Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. v.44 n.3-4 Córdoba ago./dic. 2009
ARTÍCULO INVITADO
The adaptive value of shoot differentiation in deciduous trees and its evolutionary relevance
Veit M. Dörken1 & Thomas Stützel1, 2
1 Ruhr-University Bochum, D-44780 Bochum, Germany.
2 Corresponding author: email: thomas.stuetzel@rub.de
Summary: Pronounced long shoot/short shoot differentiation is typical for deciduous angiosperm trees. It also occurs in a number of gymnosperms and very few evergreen angiosperm trees. The study of 719 angiosperm tree species (602 deciduous and 117 evergreen species) demonstrated that the deciduous condition is nearly always associated with shoot differentiation. Detailed measurements in 38 angiosperms showed that the leaf area of an entire short shoot equals the leaf area of a single long shoot leaf of the same species and individual. In the few cases where the leaf area of the short shoot is slightly larger than that of a single long-shoot leaf, the short shoot leaves shade each other and the projection of the short shoot equals the area of a single long shoot leaf. Calculations of the stem biomass needed to expose a given assimilatory surface show two interesting aspects. First, the stem biomass (dry weight) to expose leaf surface is about 10 times less in short shoots than in long shoots. Second, this biomass in long shoots and short shoots appears to be species independent. Regarding shoot structure efficiency, leaf size and shape do not matter. Some evergreen species resemble in all parameters more to deciduous species than to typical evergreen species. Phytogeographical data as well as morphological data suggest that these atypical evergreen species are derived from deciduous ancestors. As measured parameters differ markedly between all gymnosperms, except Ginkgo, and angiosperms, we suppose that the evolutionary pathway leading to shoot differentiation was different for gymnosperms and angiosperms.
Key words: Shoot differentiation; Deciduous trees; Angiosperms; Gymnosperms; Evolution; Phytogeography.
Resumen: Valor adaptativo de la diferenciación de brotes en árboles deciduos y su relevancia evolutiva. En Angiospermas arbóreas deciduas, es común encontrar un alto grado de diferenciación entre brotes largos y brotes cortos. También se presenta esta característica en un número de gimnospermas y en muy pocas angiospermas arbóreas siempreverdes. El estudio de 719 especies de angiospermas arbóreas (602 deciduas y 117 siempreverdes) demostró que la condición decidua está casi siempre asociada a la diferenciación de los brotes. Mediciones detalladas en 38 angiospermas demostraron que la totalidad del área foliar de un brote corto es semejante al área foliar de una hoja de un brote largo de la misma especie y del mismo individuo. En los pocos casos en que el área foliar del brote corto es levemente mayor que el área de una hoja de un brote largo, las hojas del brote corto se sombrean entre sí de manera que el área proyectada del brote corto se asemeja a la de la hoja del brote largo. Cálculos de la biomasa de tallo necesaria para soportar una determinada superficie asimilatoria mostraron dos aspectos interesantes. Primero, que la biomasa de tallo (peso seco) de soporte del área foliar is alrededor de 10 veces menor en brotes cortos que en brotes largos. Segundo, que esta biomasa en brotes largos y brotes cortos parece ser independiente de la especie. En cuanto a la eficiencia estructural de los brotes, el tamano y la forma de las hojas no son relevantes. Algunas especies siempreverdes se asemejan en todos sus parámetros más a las especies deciduas que a especies siempreverdes típicas. Datos fitogeográficos así como datos morfológicos sugieren que estas especies siempreverdes atípicas derivaron de ancestros deciduos. Como todos los parámetros medidos difieren notablemente entre todas las gimnospermas, excepto Ginkgo, y las angiospermas, suponemos que el camino evolutivo que condujo a la diferenciación de brotes fue diferente en gimnospermas y angiospermas.
Palabras clave: Diferenciación del eje; Árboles caducifolios; Angiospermas; Gimnospermas; Evolución; Fitogeografía.
INTRODUCTION
Long shoot/short shoot differentiation is common in deciduous angiosperm trees. In extreme cases (e.g. Cercidiphyllum, Fig. 1), each short shoot forms only a single folious leaf per year which equals the long shoot leaf in size and shape. This leads to the idea, that deciduous trees need short shoots to replace the leaves of the previous year in the same position without the need to invest in new stem tissue. Evergreen trees do not need this and a pronounced shoot differentiation should be lacking there. If the idea is true, the occational occurrence of evergreen trees with prominent shoot differentiation could probably be regarded as an indicator for a deciduous ancestry in such species. Looking at the recent distribution of deciduous and evergreen forests in the world, one might assume that deciduous gymnosperms should have been more frequent prior to the evolution of angiosperms than they are today. One might wonder whether pronounced shoot differentiation in evergreen gymnosperms could be regarded as an indicator for a descent from deciduous ancestors. Such an assumption is in conflict with the present interpretation of deciduousness in gymnosperms regarding the deciduous species as derived from evergreen ancestors (Coulter & Chamberlain, 1982). This is also the case for angiosperms, in which deciduousness is generally regarded as a derived feature (Axelrod, 1966; Wolfe, 1987) too. It should however be taken into account that the arguments about primitive and derived characters in these publications are basically morphological or typolgical. This approach tries to interpret the rare character as derived from the frequent character. The term "derived" is thus frequently used by morphologists with a different meaning than in phylogenetics.
