Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. v.44 n.3-4 Córdoba ago./dic. 2009
ANATOMÍA Y MORFOLOGÍA
Identification of six Papilionaceae species by epidermal characteristics: Microanalysis of handcomposed mixtures
Cristina Yagueddú1, Viviana Comparatore1 y Gilda Paoletti2
1 Laboratorio de Vertebrados, Departamento de Biología, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata. Funes 3250. (B7602AYJ) Mar del Plata. Provincia de Buenos Aires. Argentina;
2 Instituto Argentino de Investigaciones de las Zonas Áridas. Mendoza. Argentina.
crisyagueddu@gmail.com y vivicom99@yahoo.com.ar
Summary: The microhistological analysis quantifies the botanical composition of herbivore diets by the identification of the epidermal characters of ingested species. Many Papilionaceae form part of herbivore diets due to their high nutritive quality. Different species of this family are sometimes grouped together due to the difficulty to recognize them. In this study, the epidermis of Lotus tenuis, Medicago arabica, Medicago lupulina, Melilotus albus, Trifolium pratense and Trifolium repens was analysed. Also, it was tested if such descriptions could be useful to identify fragments of these species in hand-composed mixtures and then quantified by microanalysis. Descriptions and drawings are presented. Regressions were significant (p<0.05) for Lotus tenuis, Medicago arabica, Medicago lupulina, Melilotus albus, Trifolium pratense, and not significant (p>0.05) for Trifolium repens. The slopes of the significant regressions did not differ from 1 (p>0.05). The identification of these species when present in herbivore faeces or in digestive tract contents is possible.
Key words: Epidermis; Microhistological analysis; Herbivore diet; Fabaceae; Leguminosae; Lotus; Medicago; Melilotus; Trifolium.
Resumen: Identificación de seis especies de Papilionaceae mediante características epidérmicas: microanálisis de mezclas compuestas a mano. El análisis microhistológico cuantifica la composición botánica de la dieta de herbívoros mediante la identificación de los caracteres epidérmicos de las especies ingeridas. Dado su alto valor nutricional, muchas Papilionaceae forman parte de la dieta de los herbívoros. Diferentes especies de esta familia son a veces agrupadas debido a la dificultad para su reconocimiento. En este trabajo se analizaron las epidermis de Lotus tenuis, Medicago arabica, Medicago lupulina, Melilotus albus, Trifolium pratense y Trifolium repens. También, se testeó si dichas descripciones podrían ser útiles para identificar fragmentos de estas especies en mezclas compuestas a mano y luego cuantificadas por microanálisis. Se presentan descripciones y dibujos. Las regresiones resultaron significativas (p<0.05) para Lotus tenuis, Medicago arabica, Medicago lupulina, Melilotus albus, Trifolium pratense, y no significativa (p>0.05) para Trifolium repens. Las pendientes de las regresiones significativas no difirieron de 1 (p>0.05). La identificación de estas especies cuando están presentes en las heces o en contenidos del tracto digestivo de los herbívoros es posible.
Palabras clave: Epidermis; Análisis microhistológico; Dieta herbívoros; Fabaceae; Leguminosae; Lotus; Medicago; Melilotus; Trifolium.
INTRODUCTION
In studies of botanical composition of herbivore diets utilizing microanalysis techniques (Sparks & Malecheck, 1968), different species of the family Papilionaceae are sometimes grouped together due to the difficulty to recognize them (Comparatore et al., 2001). But this difficulty depends on which legumes are present together in the field and in the diet. These species have high quality and digestibility, and are commonly found in herbivore diets (Camezzana, 1987; Bonti et al., 1995; Martella et al., 1996; Pelliza et al., 1997). In studies of the dietary habits of Rhea americana albescens (Common Rhea) in grasslands of Buenos Aires Province, Argentina, Isacch et al. (2001) found a high percentage of Medicago sp., and Vacarezza (2001) was able to identify three different legumes.
