Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. v.42 n.3-4 Córdoba ago./dic. 2007
Morphoanatomical characterization of Cuphea glutinosa (Lythraceae) seedlings primary root structure
Francisco J. Cardinalli1, Mario A. Thevenon1 y Ana C. Martínez Tosto1
1Laboratorio de Botánica. Departamento de Biología. Facultad de Ciencias Exactas y Naturales. Universidad Nacional de Mar del Plata. Funes 3250, B7602AYJ Mar del Plata. Argentina. E-mails: cardinal@mdp.edu.ar; thevenon@mdp.edu.ar; amtosto@mdp.edu.ar
Summary: Morphoanatomical characteristics and growth patterns of root systems are genetically controlled. Cuphea glutinosa is an important species in popular medicine as a treatment for high blood pressure, as a diuretic and for industrial uses. Actually, the arrangement and relation among the tissues that make up its primary root is unknown. Therefore the objective of the present work was to characterize in axial and radial form the primary structure of this species including the limits of each root area, its characteristics and tissue relations that are present in each zone. The root tip showed a prominent root cap. The meristematic zone starts immediately above the root cap and extends up to 231 µm. C. glutinosa showed minimum variations in the diameter between elongation and maturation zones and enormous differences in tissue composition between both zones considered which indicated that the radical morphogenesis prioritizes vascular tissue formation.
Key words: Elongation zone; Maturation zone; Meristematic zone; Mucigel; Root hairs
Resumen: Características morfoanatómicas de la estructura primaria de la raíz de plántulas de Cuphea glutinosa (Lythraceae). Las características morfoanatómicas y los patrones de crecimiento de los sistemas radicales están controlados genéticamente. Cuphea glutinosa es una especie importante en medicina popular, la cual es utilizada para el tratamiento de la hipertensión arterial, como diurética y para usos industriales. Actualmente se desconoce la disposición y relación entre los tejidos que componen su raíz primaria. Por este motivo, el objetivo del presente trabajo fue caracterizar en forma axial y transversal la estructura primaria de esta especie, incluyendo la delimitación de cada zona propia de la raíz, sus características y relaciones tisulares que se presentan en cada una de ellas. El ápice radical presentó una caliptra prominente. La zona meristemática comienza inmediatamente por encima de la caliptra y se extiende hasta 231 µm. C. glutinosa mostró mínimas variaciones en los diámetro entre las zonas de elongación y maduración y grandes diferencias en la composición de los tejidos entre ambas zonas consideradas, lo cual indicó que la morfogénesis radical prioriza la formación de tejido vascular.
Palabras clave: Zona meristemática; Zona de elongación; Zona de maduración; Pelos radicales; Mucigel.
INTRODUCTION
Morphoanatomical characteristics and growth patterns of root systems are genetically controlled (Zobel, 1996). General genetic pattern determines the growth and root development. Nevertheless, it is frecuent to find differences among species of the same genera (Waisel et al. , 1997).
Dicotyledons, like Cuphea glutinosa Cham. et Schltdl., respond to a general model whose characteristic is a ramificated main axis that gives the root an axonomorfical structure (Russel, 1977). Radical growth is a result of cellular divisions and their enlargement (Burholt & Van´t Hoof, 1971). This growth originated in the tip extreme shows a pattern that lets differentiate the elongation, maturation and meristematic zones and a notable root cap (Obraucheva, 1975).
Meristematic zone of active cellular division goes with a slight enlargement which increases in basipetal direction. In the elongation zone enlargement goes with some cellular divisions. In consequence, division and elongation happens at the same time but inverse sense with respect to intensity (Ishikawa & Evans, 1995). Besides, both processes are attended by cellular differentiation (Esau, 1982).
C. glutinosa is a native species from Argentine present in Pampean steppe and high plains of Bonaerenses hills and Subandinas of Córdoba, Catamarca, San Luis, Tucumán, Jujuy and Salta (Ratera & Ratera, 1980 and Cabrera & Zardini, 1978). Besides it was found in Chaco, Corrientes, Formosa, Misiones, Santa Fé provinces and in limit countries as Brasil, Paraguay and Uruguay (Zuloaga & Morrone, 1999).
Many Cuphea species have seed oils rich in medium-chain fatty acids and represent a potencial source of both economically important medium-chain fatty acids as well as a genetic resource for the engineering of seed oils in existing crops (Leonard et al. , 1997). In popular medicine C. glutinosa is used because of their diuretic and hipotensor properties (Marzocca, 1997 and Ratera & Ratera, 1980) and for their laxative, antimalarial, emmenagogic and cordial properties (Barboza et al ., 2006).