One difficulty for such an analysis is that within gymnosperms transition series which could lead to an understanding of the evolutionary pathway of this character can hardly be found. Within a taxon the same state is displayed and others show the extreme opposite without any intermediates that could help towards an evolutionary understanding (Bresinsky et al., 2008; Dallimore & Jackson ,1966; Farjon, 1984; Kramer & Green, 1990; Krüssmann, 1983; Rehder, 1967). Many extant gymnosperms are isolated relicts which can hardly be studied in the context of character evolution (Jagels &Equiza, 2005; LePage et al., 2005; Meyer, 2005; Momohara, 2005; Richter & LePage, 2005; Parakash & Kumar, 2004; Royer et al., 2003). In a first set of investigations it was therefore tested whether shoot differentiation is really linked to the deciduous condition and absent from evergreen trees. Then we focused on genera with evergreen and deciduous species such as Acer, Quercus, Nothofagus and Prunus. In a third step we checked whether the patterns in gymnosperms coincide with those found in angiosperms.

Fig.1: Cercidiphyllum japonicum, "ideal short shoot"; every year only a single folious leaf of exactly the same size as the original long shoot leaf is formed. Theinvestment in branch biomass is the lowest in all species studied.
MATERIALS AND METHODS
For the measurements, 5 year-old mature branches (Fig. 2) were cut out from the crown of freely exposed trees in the botanical garden of the Ruhr University Bochum (51o27´north, 7o5´east, mean temperature 10.2oC, 810 mm annual precipitation, altitude 87-127 m). From these 5 year-old branches all current year branches were cut off and measured in length. Frequency length curves were drawn for all species to analyze the degree and type of shoot differentiation. Long shoot/short shoot systems were considered, when the curve showed two distinct maxima (extreme values), a not differentiated branching system was considered if the curve showed only a single maximum.
All frequency length curves represent measurements from single branches. As a botanic garden does not offer homogenous stands of many individuals of one species, we could not pool the data from different individuals. The curves obtained from different individuals of the same species were basically identical, but the curve and thus also the gap between long shoots and short shoots was slightly compressed or streched in x-direction. Thus pooling made the gap disaperaring, dispite the fact that it was obvious and prominent in all individual measurements.
A further problem arose from the fact that we have to assign the measured shoots to size classes to draw the curves. In comparison to long shoots, the length variation in short shoots is very small. Using large intervals, we would not be able to detect the variability in short shoots. Using small intervals, it may happen that a certain size class is lacking in the measurements. This can give the (wrong) impression of several maxima in the long shoot part of length frequency curves. The problem could be overcome by using different intervals or size classes for long shoots and short shoots. We decided not to use this approach, as it may introduce prejudices in an understandable and thus not reproducable way.
Then the leaf area of 50 long shoot leaves was measured. Long shoot leaves were selected at random from the entire 5 year old branch. For this, the leaves were first scanned with a standard scanner, and then the total leaf area was calculated using the tools supplied by the analySISR software package. The same was done with 50 short shoot leaves. In this case entire, randomly selected short shoots were used. The arithmetic mean of the value for the short shoot leaves together with the mean value of the number of leaves within a short shoot gives the mean surface area of an entire short shoot. This analysis was done for a total of 38 angiosperm species, 25 of them deciduous and 13 evergreen, and 18 gymnosperms, 5 of them deciduous and 13 evergreen. The full list of all species studied is available in Dörken (2008). Here we focus on species with typical shoot differentiation and those species which could be regarded as misfits within their group or in respect to our hypothesis. The bulk of further data confirming our hypothesis has been omitted here as it supplies no additional information.
In a more detailed second analysis, the present year units of short shoots as well as long shoots axis (without leaves) where dried in a drying oven to constant weight to measure the dry weight. We have related the leaf area of all leaves of a present year growth unit to the dry weight of the axis on which they are inserted (including basal parts with cataphylls) to elucidate whether the formation of short shoots can really reduce the investment in stem per leaf area compared to undifferentiated shoot systems. A total of 38 angiosperms and 18 gymnosperms (tab. 1) where analyzed in the same way.
Shoot abscission structures were analysed by SEM after fixation in FAA, dehydration in FDA (Gerstenberger & Leins, 1978) and critical point drying, and from serial sections using classical paraffin technique and subsequent safranin/astrablue staining (Gerlach, 1984). Abscission structures where studied for the species listed in tab. 2. Photographs were taken with a NIKON COOLPIX 995 in the field, with a dissection microscope ZEISS SV 11 and with a light microscope ZEISS AXIOPLAN. Both microscopes were supplied with an OLYMPUS ColourView IIR camera. As record system we used the analySISR software version 3.2 build 776. Multiple image alignment was done with the same software package.
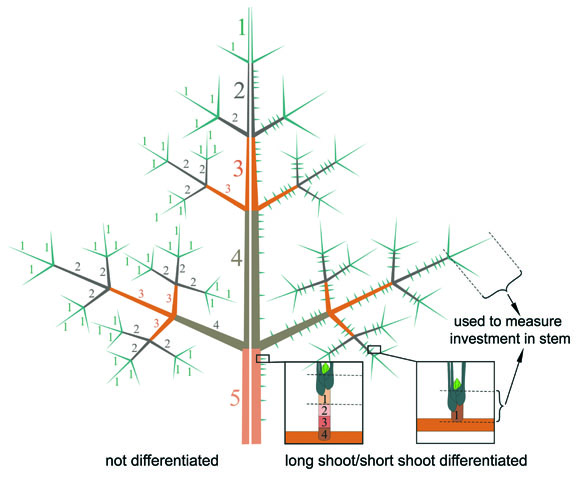
Fig 2: Not differentiated (left side) and differentiated shoot system (right side) to measure the investment in stem biomass; only the present year units of long shoots and short shoots have been used; numbers indicate the age of the shoot unit; 1= present year.
