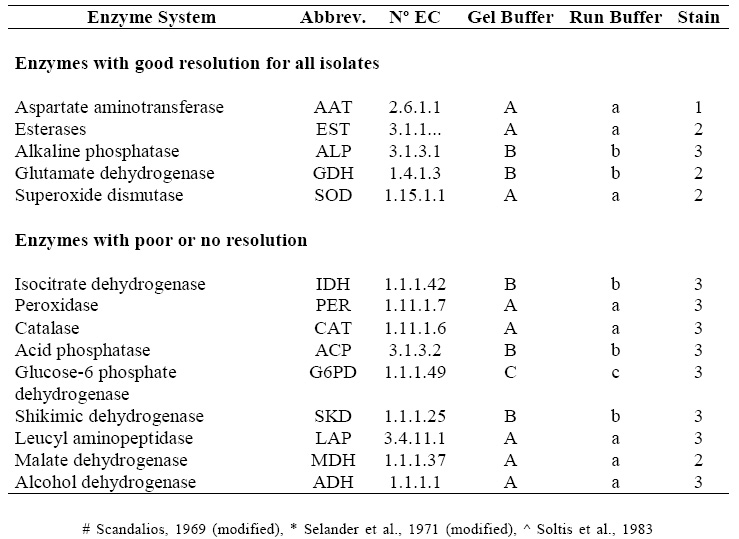
Horizontal electrophoresis on 7% polyacrylamide gels (Saidman, 1985) was the technique employed. Different buffers were used for preparing the gels and the electrophoretic run, according to the different isozyme systems analyzed (Scandalios, 1969; Selander et al., 1971; Soltis et al., 1983). Fourteen isozyme systems were analyzed. Table 2 shows the tested isozyme systems, and the buffers and enzyme staining protocols used for each one of them. Those systems having good resolution for all the isolates were selected for subsequent analyses.
Table 2. Isozyme systems tested, Nº of EC, abbreviations, gel and run buffers, enzyme staining protocols. A: lithium borate, pH 8 #, a: lithium borate, pH 8,2 #, B: Tris-citrate, pH 6,5 *, b: Tris-citrate, pH 7 *, C and c: Tris-citrate, pH 8 ^ 1: Vallejos (1983), 2: Wendel & Weeden (1989), 3: Manchenko (1994).
RESULTS
Morphological studies I. carneus (Pers.). Korf in J. W. Kimbrough and R. P. Korf (1967).
Apothecia: superficial, small, gregarious, globose at first, and entirely covered in hyaline hyphae giving it an arachnoid appearance; lenticular to pulvinate when mature, with papillose hymen due to asci emergence, pale pink to bright orange. Diam. 0.5-1.8 mm.
Asci: unitunicate, 8-spored, subclaviform, pseudoamyloid when young and with amyloid reaction of the entirely wall when mature; rounded apex and central operculum; 192-298 x 24-32 mm.
Paraphyses: simple, broadening slightly at the apex, containing guttules of orange pigments concentrated at the basal portion of paraphysis; 5-8.3 m m diam. at the apex.
Ascospores: irregularly biseriate, 1-celled, clustered at the top of the ascus, ellipsoid, hyaline, smooth when young and ornamented towards maturity with small spiny warts, irregularly distributed; 18-24 x 10-16.6 mm.
Excipulum: «globulous» texture, formed of cells pigmented at the outer basal zone, 8-10 mm diam.
Anamorph: Oedocephalum conidial state.
Habitat: on dung of various herbivores.
Holotype: not known.
Material studied: ARGENTINA, Prov. Entre Ríos, Hernández, on cow dung, 24 april 2002, Giménez 51546 (BAFC); ARGENTINA, Prov. Entre Ríos, Gualeguaychú, on cow dung, 24 april 2002, Giménez 51545 (BAFC) I. testaceus (Moug.) Korf in J. W. Kimbrough and R. P. Korf (1967) Apothecia: superficial, isolated to concrescent, globose. Pale yellowish when young, turning bright pink on maturing. Diam: 0.45-0.75 mm. Asci: Unitunicate, 8-spored, with central operculum. Amyloid, subclaviform; 190.28-230.48 x 29.48-34.84 mm. Paraphyses: simple, regularly septate and broadening at the apex.