In previous works, Martínez Tosto et al. (2003) and Yagueddú & Martínez Tosto (2005) analized the anatomic variations and cellular contains in stems of this species and Yagueddú et al. (2006) described the morphology and architecture of C. glutinosa plants aerial portion. Nevertheless, it has not made any study about radical system nor relation and disposition between tissues that compound their primary root. Into this context, the aim of the present work was to characterize the primary root structure of C. glutinosa in axial and radial direction including the limits of each root zone, their characteristics and tissue relations present in each zone.
MATERIAL and METHODS
Germination and seedlings growth
Seeds of C. glutinosa were collected in their natural habitat of Sierra de los Padres corresponding to Sistema de Tandilia (37º 56´45" S - 57º 46´45" W) Buenos Aires province and were put to germinate on water saturated filter paper in petri dishes. After six days it was verified an 80% of germinated seeds by the presence of radicle. Seedlings remained during 14 days in petri dishes, time required for reaching an adequate growth to be able to work on them. At that time, roots average length was 17.5 +/- 0.54 mm and had small cotyledons in expansion. Seedlings were transferred to test tubes with Hoagland nutritive solution (50 %) on a metal paper which also covered wholly the tubes to avoid the incidence of light on the roots development. They were kept in controlled conditions with a period of light of 12 hours and a radiation of 220 mE at a temperature of 15 +/-2 ºC. Seven days later, 15 plants were taken as representatives. They had fully developed cotyledons, on a 10 +/- 0.31mm hypocotyle average length that had on its extreme the first pair of leaves emerging from a short epicotyle. Over the samples the length and diameter of primary root elongation and maturation zones were measured. Besides we observed and measured with stereoscopic microscope the mucigel that covers the root cap and the area adjacent to it.
Histological techniques
Seedlings were cut to separate the aerial parts from the subterranean system. Roots were immediately fixed in glutaraldehyde 3%, in cacodilato de sodio buffer 0.1 pH 6.8 during 48 hours at 4ºC. Later they were washed three times in the same buffer. Post fixing was made with osmiun tetroxide (OsO4) 2%, using the same buffer and conditions for fixing and washing.
Roots were embedded in epoxy according to procedures described by Spurr (1969). Longitudinal and transverse sections of 200 nm were made with ultramicrotome and colored with toluidine blue (Sakai, 1973). Photographs were taken on a Nikon Eclipse 2000 optical microscope with a Nikon Coolpix 990 digital camera.
Primary structure.
Root zones In axial direction, root cap, meristematic zone (MeZ), elongation zone (EZ) and maturation zone initiation (MaZ) were identified and characterized. In transverse direction were made measurements on the limit between MeZ and EZ and in MaZ that showed developed root hairs. As reference (point cero) the tip of the radical meristem was taken. Rost & Baum (1988) determined the upper limit of MeZ at the point where the cortical parenchyma cells nucleus had a distance equivalent to twice the nuclear diameter. In this work we considered this point where cortical cells duplicate their length in axial direction.
The limit between EZ and MaZ was defined as the point where cortical parenchyma cells ceased their elongation which corresponded to the beginning of root hairs. In both levels, root total diameter and vascular cylinder were determined. From these, the area corresponding to cortical parenchyma cells and tissues relations were calculated.
Root hairs
On radical system of samples the rizoderm length of primary root was determined. Over semi-thin cross sections root hairs in the rizoderm at 1300 µm from the tip were observed and characterized.
RESULTS and DISCUSSION
Axial characterization The primary root had an 37 mm average length without visible ramifications and abundant root hairs.
The axial characteristics referred to dimensions of each primary root zone are showed in Table 1 and Fig.1.
Table 1. Length average values of different zones of Cuphea glutinosa primary root in axial direction.