Table 1: Leaf area and dry weight of current-year growth units. For Acer platanoides and Ginkgo biloba three individuals of markedly different ages were used to estimate age effects. Tree age was estimated as exact data were not available in most cases.
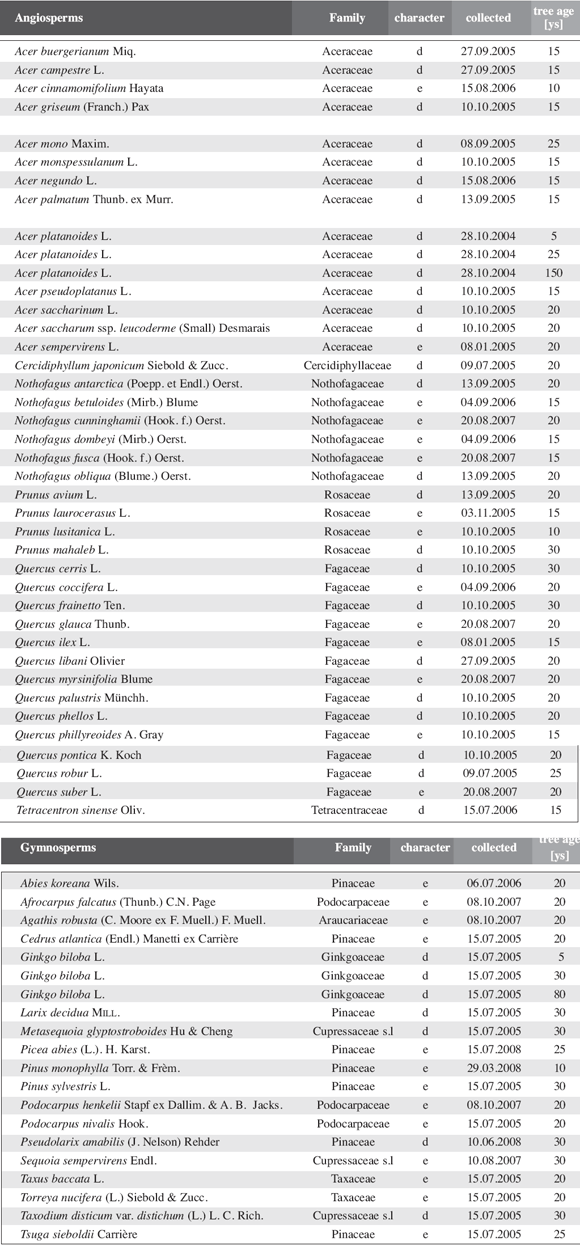
Table 2: Gymnosperm species for which the abscission of leaves and shoots was studied using a dissecting microscope, by SEM or serial sectioning.
RESULTS
Length frequency diagrams for a representative set of species are shown in Fig. 3 for deciduous species and in Fig. 4 for evergreen species. Typically, deciduous species have differentiated shoots, with a marked short shoot peak comprising about 80 to 90% of all shoots of the present year (Fig. 3a,b). The long shoots are much less and separated from the short shoot peak by a pronounced gap (Fig. 3a-d). Only in some species there is no clearly delimited length gap between short shoots and long shoots (Fig. 3e,f). In Metasequoia glyptostroboides the curve shows about the pattern for evergreen species gradually rising to a maximum and then going down again. The maximum is slightly shifted to the lower end of the curve, but is not at the lower end of the range (Fig. 3c). Nothofagus obliqua (Fig. 3f) shows more or less the typical short shoot peak, although the short shoot peak is not placed at the low end of the length scale and there is no distinct gap in the curve. In typical evergreen species (Fig. 4), the length distribution curve has only a single maximum. The peak value for the single peak in undifferentiated systems (Fig. 4a-d) reaches about the value for long shoots in differentiated systems and is about one dimension lower than for the short shoot peak in differentiated systems. This single peak is placed about in the middle of the curve if it is well developed (Fig. 4a). In many cases there is however a broad range of values without a prominent maximum (Fig. 4d). Sequoia sempervirens (Fig. 4c) does not really fit among the evergreen species, since it has a slight gap in the curve. For the shorter shoots the curve is lower than for typical deciduous species with shoot differentiation and the maximum is not at the low end of the range. Nothofagus dombeyi (Fig. 4f) shows a curve that resembles much more the curve for deciduous species with a shoot length frequency curve with two peaks (Fig. 3a,b,d,e) However, the gap is smaller, the mean length of the short shoots is longer and there is more variation in length of short shoots than in typical deciduous species. Evergreen species with curves similar to those for typical deciduous species are Cedrus atlantica (Fig. 5a) and Pinus sylvestris (Fig. 5b).
For the deciduous angiosperms species listed in (Table 3) the leaf area was measured. The leaf area of a single long shoot leaf was then compared to the leaf area of an entire short shoot. While the ratio between the area of a long shoot leaf and the leaf area of an entire short shoot is relatively constant and close to 1, exceptions are rare and range from 0.4 to 2.5. The absolute leaf areas vary considerably even within a species. These correlations are illustrated in Fig. 6, showing that short shoot leaves are considerably smaller and have petioles of a length that avoids shading within a short shoot.
The length frequency curves also depend from the age of the tree (Fig. 7). This is illustrated for Ginkgo biloba (Fig. 7d-f) and Acer platanoides (Fig. 7a-c) for a juvenile, a young but mature tree and an old tree that has reached about its maximum height for the growth site. The absolute length of the long shoots decreases with age and old individuals produce more or less exclusively short shoots.