There are few descriptions of the epidermal characteristics of legumes present in grasslands of Buenos Aires Province, Argentina (Arambarri & Colares, 1993; Manganaro, 1923; Stenglein et al., 2003; Yagueddú & Cid, 1992). Besides, there are no comparisons among different legumes that allow their recognition in mixtures. Therefore, in this study, the epidermis of Lotus tenuis, Medicagoarabica, Medicago lupulina, Melilotus albus, Trifolium pratense and Trifolium repens was analysed. Also, it was tested if such descriptions could be useful to identify fragments of these species in hand-composed mixtures. It is appropriate to make clear that these are cosmopolite plants. Besides they are cultivated in pastures.
MATERIALS AND ETHODS
Plants of Lotus tenuis Waldst. & Kit, Medicago arabica (L.) Huds., Medicago lupulina L., Melilotus albus Medik, Trifolium pratense L. and Trifolium repens L., were collected from three differents sites in grasslands of Balcarce, Buenos Aires Province, Argentina. Fourty fully expanded young and mature leaves per species were selected and diafanize dusing the technique of Dizeo de Strittmater (1973) and mounted in gelatine-glycerine. The following epidermal characteristics of adaxial and abaxialsurface of the leaflets were analysed using all histologycal slides: type of trichomes and stomata complex, form of the epidermal cells and cell wall patterns. Length and width (µm) of epidermal cells, and length, width and density (number/mm2) of stomata and trichomes, were estimated based on ten random measurements per species with a Leica ATC 2000 light microscope equipped with an ocular micrometer. Qualitative microcharacters and measurements are presented in tables.
Original drawings of the epidermal tissue were carried out with an Olympus CH3 light microscope equipped with a camera lucida. Besides, to test if the epidermal characteristics described for each species could be useful to identify them, 15 mixtures were hand composed with dried and ground material. Each mixture had a different combination and proportion of three of the studied species. They were cleared in 50% sodium hypochlorite (NaOCl) for 5 to 10 min. When the material turned yellowish, three washes were carried out in distilled water to remove the sodium hypochlorite. The transparent mixture was mounted in gelatine-glycerine. The percentageof each species in the mixture was quantified by the microanalysis technique (Sparks & Malechek,1968). Regression analyses were performed for each species with these data and the real percentage of the species in the mixtures. Graphs are presented. By means of a Student's t test, Ho: B=1 was tested. STATISTICA 5.5 Program was used.
RESULTS
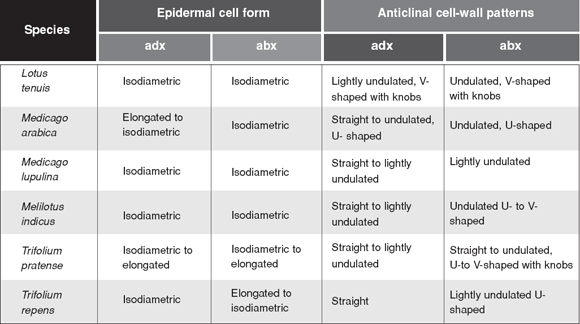
There were no differences between young and mature leaves (Fig. 1, 2). The epidermal cells were, in general, isodiametric. But Medicago arabica, Trifolium pratense and Trifolium repens also presented elongated to isodiametric epidermal cell form (Table 1). The anticlinal cell walls of all studied species were curved and undulated U- or V-shaped on the abaxial surface, and straight and straight to curved in the adaxial one (Table 1).