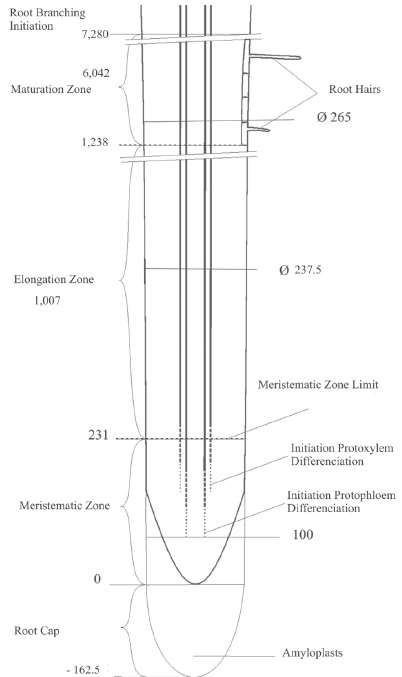
Fig. 1. Schematic drawing of root longitudinal section showing the different zones. Values are expressed in µm.
The root tip of C. glutinosa showed a prominent root cap that showed a clear limit to radical tip. The central zone presented a columella of 4 rows of 6 cells each other with increasing sizes in acropetal direction (Fig. 2).

Fig. 2. Longitudinal section of root tip. Co: columella; Mu: mucigel; Am: amiloplast. Scale bar: 15 µm.
From the tip of the primary root to the area immediately above the root hairs zone could be observed a notable mucigel with a hardly uniform thickness between 1- 2 mm . Similar results were observed by Samtsevich (1968) in barley roots growing in nutritive solutions that showed a mucigel of 0.9 mm thick. However, Nye & Tinker (1977) working with species growing in nutritive solutions observed that the thickness of the mucigel varied between 10 - 200 µm. Leisser (1968) suggests that the mucigel protects the root cap and radical meristem from dehydration. Dart & Mercer (1964) consider mucigel is a protection to bacteria multiplication which helps root growth. Yagueddú et al. (2006) observed that C. glutinosa grew in litic hapludol soils at the top of hills or in their hillsides with a thin superficial horizon (9 - 30 cm ) in rock direct contact. The conspicuous mucigel present in C. glutinosa could be a natural fundamental strategy for roots survival that frequently develop in very shallow soils with a minimum capacity to retain moisture and in consequence are exposed to great water variations.
The MeZ starts immediately above root cap and extends up to 231 µm, point where each layer of cortical parenchyma duplicate their length. (Fig. 3 A y B).
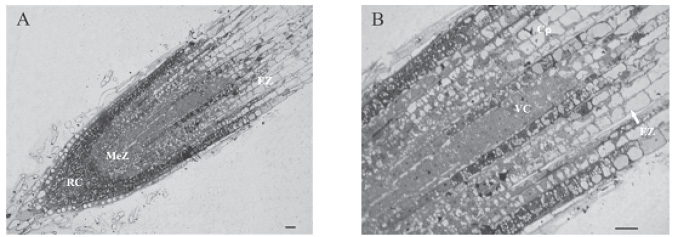
Fig. 3. Longitudinal section of primary root of Cuphea glutinosa . ( A ) General view showing the different zones: root cap (RC), meristematic zone (MeZ) and elongation zone (EZ); (B) limit detail between MeZ and EZ (arrowed), point where the cortical parenchyma cells (Cp) duplicate their length. (VC): vascular cylinder. Scale bars: A and B 20 µm.
As well the shape and size of meristems change with the length of the root (Rost & Baum, 1988), C. glutinosa showed a MeZ of 231 µm average length for roots of 37 mm average length, small compared with Pisum sativum , that had a MeZ of 1,900 µm with a root 20 mm long (Rost & Baum, 1988). However, it is somewhat bigger than Marsilea coromandelica , a pteridophyte with a 210 µm MeZ (Charlton, 1983).
Above the MeZ the primary root showed the EZ that reached a length of 1,007 µm (Table 1 and Fig. 1). Its lower portion showed a vascular cylinder with totally differentiated phloematic cells, a xylem ending its differentiation and a cortical zone with parenchymatic cells in elongation. EZ started when cortical parenchyma cells duplicated their length (2x). Further, they changed their square shape to rectangle along the zone. This results contrast with the ones obtained by Balu ka et al. (1990) that worked with corn root tips and observed that in EZ close to MeZ cells adopted a rectangle shape until they reach an isodiametric shape using the term «posmitotic isodiametric zone» to characterize this fact.
Beginning EZ and in basipetal direction the size relations were 1.5x, 1.35x, 1.25x, until maintaining constant its length with a maximum between 60 - 75 µm being the most frequent size 62.5 µm (Fig. 3 A and B). These tendencies agree with the ones obtained by Ishikawa & Evans (1993) within corn roots EZ.