The dry weight of annual growth units per leaf and leaf area is given for undifferentiated shoot systems in Table 4. The stem biomass is not related to the leaf weight but to the leaf area supported by the branch.
A similar calculation is presented in Table 5 for a differentiated shoot system. The cost in biomass per cm2 leaf area is for long shoots about the same as for the shoots of an undifferentiated shoot system. For short shoots the cost is only between 10 and 40% (mean 17,1%) of the cost measured for long shoots.
In respect to the relative cost in biomass per leaf area for long and short shoots two evergreen species (Acer sempervirens and Nothofagus dombeyi) coincide well with the bulk of deciduous species with differentiated shoot system. This becomes even more evident comparing Nothofagus dombeyi with other evergreen and deciduous species of Nothofagus such as N. antarctica (Fig. 8a), N. betuloides (Fig. 8b), N. cunninghamii (Fig. 8c)and N. fusca (Fig. 8d).
This relative costs is more or less constant for typical differentiated shoot systems and does not depend on the size or shape of the leaves. It does not matter in this respect whether the leaves are rather big (Acer platanoides) or relatively small (Nothofagus antarctica) or whether they are simple or compound (Acer negundo).
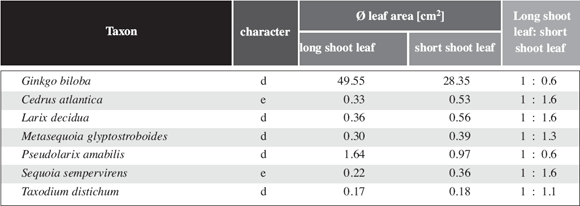
In evergreen gymnosperms without shoot differentiation, the relative cost in shoot dry weight per shoot leaf area is very similar to the values found for angiosperms (Table 5). In gymnosperms with shoot differentiation, leaf size varies considerably less between long shoot leaves and short shoot leaves than in angiosperms (Table 3). The same effect is visualized in Fig. 6c,d. Except for Ginkgo biloba, which is very "angiosperm like" in this respect, the leaf area of the entire short shoot in gymnosperms with shoot differentiation is between 13.4 and 80.8 times more than the leaf area for a long shoot leaf (Table 3). While in deciduous angiosperms any axil of a well developed long shoot leaf bears a short shoot in the following year, in deciduous gymnosperms only a minor part of the axils of long shoot leaves produces short shoots in the following year. Nevertheless, in the latter group the surface area of a single short shoot equals about the entire long shoot leaf area. Except for Ginkgo, gymnosperms with differentiated shoots differ markedly from angiosperms with differentiated shoots whether they are deciduous or not. This applies also for the amount of biomass to be formed to expose a given leaf area (Table 5). To some degree this is compensated by the fact that the number of long shoot leaves exceeds by far the number of short shoots formed in the following year. So in the end the relative cost per short shoot leaf area is only slightly less than in angiosperms (see last column in Table 5).
In Metasequoia, Sequoia, Taxodium and Glyptostrobus entire short shoots are abscised. While this occurs after one season in three of these taxa, which are therefore deciduous, Sequoia keeps the short shoots for more than one season and is therefore evergreen. In Sequoia and probably also in Sequoiadendron there is however a pronounced fall of short shoots in autumn and it needs heavy winds to blow the shed short shoots from lower branches where they get often deposited after shedding. In all four species short shoots are replaced from descendent serial accessory buds (Fig. 9). In the following year the new short shoots originate from the axils of basal bud scales of these accessory buds . In subsequent years the renewals rise also from the axils of bud scales.
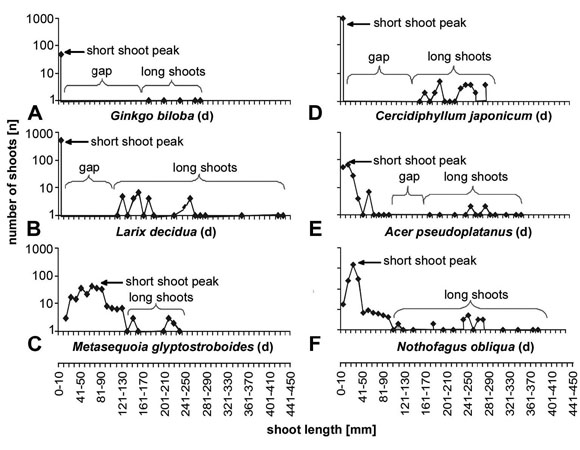
Fig. 3: Length/frequency curves for some deciduous species. Deciduous species show generally a well developed shoot differentiation indicated by a pronounced short shoot peak and a distinct more (fig. a,d) or less (fig. b,e) prominent gap. The long shoots cover a rather broad range and do not display a pronounced peak. Metasequoia differs from this pattern in having a relatively uniform distribution curve with the maximum shifted to the lower end of the curve. Nothofagus obliqua has a pronounced short shoot peak, but lacks also the gap in the curve.

Fig. 4: Length frequency curves for some evergreen species. a, b, d, e - typical evergreen species show a flat curve with a broad maximum near the middle of the curve. Sometimes the maximum seems to be shifted to the low values (fig.3c) but in the case of Taxus, this may be also an effect of crossings with one of the numerous cultivars. c,f - the evergreen species Sequoia sempervirens and Nothofagus dombeyi show curves that are very similar to those of seasonal species.