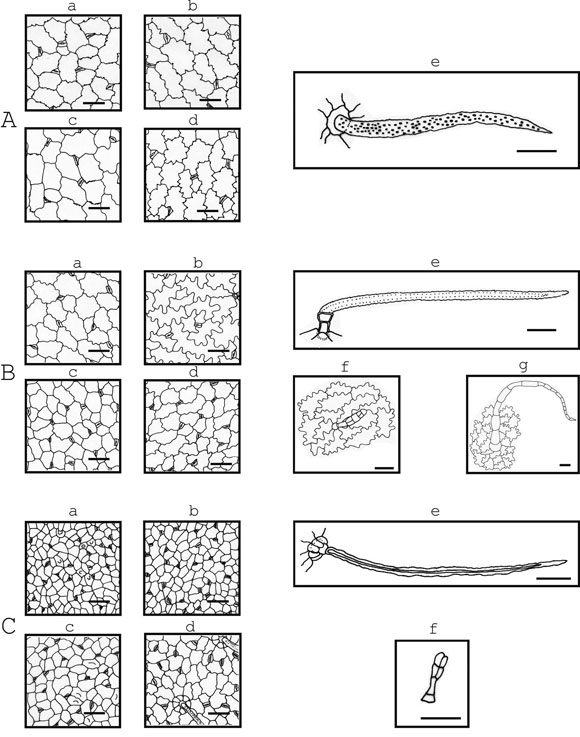
Fig. 1. Drawings of epidermal tissue of: A, Lotus tenuis Waldst. & Kit; B, Medicago arabica (L.) Huds.; C, Medicago lupulina L.; a & b, young leaflet; c & d, mature leaflet; a & c, adaxial epidermis; b & d, abaxialepidermis. Presenting papillae: C, a & c. Trichomes: e, typical uniseriated eglandular trichomes; f, glandulartrichomes; g, uniseriated eglandular multicellular trichomes. Scale bar = 50 µm
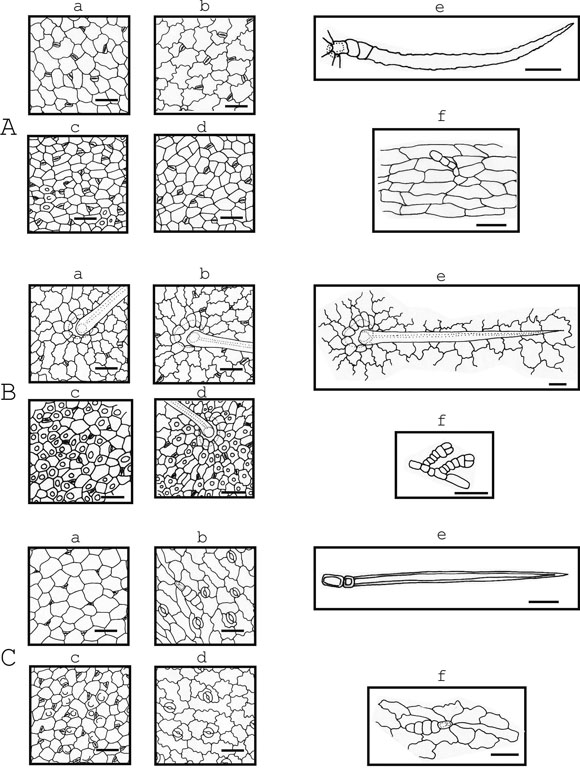
Fig. 2. Drawings of epidermal tissue of: A, Melilotus albus Medik; B, Trifolium pratense L.; C, Trifolium repens L. a & b, young leaflet; c & d, mature leaflet; a & c, adaxial epidermis; b & d, abaxial epidermis. Presenting papillae: A, c; B, c & d; C, c. Trichomes: e, typical uniseriated eglandular trichomes; f, glandulartrichomes. Scale bar = 50 µm
Table 1. Leaflets epidermal cells qualitative microcharacters. adx=adaxial epidermis; abx=abaxial epidermis.
The outside periclinal surface of each epidermal cell in all of the six studied species was convex and sometimes beared papillae (Fig. 1, 2). When present, papillae in Medicago arabica, Medicago lupulina, Melilotus albus and Trifolium pratense, were in both epidermal surfaces. Sometimes, papillae were in leaflet margins and in the costal zones. Whereas, in Lotus tenuis and Trifolium repens, papillae were observed in the adaxial epidermis.