The point where two superposed cortical parenchyma cells showed the same length determined the end of EZ and marked the beginning of the MaZ that was verified at 1,238 µm from the tip (Fig. 1).
The extensive MaZ had a 6,042 µm average length. The inferior limit showed constant length cortical cells and the presence of root hairs. This limit was pointed by the beginning of radical primordia formation and determined the beginning of ramifications zone.
The presence of root hairs and their persistence vary according to the species and the environment conditions (Nye & Tinker, 1977). In this report the environmental effects were given by the proper conditions of hydroponic farm chemically balanced and without mechanical impedances. Generally in hydroponia the development of root hairs is limited and in some cases there are none (Von Gutemberg, 1968). However, C. glutinosa showed a great amount of these with various lengths and thicknesses even when the were grown first in petri dishes on water saturated filter paper and then in hydroponia. According to Vartanian et al. (1983), moisture has effects on the extension of root hairs but not on differentiation process in radical meristem protoderm.
In some transverse sections at approximately 1,300 µm from the root tip we observed root hairs in longitudinal direction (Fig. 4). Their average diameters and lengths were 10 µm and 40 µm respectively. The diversity of lengths and diameters did not permit establishing a correlation between both variables. However, it was observed that the trichoblasts were located on 2 or 3 subjacent cortical cells which would resemble to type 3 characterized by Kim et al. (2006).
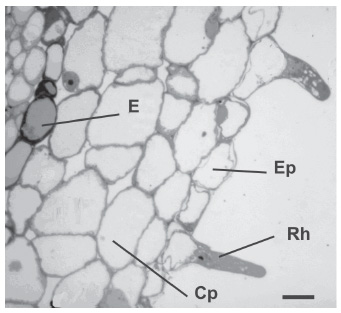
Fig. 4. Transverse section of a root with root hairs in longitudinal view. E: endodermis; Ep: epidermis; Rh: root hairs; Cp: cortical parenchyma cell. Scale bar: 15 µm.
Radial characterization
Transverse sections obtained in EZ approximately 1,100 µm from de root tip showed an uniseriate endodermis of 19 to 20 ovall cells. The uniseriate pericycle was composed of 23 cells totally rounding the tetrarch vascular cylinder. These cells presented their major axis parallel to organ surface of between 12 and 15 µm and a minor axis perpendicular to the previous one of between 5 and 6.25 µm. Pericycle portions opposite to the protoxilematic poles are potencial places of radical primordial initiation and they showed rounded cells with a diameter between 7.5 and 12.5 µm. (Fig. 5).

Fig. 5. Transverse section in the elongation zone (EZ) at 1,100 m m from the root tip. E: endodermis; Pe: pericycle; Pp: protoxilematic poles; Ep: epidermis; Cp: cortical parenchyma. Scale bar: 15 µm.
Transverse sections described in Table 2 are depicted by dotted lines in Fig.1. MeZ upper sections showed a cortical parenchyma composed of small rounded cells with fine walls and small intercellular spaces. The cytoplasm was homogeneous without organelles.

Table 2. Characterization in transverse sections of meristematic and maturation zone. Ratios among different zones respect to total surface and ratios between zones. VC: Vascular Cylinder Cp: Cortical Parenchyma ( 1 ) Transverse sections at the beginning of the elongation zone. ( 2 ) Transverse sections in maturation zone at 1200 µm from the tip.
The limit of EZ and MaZ showed an extended cortical parenchyma with very big cells, thin walls, notable intercellular spaces and citoplasmatic inclusions (Fig. 5).
The results of EZ and MaZ observed in radial direction were presented in Table 2. The points observed in each zone (Fig.1) were separated 1,269 µm. In this zone was produced the most length increase with a thickness increase superior to 11 % which was verified by the small growth in root diameter. However, the internal tissue ratios varied remarkably between both zones. The vascular cylinder surface in EZ was increased 140.71 % when it reached MaZ, while cortical parenchyma only 7.25%.
Stamp (1984) and Kiel & Stamp (1992) working on mature zones cross sections of corn primary roots of 20 genotypes proved that low temperatures increased the radical diameters. However, the tissue ratio (vascular cylinder/root total area) was practically constant and close to 0.2. The results of the present work were quite coincident with those of Kiel & Stamp (1992) for MaZ since presented a ratio 0.25 (Tabla 2). However, when the tissular ratio in EZ was compared it differed remarkably, being the value 0.129. We could deduct that when increases in roots size were caused by thermal effects, the volume ratios among tissues are almost invariable. However, when increases were due to root natural development there were big changes. That is the case of C. glutinosa which showed minimum variations in the diameter between EZ and MaZ and large differences in tissue composition between both zones.