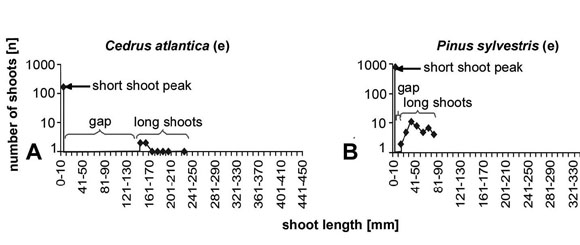
Fig. 5: The evergreen taxa Cedrus and Pinus show length/frequency curves that are typical for seasonal angiosperms with a pronounced short shoot peak and a gap between the short shoots and the long shoots.
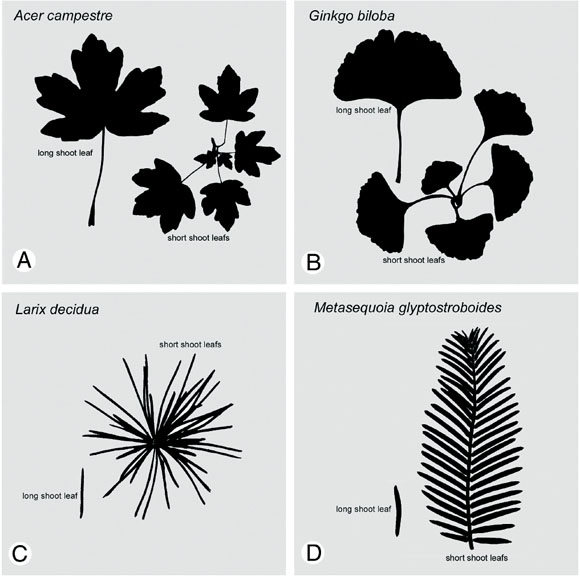
Fig. 6: Long shoot leaf and entire short shoot in 1 angiosperm and 3 gymnosperm species with differentiated shoot system; a,b - the leaves of the entire short shoot replace the long shoot leaf of the precious year; c, d - the short shoot exceeds the leaf area of a long shoot leaf by about the factor 100.
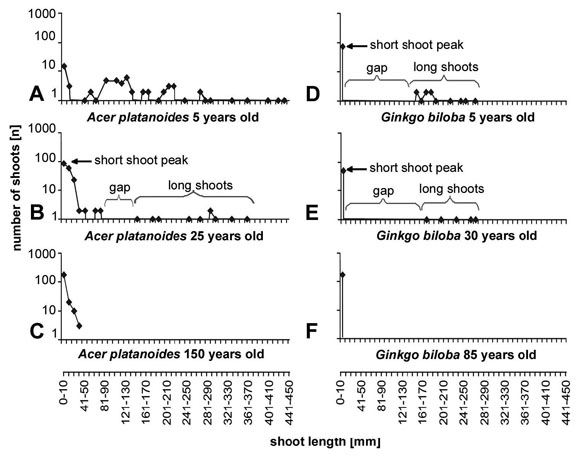
Fig. 7: Length frequency curves for tress of different age. The curves illustrate that the shoot differentiation is most prominent in the juvenile phase. In older stages the gap between long shoots and short shoots gets shorter and finally disappears and the variation of the shoot length is reduced to about what occurs in short shoots of young plants.
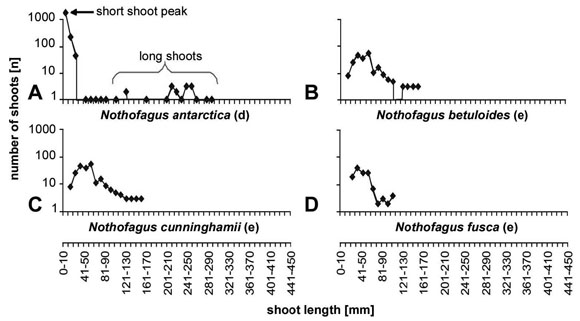
Fig. 8: Length frequency curves for different species of Nothofagus; the deciduous N. antarctica is differentiated in long and short shoots; in the evergreen species a shoot differentiation is lacking.
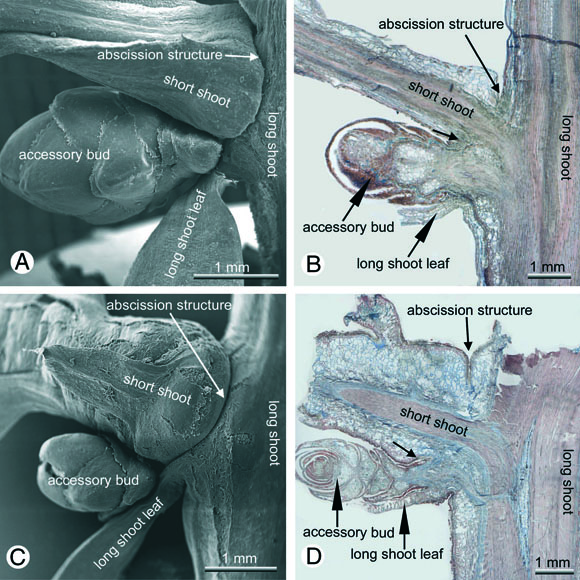
Fig. 9: Shoot abscission in Cupressaceae s.l.; a,b Metasequoia glyptostroboides, c,d Sequoia sempervirens; from the accessory bud the short shoot will be replaced after shedding.
Table 3: Leaf area of an individual leaf of one long shoot, leaf area of an entire short shoot and ratio between these two leaf areas for evergreen (e) and deciduous (d) species.