Highly pigmented, with great accumulation of orange pigments. Diam: 6.7-7.15 mm. Ascospores: ellipsoid to rounded with small, irregularly distributed ornamentation; 16.25-19.5 x 10.4-11.7 mm.
Excipulum: made of globose cells, 15.6-26 x 13-22.75 mm.
Anamorph: Oedocephalum conidial state.
Habitat: not coprophilous, found on a wide range of decomposing cellulose substrates.
Type specimen: not known; type locallity: probably France. Material studied: ARGENTINA, Ciudad Autónoma de Buenos Aires, on old paper, 12 october 2002, Ranalli 51547 (BAFC).
Notes: Quantitative morphological characteristics, such as asci and ascospore sizes, have always been very important to describe a species and can be used to define species phylogenetically. In the case of these species, there is a noticeable overlap between different features, making species boundaries unclear.
Isozyme systems The isozyme systems that provided a good resolution for all isolates are: AAT, EST, ALP, GDH and SOD. The remaining systems (IDH, PER, CAT, ACP, G6PD, SKD, LAP, MDH & ADH) showed poor resolution and were excluded.
Monosporic strains of the independent taxonomic unit I. granulipolaris were used as a control.
Figure 1 shows the electromorphs for the five systems. Ten electromorphs were detected for the five systems. I. granulipolaris showed a characteristic band pattern for each system tested, while the other two species under study always showed a characteristic pattern for each system, but not a single difference was found between them. No isoenzymatic differences were found among strains from the same geographical location. All the systems tested were monomorphic.
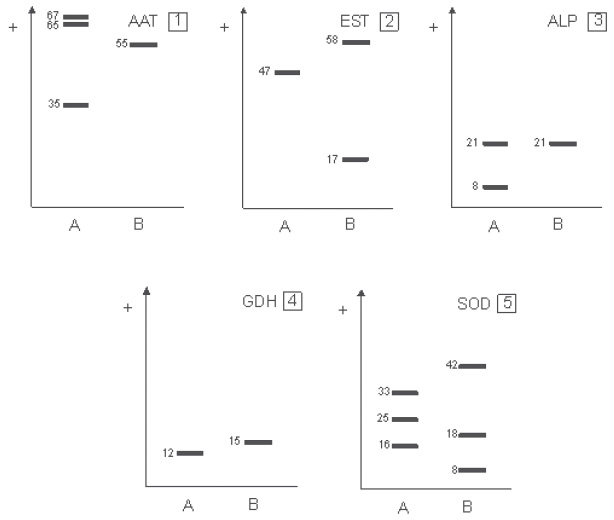
Fig. 1. Diagrams of electromorphs ( A-B ) identified in each system. A: I. granulipolaris (IG); B: I. carneus (TJ y ER) and I. testaceus (IT). 1: aspartate aminotransferase (AAT); 2: Esterases (EST); 3: alkaline phosphatase (ALP); 4: glutamate dehydrogenase (GDH); 5: superoxide dismutase (SOD).
DISCUSSION
During this study, we found Oedocephalum as the anamorph form for both species when they were growing at room temperature (28ºC approximately) on TD culture medium. The morphological differences described by Kimbrough et al. (1969) for these species did not match those we found during this study. We found that although there is a large overlap between the measurements of the two species, I. testaceus apothecia were altogether considerably smaller than I. carneus apothecia, and the same was true for asci and ascospore sizes.
However, as the different measurements did overlap, it is hard to use these characters for identifying species, we consider that the fact of two groups (or population) of organisms develop on different habitats doesn´t seem to be a significant character for species delimitation.