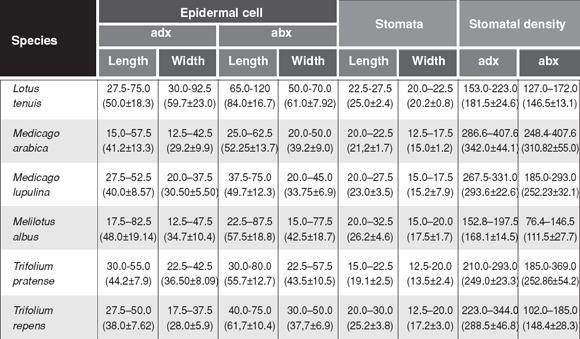
The leaves of all species were amphistomatic and had anisocytic and anomocytic types of stomatal apparatus (Fig. 1, 2). Lotus tenuis, Medicago lupulina, Melilotus albus and Trifolium pratense presented sunken stomata in adaxial and abaxial epidermis. Medicago arabica and Trifolium repens had sunken stomata in adaxial epidermis, but in the abaxial one the latter presented stomata over the level of the other epidermal cells and the former had both stomata positions in the same leaflet. The sunken stomata in all the species were surrounded by three or four epidermal cells, which form triangular or trapezoidal spaces over them (Fig. 1,2). Stomata length varied from 15 to 30 µm and the width from 12.5 to 22.5 µm in all the species. Stomata density (number/mm2) ranged from 152.8to 407.6 in the adaxial epidermis and from 76.4 to 407.6 in the abaxial one. Whereas Medicago arabica and Trifolium pratense had similar stomatal density in the adaxial and abaxial epidermis, the rest of the species presented a higher density on the adaxial one (Table 2).
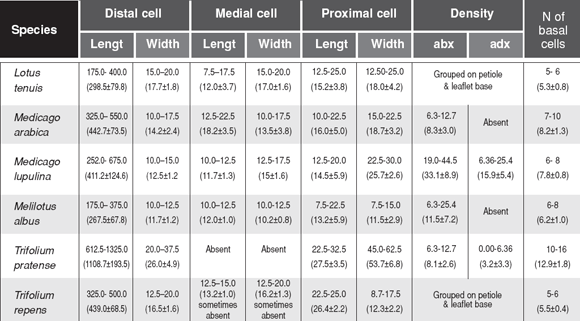
Table 2. Minimum and maximum sizes of epidermal cells and stomata (µm) and stomatal density (number/mm2). adx = adaxial epidermis; abx = abaxial epidermis (mean ± standard deviation).
Two types of trichomes were found: eglandular and glandular. Among the eglandular trichomes two types were observed: the typical uniseriated trichome and a multicellular uniseriated trichome (the latter only in Medicago arabica). All studied species presented the typical uniseriated trichomes of the Papilionaceae with different density and size of the cells that compose it (Table 3). The setrichomes, in general, present a couple of short proximal cells and one long distal cell, except in Trifolium pratense which presents only one short proximal cell (Fig 2: B). This latter characteristic was observed, sometimes, in the typical uniseriated trichomes of Trifolium repens. Lotus tenuis and Trifolium repens presented these eglandular trichomes in both epidermis grouped in the petiolules and, besides, the former species presented them at the base of the leaflets. In Medicago arabica and Melilotus albus these trichomes were absent in the adaxial epidermis.
Table 3. Typical eglandular uniseriated trichomes: Cells sizes (µm), density (number/mm2) and number ofbasal cells. adx = adaxial epidermis; abx = abaxial epidermis, (mean ± standard deviation).
Trifolium pratense presented the longest typical uniseriated trichomes (635 to 1357.5 µm) while in the other species the total length oscillated from 195 to 707.5 µm (Table 3). Also, Trifolium pratense presented the widest proximal short cell (from 45 to 62.5 µm) while the remainder species presented values from 7.5 to 30 µm (Table 3). The highest trichome density was found in Medicago lupulina in the abaxial epidermis (from 19 to 44.5 trichomes/mm2), and was 33.5 to 57% lower in the adaxial one (Table 3). Besides the typical uniseriated trichomes, Medicago arabica presented scarce and large eglandular uniseriated trichomes (from 460 to 930 µm length) constituted by a variable number of cells (from 12 to18) with thin walls, and located at the base of the leaflets and in the petiolules (Fig. 1: B).
In regard to glandular trichomes, the ones of Medicago arabica and Medicago lupulina are formed by one short proximal cell, a foot constituted by one cell aproximately twice longer than the proximal cell, and a head with several cells. The head of the glandular trichomes of Medicago arabica has five to seven cells, some subapical arranged in a form biseriate (Fig. 1: Bf). But the head of the ones of Medicago lupulina has three to four cells. When there are three, the apical ones are arranged in a biseriate form, and when there are four they are disposed in a biseriate form (Fig. 1: Cf).