Table 4: Shoot biomass per leaf and per cm2 leaf area in some evergreen (e) and deciduous (d) species with undifferentiated shoot system.
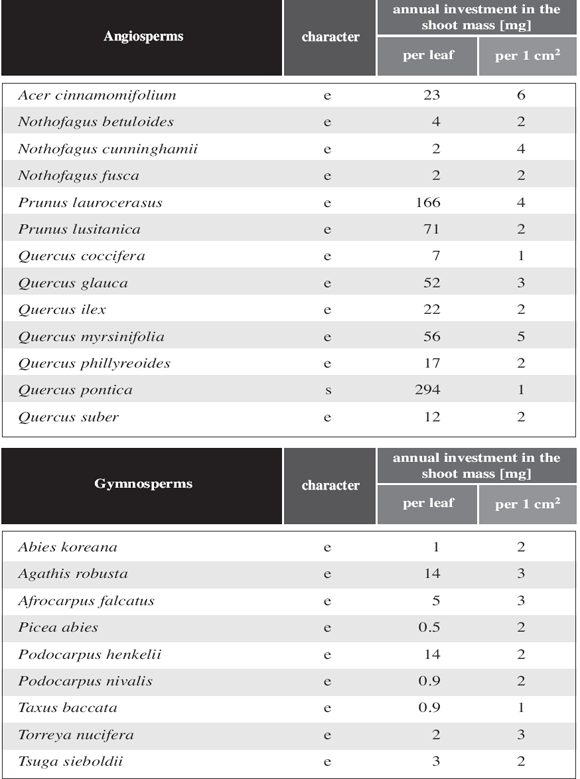
Table 5: Shoot biomass per leaf area for long shoots and relative cost per leaf area for short shoots and cost of short shoots; evergreen (e) and deciduous (d) species.
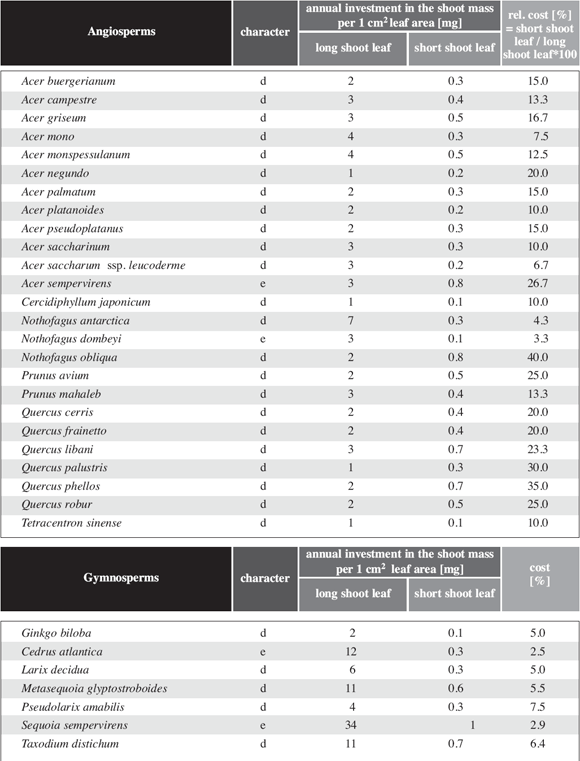
Table 6: Leaf area of long shoot leaves and short shoot leaves of some gymnosperms. Unlike the angiosperms the leaf size is quasi identic.
DISCUSSION
There is some ambiguity in the use of the terms long shoot and short shoots themselves. The length frequency curve for a species with clear differentiation between long shoots and short shoots is exemplified in Ginkgo biloba (Fig. 3a) or Larix decidua (Fig. 3c) Both species show a clear short shoot peak. As there are generally more than 10 times more short shoots than long shoots, the short shoot peak is very pronounced and narrow while the long shoot maximum is much lower and this part of the curve is much wider. In species with clear shoot differentiation, both peaks are separated by a clear gap. In other species, the gap may be less prominent or even lacking, it is however essential, that there are two peaks clearly separated at least by a local minimum. This requirement is not met in Metasequoia glyptostroboides (Fig. 3c) and only imperfectly so inNothofagus obliqua (Fig. 3f). It seems that the terms long shoot and short shoot in Metasequoia are not applied based on shoot length. Instead, the persisting shoots that abscise individual needles are called long shoots while those shoots that are abscised entirely together with the needles are called short shoots. In Notofagus obliqua (Fig. 3f) the terminology is applied according to the situation in other deciduous species of Nothofagus that show a much more typical shoot differentiation.
In textbooks and diagnostic keys (e.g. Bresinsky et al., 2008) we find however often phrases like"...has only long shoots..", in descriptions for Picea and Abies. The contrasting character or lead in keys is in this case "differentiated in long shoots and short shoots", e.g. for Pinus and Larix. Looking at the length distribution curve for Abies (Fig. 4a) and Larix (Fig. 3b), one can state, that the entire curve for Abies coincides nicely with the curve part for long shoots in Larix in being relatively flat and broad and having its maximum more or less in the middle instead of at the lower end. In this case the use of the term long shoot in species without a shoot differentiation is on a comparative basis as well. It is however an interspecific comparison instead of the more common intraspecific comparison. The results of interspecific comparisons depend on the species to be compared. The intraspecific comparison, as for Larix, Cedrus and Pinus, is independent from such effects.