These important tissue ratios variations at different distances from the root tip indicated that the radical morphogenesis prioritizes the vascular tissue formation against cortical paranchymatical tissue. This is clearly showed in Fig.1 and in tissular ratios of Table 2.
Over 7,280 µm it was observed the formation of radical primordial. Its beginning was the periclinal divisions of pericyclical cells followed by anticlinal and periclinal divisions that were conforming anatomically and physiologically the radical primordium (Fig. 6). In this zone was observed the initial development of radical primordium and it looked dense as result of the pericycle initials divisions. This responds to development state number 1 according to the scale depicted by Blakely et al . (1982). At this instance the length of pericyclical perturbation area in the place of primordium was 75 µm beginning involving between 6 and 8 hardly regular cells of 10 µm diameter.

Fig. 6. Transverse section in maturation zone (MaZ). Pr: radical primordium. Scale bar: 15 µm.
During the primordial development the vascular tissue of the primary root were connected to the lateral ramification. However, the earlier state observed did not allow to recognize the vascular connections which would appear in the five state when the root cap of radical primordium emerged through the radical surface and the root cap was evident Blakely et al., 1982).
ACKNOWLEDGEMENTS
This work was supported by grants provided by Universidad Nacional de Mar del Plata, Argentine. We thank to Lic. Mónica Opedisano for her support in samples processes and Professor Juan Roig for helping to translate the manuscript.
BIBLIOGRAPHY
BARBOZA, G. E., J. J. CANTERO, C. O. NUÑEZ Y L. ARIZA ESPINAR. 2006. Flora Medicinal de la Provincia de Córdoba ( Argentina). Museo Botánico de Córdoba. Gráficamente ediciones, Córdoba, Argentina. [ Links ]
BALUSKA, F., S. KUBICA & M. HAUSKRECHT. 1990. Postmitotic «isodiametric» cell growth in the maize root apex. Planta 181: 269-274. [ Links ]
BLAKEL, Y. L., M. M. DURHAM, T. A. EVANS & BLAKELY, R. M. 1982. Experimental studies on lateral root formation in radish seedling roots: 1. General methods, developmental stages and spontaneous formation of laterals. Bot. Gaz. 143: 341-352. [ Links ]
BURHOLT, D. R. & J. VAN'T HOF. 1971. Quantitative thermal-induced changes in growth and cell population kinetics of Helianthus roots. Am. J. Bot. 58(5): 386-393. [ Links ]
CABRERA, A. L. & E. M. ZARDINI. 1978. Manual de la Flora de los alrededores de Buenos Aires. Acme, Argentina. [ Links ]
CHARLTON, W. A. 1983. Lateral root initiation. In: WAISEL, Y., A.ESHEL & U. KAFKAFI (eds.), Plant Roots. The Hidden Half , pp. 149-173. Marcel Dekker, Inc. [ Links ]
DART, P. J. & F. V .MERCER. 1964. The legume rhizosphere. Archiv fur Mikrobiologie 47: 344-378. [ Links ]
ESAU, K. 1982. Anatomía de las plantas con semilla. Hemisferio Sur, Argentina. [ Links ]
ISHIKAWA, H. & M. L.EVANS. 1993. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 102: 1203-1210. [ Links ]
ISHIKAWA, H. & M. L. EVANS. 1995. Special Zones of Development in Roots. Plant Physiol. 109:725-727. [ Links ]
KIEL, C. & P. STAMP. 1992. Internal root anatomy of Maize Seedlings (Zea mays L.) as influenced by Temperature and Genotype. Ann. Bot. 70: 125-128. [ Links ]
KIM, D. W., S. H. LEE, S. -B. CHOI, S. -K. WON, Y. -K. HEO, M. CHO, Y. -II. PARK & H. -T. CHO. 2006. Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell 18: 2958-2970. [ Links ]
LEISER, A. T. 1968. A mucilaginous root sheath in Ericaceae. Am. J. Bot. 55: 391-398. [ Links ]