Melilotus albus glandular trichomes present one short proximal cell, a foot formed by two slightl yelongated cells so arranged uniseriate and a head formed by one to four cells generally disposed uniseriate (Fig. 2: Af). The ones of Trifolium pratense present a short proximal cell from isodiametric to slightly elongated, a foot one celled, sometimes two to four celled so arranged uniseriate, and a head with four cells disposed uniseriate (Fig. 2: Bf). The ones of Trifolium repens present one short proximal cell which continues with a slightly elongated cell foot and the head formed by four cells arranged uniseriate to six cells some of them arranged in a form biseriate (Fig. 2: Cf).
Lotus tenuis was the only species that lacked glandular trichomes and Trifolium pratense presented them only sometimes at the margin of the leaflets. Medicago arabica, Melilotus albus and Trifolium repens did not present glandular trichomes in the adaxial epidermis. In general, the density of glandular trichomes respect to the typical eglandular uniseriated ones was smaller and more variable. Glandular trichome characteristics can be seen in Figures 1 and 2, and Table 4.
Table 4. Glandular trichomes: Minimum and maximum sizes of cells (µm), density (number/mm2) and number of head and basal cells. adx=adaxialepidermis; abx = abaxial epidermis, (mean ± standard deviation).
Figure 3 shows the curves of the regression analysis of each of the studied species. Regressions were significant (p<0.05) for Lotus tenuis (n=8, p=0.000594), Medicago arabica (n=7, p=0.013327),
Fig. 3. Regression curves (95% confidence) of the studied species. Independent variable (X): percentage ofthe species in the mixture (actual %). Dependant variable (Y): quantified percentage of the species (estimated %).
Medicago lupulina (n=7, p=0.000040), Melilotus albus (n=7, p=0.043359), Trifolium pratense (n=8, p=0.000627), and not significant (p>0.05) for Trifolium repens (n=8, p=0.180552). The slope of the significant regressions did not differ from 1(p>0.05), Lotus tenuis p=0.45592686, Medicago arabica p=0.4779881, Medicago lupulina p=0.650604637, Melilotus albus p=0.11459676 and Trifolium pratense p=0.071558494.
In the analysed mixtures, Medicago arabica, Medicago lupulina, Melilotus albus and Trifolium pratense, had no difficulty to be recognized because of the density, length and shape of typical uniseriated eglandular trichomes. Neither did Lotus tenuis due to the large isodiametric epidermal cells with anticlinal cell wall pattern undulated, V-shaped with knobs thickening ornamentation (Dilcher, 1974). Trifolium repens presented some difficulties to be identified in the mixtures because of the density and distribution of the two types of trichomes in the leaflets. Glandular ones, scarce and scattered in the leaflets surface; and uniseriated hairs, more scarce and grouped at the base of the leaflets; so many fragments in ground material lacked hairs. Also, the shape of the epidermal cells was not enough to recognize this legume without mistake. In fact, all these species had isodiametric cells and straight anticlinal cell walls in the adaxial epidermis, and elongated to isodiametric cells with lightly undulated U-shaped anticlinal cell walls in the abaxial one. These were similar for Medicago arabica and Medicago lupulina, Melilotus albus, Trifolium pratense and Trifolium repens. This caused difficulties in the recognition of some mixtures with Medicago arabica and Medicago lupulina, and Melilotus albus, whereas we had no difficulties with Lotus tenuis and Trifolium pratense. In spite of the similar characteristics of the epidermal cells of the two last named species, the presence of the larger uniseriated trichomes in Trifolium pratense was very helpful in its recognition.
DISCUSSION AND CONCLUSIONS
The degree of undulation of the wall of the epidermal cells is characteristic of each species. But it can vary with environmental conditions (moisture, draught, shadow), and with phenologycal stage (Stace, 1965). The maximum growth of the undulations ceases before the cells (and leaf) reachtotal size (Stace, 1965). This could be the reason of the small differences in cell wall undulation between young and mature leaves.