For angiosperms two results are especially surprising. First the entire short shoot replaces the long shoot leaf rather precisely. It ranges from 1:0.5 to 1:1.5 (Table 3). Even the rare extreme values do not go farther than 1:0.4 and 1:2.5. This is very low and covers about the variation that occurs within long shoot leaves. The extreme values might be also an effect of the sometimes limited availability of material. The other surprising fact is that the investment in biomass per long shoot leaf area is about the same (ranging only from 1-7mg/cm2) for all deciduous species studied. It appears that all species are equally well adapted to the local situation of Bochum and that the size and shape of the leaves has no evolutionary meaning in this respect. This should be tested by carrying out similar studies in environments with markedly different climate. Such studies should include at least some of the species studied here to guarantee that the results are not affected by the selection of taxa under study. Nothofagus in Patagonia or Tierra del Fuego might be a promising example. The invested shoot biomass per short shoot leaf area ranges from 0.1-0.8mg/cm2.
Beogeographical data (e.g. Romero, 1986; Tanai, 1986; Hill & Jordan, 1993; Swenson et al., 2000; Hill, 2001; Knapp et al., 2005; Heads, 2006; Gibbs, 2006) might indicate that Nothofagus dombeyi might be derived from a deciduous ancestry. This would support our initial idea, that shoot differentiation in evergreen species is generally an indicator of a deciduous ancestry. The ramification patterns given for Nothofagus dombeyi by Puntieri et al. (2003) fit with our findings. The author(s) study however also other species than we did and with a different goal. A direct comparison of the data from natural stands with our garden data was therefore not possible.The evergreen Acer sempervirens fits into this scheme in a similar way. Spatial patterns as well as the generell accepted taxonomic relationship suggests that the well established shoot differentiation is the remainder of a deciduous ancestry in this species too. In gymnosperms however the only species for which length frequency curves as well as leaf surface and stem biomass data coincide with those for angiosperms is Ginkgo biloba. All other gymnosperms with shoot differentiation differ markedly from angiosperms in all these aspects.
It is therefore unlikely that the same evolutionary pathway or mode of directed selection as in angiosperms led to differentiated shoot systems in gymnosperms. At present we have no testable hypothesis for gymnosperms in this respect. To understand the shoot morphology of Pinus, it might be helpful to study the variability of shoot formation in deciduous and evergreen mature species of Berberis. In this genus we find also long shoots with non-assimilatory leaves (thorns) and sylleptic growth of short shoots. But while Pinus does not show any variability that might help towards and evolutionary understanding of this type of shoot differentiation, Berberis covers a wide range of morphological types.
The fact that we could not prove our initial hypothesis, that in pre-angiosperm times more gymnosperms have been deciduous and consequently recent gymnosperms might be derived from a deciduous ancestry does not imply that this hypothesis would necessarily be wrong. During our study we found deciduous Cupressaceae such asMetasequoia, Taxodium and Glyptostrobus show an abscission of entire short shoots which can be found in evergreen Cupressaceae as well. In Sequoia sempervirens (Fig. 9c,d) this abscission occurs together with the formation of serial accessory buds as in the deciduous species, e.g. Metasequoia glyptostroboides (Fig. 9a,b). In all other genera and species of Cupressaceae studied (Dörken & Stützel, in press), we found a morphologically similar abscission zone, but abscission takes only place in an irregular mannor or even not at all. Such a morphologically well performed but non-functional abscission structure can probably only be understood as a reminder of a deciduous ancestry.
Therefore the idea of a more widespread deciduous ancestry in gymnosperms is still of interest. It has however to be stated, that the morphometric data for gymnosperms and angiosperms are so markedly different, that the adaptative pathway to differentiated shoot systems has most likely been a different one in angiosperms and gymnosperms, the latter one still being largely not understood.
ACKNOWLEDGEMENTS
We grateful acknowledge the support of the Botanic Gardens of Bochum, Bonn, Düsseldorf, and Dortmund, as well as the Palmengarten Frankfurt a.M. and many other Botanic Gardens. The numerous and detailed comments on an earlier version of the manuscript by an anonymous reviewer helped us to improve the manuscript significantly.