Isozyme analysis of all strains of the same species produced the same pattern. Iodophanus species, like many coprophilous species, are homothallic (non-outcrossing), probably as an adaptation to its substrate determining its reproductive isolation. The main source of variation of the homothallic form of a haploid organism is mutation. Thus, while a homothallic organism produces cloned descendants, a heterothallic organism produces variable descendants via recombination (Rayner, 1990). The process of change in homothallic populations will thus be slower because the variation among individuals of the populations, and among populations themselves, will be mainly due to the only source of variation. This may explains why no intraspecific variation was found in our study. Nevertheless, mutation and selection could lead to genetic divergence and increase the level of variability in a homothallic population, but in a longer period of time. The correlation between the degree of enzymatic varability and the type of reproduction and the habitat of organism has been studied by oyhers authors. Ramos (1998) worked with Saccobolus and Suárez et al. (2006) worked with Coprotus , both homotallic coprophilous fungi, also obtaining low isoenzymatic variability.
During this study we have not found any characteristic enabling a boundary to be established between them. In addition to this, isozyme analysis was useful and appropriate for separating I. carneus and I. testaceus from I. granulipolaris, but no system was found to separate I. carneus and I. testaceus from each other. So, at the lack of a strong character which permit us to delimit each species, and the impossibility of observe any type specimen we have to agree with those authors that place I. carneus and I. testaceus in the same taxa.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and University of Buenos Aires, Argentina.
BIBLIOGRAPHY
ANDERSON, J. C., D. M. PETSCHE & M. L. SMITH. 1987. Restriction fragment polymorphism in biological species of Amarilla mellea . Mycologia, 79: 69-76. [ Links ]
BRUNS, T. D., T. J. WHITE & J. W. TAYLOR. 1991. Fungal molecular systematics. Ann. Rev. Ecol. Syst. 22: 525-464. [ Links ]
CINTO, I. E. & D. A. DOKMETZIAN. 2006. Iodophanus granulipolaris (Ascomycota-Pezizales): primera cita para la Argentina.Un estudio morfológico y fisiológico. Hickenia 3, 62: 277-284. [ Links ]
COTTY, P. J. & I. J. MISAGHI. 1985. Effect of light on the behavior of Alternaria tagetica in vivo and in vitro. Phytopathology , 75: 366-370. [ Links ]
DENNIS, R. W. G. 1968. British ascomycetes. J. Cramer. Lehre [ Links ]
DESSAUER, H.C., C. J. COLE & M. S. HAFNER. 1984. Collection and storage of tissues. in: HILLIS, D. M. & C. ORITZ (eds.), Molecular systematic , pp. 21-45. Sinauer Assoc. Inc. MA. USA.DIORIO, L. A., D. A. [ Links ]
DOKMETZIAN & F. FORCHIASSIN. 1995. Desarrollo de Iodophanus carneus ante distintas calidades de luz. Revista Argentina de Microbiología. 27: 71-79 [ Links ]
DOVERI, F. 2004. Fungi Fimicoli Italici . Associazione Micologica Bresadola. Trento. Italy [ Links ]
GAMUNDÍ, I. J. & M. E. RANALLI. 1964. Estudio sistemático y biológico de las Ascoboláceas de Argentina I. Nova Hedwigia 7: 517-533. [ Links ]
HARRINGTON, F. .A. & D. M. RIZZO. 1999. Defining species in the fungi. In: WORRALL, J. J. (eds.) Structure and dynamics fungal populations , pp. 43-71. Kluwer Press, Dordetcht, The Netherlands. [ Links ]