Although all species presented papillae, this does not constitute a constant characteristic, as they were not present in all the leaves of the same species, so they cannot be used as a diagnostic character. The variation of the presence of papillaeis related to environmental conditions (Fahn, 1990;Farooqui et al., 1997). Metcalfe & Chalk (1950) pointed out the abaxial epidermis papillose for Lotus and Trifolium species but they did not mention the presence of papillae in species of Medicago and Melilotus or in the adaxial epidermis of all the segenera. Our results do not agree with Metcalfe & Chalk (1950) with respect to Lotus tenuis and Trifolium repens because we recorded the presence of papillae in the adaxial epidermis and they did in the abaxial one. But this coincides with what we found in Trifolium pratense. These differences are probably due to the fact that papillae are not a constant character.
Stomata position in relation to the rest of the epidermal cells was not useful to recognize fragments in ground material.
All six species presented typical uniseriated eglandular trichomes, with a variable number of short basal cells, accompanied by an elongated terminal cell. This type is typical in Papilionaceae according to Uphof (1962) and Metcalfe & Chalk (1950). But in this study it was necessary to establish with more details the characteristics offered by the cells which conform this trichomes in order to use them to recognize each of these species.
According to Arambarri & Colares (1993), Lotus tenuis showed epidermal cells with undulated anticlinal and convex external cell walls, and sunken stomata surrounded by three or four epidermal cellswhich form a triangular or trapezoidal space over them. Besides, we observed the undulated V-shaped with knobs thickening ornamentation, similar to Stenglein et al. (2003) description. These characteristics permit the recognition of Lotus tenuis without difficulties. Also, we observed papillae in some leaflets and the presence of typical uniseriated eglandular trichomes in the petiolules. The presence of papillae was not mentioned by Arambarri & Colares (1993) nor by Stenglein et al. (2003), but the presence of typical uniseriated egalndular trichomes coincides with Stenglein et al. (2003).
Although Trifolium repens has epidermal characteristics that permit its recognition, over-estimation or under-estimation in its quantification in the hand-composed mixtures depended on the other species present. By another hand, it must beconsidered that it is very improbable that the six species appear together at the same place as available for herbivores.
We can conclude that the main characteristics that help us in the recognition of these species are the form of the anticlinal epidermal cell walls, and the density, dimensions and form of the typical uniseriated eglandular trichomes. According to the epidermis descriptions and the statistical analysis performed, it is possible to recognize these species when present in herbivore faeces or in digestive tract contents. Furthermore, these descriptions were valuable to identify legumes in a rhea's fecal diet study (Comparatore & Yagueddú, 2007).
ACKNOWLEDGEMENTS
We thank M. Sci. Jorge Castaño (EEA INTABalcarce) for the collection of some species in the field. Financial support was provided by Universidad Nacional de Mar del Plata, Project15/E-238.
BIBLIOGRAPHY
1. ARAMBARRI, A. M. & M. N. COLARES. 1993. Lotus corniculatus L. and Lotus tenuis Waldst. et Kit (Leguminosae). Anatomy of the leaf. Lotus Newsletter 24: 38-39. [ Links ]
2. BONTI, E. E.; R. M. BOO & L. I. LINDSTRÖM. 1995. Composición botánica de dietas de vacunos (Bos taurus) y vizcachas (Lagostomus maximus) en el sur del Caldenal. Memorias de la XIV Reunión Latinoamericana de Producción Animal y XIX Congreso Argentino de Producción Animal, Mar del Plata, Argentina: 108. [ Links ]