LEONARD, J. M., M. B. SLABAUGH, & S. J. KNAPP. 1997. Cuphea wrightii thioesterases have unexpected broad specificities on satured fatty acids. Plant Mol. Biol. 34: 669-679. [ Links ]
MARTÍNEZ TOSTO, A. C., C. YAGUEDDÚ & M. O. ARRIAGA. 2003. Anatomical differences between mature and young stems in plants of Cuphea glutinosa Cham. et Schlecht. (Lythraceae) from dry and wet places. Biocell 27: 243. [ Links ]
MARZOCCA, A. 1997. Vademécum de malezas medicinales de la Argentina indígenas y exóticas. Orientación Gráfica Editora, Argentina. [ Links ]
NYE, P. H. & P. B. TINKER. 1977. Solute movement in the soil-root system. In: ANDERSON, D. J., P. GREIG-SMITH & F. P. PITELKA (eds.), Studies in Ecology, Vol. 4 Blackwell Scientific, Oxford, UK. [ Links ]
OBROUCHEVA, N. V. 1975. Physiology of growing root cells. In: TORREY, J. G. & D. T. CLARKSON (eds.), The development and function of roots, pp. 279-298. Academic Press, England. [ Links ]
RATERA, E. L. & M. O. RATERA. 1980. Plantas de la flora argentina empleadas en medicina popular. Hemisferio Sur, Argentina. [ Links ]
ROST, T. L. & S. BAUM. 1988. On the correlation of primary root length, meristem size and protoxylem tracheary element position in pea seedlings. Am. J. Bot.75(3): 414-424. [ Links ]
RUSSELL, R. S. 1977. Plant root system. Their function and interaction with the soil. McGraw-Hill Book Company (UK) Limited, England. [ Links ]
SAKAI, W. S. 1973. Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Tech. 48(5): 247-249. [ Links ]
SAMTSEVICH, S. A. 1968. Gel-like excretions of plant root and their influence upon soil and rhizosphere microflora. In: GHILAROV, M. S., V. A.KOVDA, L. N. NOVICHKOVA-IVANOVA, L. E. RODIN & V. M. SVESHNIKOVA (eds.), Methods of productivity studies in root systems and rhizosphere organisms, pp 200-204. Nauka Publishing House, Leningrad. [ Links ]
SPURR, A. R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ult. Res. 26: 31-43. [ Links ]
STAMP, P.1984. Chilling tolerance of young plants - demonstrated on the example of maize (Zea mays L). In: GEISLER, G. (ed.), Advances in agronomy and crop science n° 7. Berlin, Paul Parey. [ Links ]
VARTANIAN, N., D. S. WERTHEIMER & H. COUDERC. 1983. Scanning electron microscopic aspects of short tuberized roots, with special reference to cell rhizoderm evolution under drought and rehydration. Plant Cell Env. 6: 39-46. [ Links ]
VON GUTEMBERG, H. 1968. Der primäre Bau der Angiospermenwurzel. In: LINSBAUER, K. (ed.), Handbuch der Pflanzenanatomie, 2nd.ed.,vol. 8, part 5. Borntraeger, Berlín. [ Links ]
WAISEL,Y., A. ESHEL & U. KAFKAFI. 1996. Plant Roots. The Hidden Half. Marcel Dekker, Inc, New York. [ Links ]
YAGUEDDÚ, C., V. M. COMPARATORE, F. J. CARDINALI, A. C. MARTÍNEZ TOSTO, & S. V. BEVACQUA. 2006. Cuphea glutinosa (Lythraceae) en sierras del Sistema de Tandilla: morfología y ambiente. Bol. Soc. Argent. Bot. 41 (3-4): 285-292. [ Links ]
YAGUEDDÚ, C. & A. C. MARTÍNEZ TOSTO. 2005. Variations in cellular contents of Cuphea glutinosa (Lythraceae) plant stems that grow in different places in Sierra de los Difuntos. Biocell 29: 145. [ Links ]
ZOBEL, R. W. 1996. Genetic control of root systems. In: WAISEL, Y., A. ESHEL & U. KAFKAFI (eds.), Plant Roots. The Hidden Half, cap.2, pp 21-30. Marcel Dekker, Inc. [ Links ]
ZULOAGA, F. O. & O. MORRONE. 1999. Catálogo de las plantas vasculares de la República Argentina. Missouri Botanical Garden Press. [ Links ]
Recibido el 21 de Marzo de 2007,
aceptado el 03 de Septiembre de 2007.