JENG, R. S. & J. C. KRUG. 1977. New records and new species of coprophilous Pezizales from Argentina and Venezuela. Can. J. Bot. 55: 2987-3000 [ Links ]
KIMBROUGH, J. W. 1970. A new species of Iodophanus from Ceylon. Bull. of the Torr. Bot. Club 97: 377-379 [ Links ]
KIMBROUGH, J. W. & R. P. KORF. 1967. A synopsis of the genera and species of the tribe Theleboleae (= Pseudoascobolaceae). Amer. J. Bot. 54: 9-23. [ Links ]
KIMBROUGH, J.W., E. R. LUCK ALLEN & R. F. CAIN. 1969. Iodophanus , the Pezizeae segregate of Ascophanus (Pezizales). Amer. J. Bot 54: 1187-1202. [ Links ]
KOHN, L. M. 1992. Developing new characters for fungal systematics: an experimental approach for determining the rang of resolution. Mycologia . 84: 139-153. [ Links ]
MANCHENKO, P. 1994. Handbook of detection of isozymes on electrophoretic gels . CRS Press, Boca Raton, Florida, USA. [ Links ]
RAMOS, A. M. 1998. Estudios quimiotaxonómicos en especies del género Saccobolus (Ascobolaceae, Pezizales). Ph.D. thesis. Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina [ Links ]
RAYNER, A. D. M. 1990. Natural genetic transfer systems in higher fungi. Transactions Mycological Society , Japan, 31: 75-87. [ Links ]
SAIDMAN, B. O. 1985. Estudio de la variación alozímica en el género Prosopis . Ph.D. thesis. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires, Argentina [ Links ]
SCANDALIOS, J. G. 1969. Genetic control of multiple molecular forms of isozymes in plants, a review. Biochemical genetics , 3: 37-79. [ Links ]
SCHUMACHER, T. 1992. New or noteworthy discomycetes. 2. five new operculate discomycetes (Pezizales) from the Dovre Mountains, Central South Norway. Mycotaxon , 43: 33-47 [ Links ]
SEAVER, F. J. 1916. Bermuda fungi. Memoirs of the New York Botanical Garden, 6: 501-511. [ Links ]
SELANDER, R.K., M. H. SMITH, S. Y. YANG, W. E. JOHNSON & J. B. GENTRY. 1971. IV. Biochemical polymorphism and systematics in the genus Peromyscus. I. Variation in the old-field mouse ( Peromyscus polionotus ). Univ. Texas Publ . 103: 49-90 [ Links ]
SOLTIS, D. E., C. H. HAUFLER, D. C. DARROW, & C. J. GASTONY. 1983. Starch gel electrophoresis of Ferns. A compilation of grinding buffers, gel and electrode buffers, and staining schedules. Amer. fern J. 73: 9-27. [ Links ]
SUAREZ, M. E., M. E. RANALLI, D. A. DOKMETZIAN & A. M. RAMOS. 2006 Characterization of three species of the genus Coprotus (Ascomycota) by isozyme analysis. Mycotaxon 97: 257-273. [ Links ]
THIND, K. S. & K. S. WARAITCH. 1971. The Pezizales of India. XII. Res. Bull. Panjah Univ. (N. S.) , 22: 109-123. [ Links ]
THIND, K. S. & S. C. KAUSHAL. 1973. Three species of Iodophanus from Western Himalayas. Indian Phytopathology, 31: 343-347 [ Links ]
VALLEJOS, C. E. 1983. Enzyme activity staining. In: TANKSLEY, S. D. & T. J. ORTON (eds.), Isozymes in Plants Genetics and Breeding , pp. 469-516. Elsevier, Amsterdam. [ Links ]
WENDEL, J. F. & N. F. WEEDEN. 1990. Visulization and interpretation of plants isozymes. In: D. E. SOLTIS & P. S. SOLTIS (eds.), Isozyme in Plant Biology , pp. 5-45, Chapman and Hall, London [ Links ]
ZAMBINO, P.J. & T. C. HARRINGTON. 1989. Isozyme variation within and among host-specialized varieties of Leptographium wagneri . Mycologia , 81: 122-133. [ Links ]
Recibido el 28 de Junio de 2007,
aceptado el 30 de Agosto de 2007.