3. CAMEZZANA, O. M. 1987. Ecología alimenticia del Ñandú Petiso de la Patagonia (Pterocnemia pennata). Tesis Doctoral. Universidad Nacional de La Plata. Argentina. [ Links ]
4. COMPARATORE, V. M.; C. YAGUEDDÚ & L. P. HERRERA.2001. Hábito alimentario del Ñandú Común (Rheaamericana) en un agroecosistema bonaerense. Libro de resúmenes de la I Reunión Binacional de Ecología (XX Reunión Argentina de Ecología y X Reunión de la Sociedad de Ecología de Chile), Bariloche, Argentina: 86. [ Links ]
5. COMPARATORE, V. M. & C. YAGUEDDÚ. 2007. Diet of the Greater Rhea (Rhea americana) in an agroecosystem of the Flooding Pampa, Argentina. Ornitología Neotropical 18: 187-194. [ Links ]
6. DILCHER, D. L. 1974. Approaches to the identification of angiosperm leaf remains. Bot. Rev. 40: 1-151. [ Links ]
7. DIZEO DE STRITTMATER, C. 1973. Nueva técnica de diafanización. Bol. Soc. Argent. Bot. 15: 126-129. [ Links ]
8. FAHN, A. 1990. Plant anatomy. 4th. ed., Pergamon Press, Oxford. [ Links ]
9. FAROOQUI, A.; K. KULSHRESHTHA; S. N. SINGH; S. A. FAROOQUI; M. YUNUS & K. J. AHMAD. 1999. Foliar metal content and changes in epidermal traits of Lagerstroemia parviflora (L.) Roxb. Environmental Monitoring and Assessment 48: 107-115. [ Links ]
10. ISACCH, J. P.; M. S. BÓ; V. M. COMPARATORE; L. P. HERRERA; R. J. VARGAS & M. M. MARTÍNEZ. 2001. Las aves de los pastizales costeros del sudeste de la Provincia de Buenos Aires. In: Iribarne, O. (Ed.). Reserva de Biosfera Mar Chiquita: Características físicas, biológicas y ecológicas, pp. 269-285. Editorial Martín, Mar del Plata, Argentina. [ Links ]
11. MANGANARO, A. 1923. Caracteres histológicos genéricos y específicos de las leguminosas bonaerenses, extrabonaerenses y exóticas. Revista del Museo de La Plata 27: 221-252. [ Links ]
12. MARTELLA, M. B.; J. L. NAVARRO; J. M. GONNET & S. A. MONGE. 1996. Diet of Greater Rhea in an agroecosystem of Central Argentina. Journal of Wildlife Management 60: 586-592. [ Links ]
13. METCALFE, C. R. & L. CHALK. 1950. Leguminosae. In: Anatomy of the Dicotyledons. pp. 476-501. 1st. ed. Oxford: Clarendon Press. [ Links ]
14. PELLIZA, A; P. WILLEMS; V. NAKAMATSU; A. MANERO & R. SOMLO. 1997. Atlas dietario de herbívoros patagónicos. Proyecto PRODESAR-INTA-GTZ, EEA Bariloche, EEA Trelew, EEA Santa Cruz. Apoyo FAO-UNESCO/ MAB, S.C. de Bariloche. [ Links ]
15. SPARKS, D. R. & J. C. MALECHECK. 1968. Estimating percentage dry weight in diets using a microscopic technique. Journal of Range Management 21: 264-265. [ Links ]
16. STACE, C. A. 1965. Cuticular studies as an aid to plant taxonomy. Bulletin of the British Museum (Natural History). Botany. 4: 1-78. [ Links ]
17. STENGLEIN, S. A.; M. N. COLARES; A. M. ARAMBARRI; M. C. NOVOA; C. E. VIZCAÍNO & L. KATINAS. 2003. Leaf epidermal microcharacters of the Old World species of Lotus (Leguminosae: Loteae) and their systematic significance. Austr. J. Bot. 51: 459-469. [ Links ]
18. UPHOF, J. C. T. 1962. Plant hairs. In: Encyclopedia of Plant Anatomy IV, 5: 1- 206. 2nd ed. Gebrüder Borntraeger, Berlin. [ Links ]
19. VACAREZZA, G. P. 2001. Uso del recurso alimentario del Ñandú (Rhea americana L.) en la pampa deprimida bonaerense, y sus relaciones con herbívoros domésticos.Tesis Magister en Investigación Biológica Aplicada. Facultad de Agronomía, Universidad Nacional del Centro de la Provincia de Buenos Aires. [ Links ]
20. YAGUEDDÚ, C. & M. S. CID. 1992. Caracteres epidérmicos de dicotiledóneas de la pampa deprimida bonaerense de utilidad en microanálisis de dietas. Revista Argentina de Producción Animal 12: 265-279. [ Links ]
Recibido el 12 de diciembre de 2008.
Aceptado el 24 de junio de 2009.