BIBLIOGRAPHY
1. AXELROD, D. I. 1966. Origin of deciduous and evergreen habits in temperate forests. Evolution 20: 1-15. [ Links ]
2. BRESINSKY A., C. KÖRNER, J. W. KADEREIT, G.NEUHAUS & U. SONNEWALD. 2008. Strasburger, Lehrbuch der Botanik, 36. Auflage. Spektrum, Akademischer Verlag Heidelberg. [ Links ]
3. COULTER, J. M. & C. J. CHAMBERLAIN. 1982. Morphology of Gymnosperms. Revised Edition. pp. 225-226. Central Book Depot, Allahabad. [ Links ]
4. DALLIMORE, W. &. A. B. JACKSON. 1966. A Handbook of Coniferae and Ginkgoaceae. 4th edition. Edward Arnold Publ., London. [ Links ]
5. DÖRKEN, V. 2008. Saisonalität und Langtrieb-/Kurztrieb-Differenzierung in Gymnospermen, ursprünglich oder abgeleitet? Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften der Fakultät für Biologie und Biotechnologie der Ruhr-Universität Bochum. http://wwwbrs. ub.ruhr-uni-bochum.de/netahtml/HSS/Diss/Doerken VeitMartin/diss.pdf [ Links ]
6. DÖRKEN, V. & TH. STÜTZEL. In press. Seasonality in Cupresaceae, primitive or derived. Feddes Rep. [ Links ]
7. FARJON, A. 1984. Pines, Drawing and Descriptions of the Genus Pinus. E. J. Brill, Leiden. [ Links ]
8. GERLACH, D. 1984. Botanische Mikrotomtechnik, eine Einführung, 2. Aufl. Thieme, Stuttgart. [ Links ]
9. GERSTERBERGER, P. & P. LEINS. 1978. Rasterelektronenmikroskopische Untersuchungen an Blütenknospen von Physalis philadelphia (Solanaceae). Ber. Deutsch. Bot. Ges. 91: 381-387 [ Links ]
10. GIBBS, G. 2006. Ghosts of Gondwana. Potton Publishing, Nelson, New Zealand. [ Links ]
11. HEADS, M. 2006. Panbiogeaography of Nothofagus (Nothofagaceae): Analysis of the main species. J. Biogeogr. 33: 1066-1075. [ Links ]
12. HILL, R. S. & G. J. JORDAN. 1993. The Evolutionary history of Nothofagus (Nothofagaceae). Austr. Syst. Bot. 6: 111-126. [ Links ]
13. HILL, R. S. 2001. Biogeography, evolution and palaeoecology of Nothofagus (Nothofagaceae): The contribution of the fosssil record. Austr. J. Bot. 49: 321-332 [ Links ]
14. JAGELS, R. & M. A. EQUIZA. 2005. Competitive Advantages of Metasequoia in Warm Latitudes. In: LEPAGE, B. A., CH. J. WILLIAMS & H. YANG (eds.), The Geobiology and Ecology of Metasequoia, pp. 335-349. Springer, Berlin, Heidelberg. [ Links ]
15. KNAPP, M., K. STÖCKLER, D. HAVELL, F. DELSUC, F. SEBASTIANI & P. J. LOCKHART. 2005. Relaxed molecular clock provides evidence for long-distance dispersal of Nothofagus (southern beech). PLoS. Biol. 3: 38-43. [ Links ]
16. KRAMER, K. U. & P. S. GREEN. 1990. The Families and Genera of Vascular Plants, Pteridophytes andGymnosperms, edited by K. KUBITZKI. Springer Verlag, Heidelberg. [ Links ]
17. KRÜSSMANN, G. 1983. Handbuch der Nadelgehölze, 2. neubearbeitete Auflage. Verlag Paul Parey, Berlin und Hamburg. [ Links ]
18. LEPAGE, B. A., H. YANG & M. MATSUMOTO. 2005. The Evolution and Biogeographic history of Metasequoia. In: LEPAGE, B. A., CH. J. WILLIAMS & H. YANG (eds.),The Geobiology and Ecology of Metasequoia, pp. 3-114. Springer, Berlin, Heidelberg. [ Links ]
19. MEYER, H. W. 2005. Metasequoia in the Oligocene Bridge Creek Flora of Western North Amerika: Ecological Implications and the History of Research. In: LEPAGE, B. A., CH. J. WILLIAMS & H. YANG (eds.),The Geobiology and Ecology of Metasequoia, pp. 159-186. Springer, Berlin, Heidelberg. [ Links ]
20. MOMOHARA, A. 2005. Palaeoecology and History of Metasequoia in Japan, with Reference to its extinction and Survival in East Japan. In: LEPAGE, B. A., CH. J. WILLIAMS & H. YANG (eds.), The Geobiology and Ecology of Metasequoia, pp. 115-136. Springer, Berlin, Heidelberg. [ Links ]
21. PRAKASH, N. & M. KUMAR. 2004. Occurrence of Ginkgo Linn. in early cretaceous deposits of South Rewa Basin, Madhya Pradesh. Curr. Sci. 87: 1512-1515. [ Links ]
22. PUNTIERI, J. G., M. S. SOUZA, C. BRION, C. MAZZINI,& D. BARTHÉLÉMY. 2003. Axis differentiation in two south american Nothofagus species. Ann. Bot. 92: 589-599. [ Links ]
23. REHDER, A. 1967. Manual of cultivated trees and shrubs. 2nd edition. The Macmillian Company, New York. [ Links ]
24. RICHTER, S. L. & B. LEPAGE. 2005. A High-Resolution Palynological Analysis, Axel Heiberg Island, Canadian High Arctic. In: LEPAGE, B. A., CH. J. WILLIAMS & H. YANG (eds.), The Geobiology and Ecology of Metasequoia, pp. 137-158. Springer, Berlin, Heidelberg. [ Links ]
25. ROMERO, E. J. 1986. Fossil evidence regards the evolution of Nothofagus Blume. Ann. Missouri Bot. Gard. 73: 276-283. [ Links ]
26. ROYER, D. L., L. J. HICKEY & S. L. WING. 2003. Ecological conservatism in the "living fossil" Ginkgo. Palaeobiology 29: 84-104. [ Links ]
27. SVENSON, U., R. S. HILL, & S. MCLOUGHLIN. 2000. Ancestral area analysis of Nothofagus (Nothofagaceae) and its congruence with the fossil record. Austr. Syst. Bot. 13: 469-478. [ Links ]
28. TANAI, T. 1986. Phytogeographic and phylogenetic history of the genus Nothofagus BL. (Fagaceae) in the southern hemisphere. J. Fac. Sci. Hokkaido Univ., Ser. IV 21: 505-582. [ Links ]
29. WOLFE, J. A. 1987. Late Cretaceous-cenozoic history of deciduousness and the terminal Cretaceous event. Palaeobiology 13: 215-226. [ Links ]
Recibido el 8 de abril de 2009
Aceptado el 21 de octubre de 2009.














