Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Boletín de la Sociedad Argentina de Botánica
versão On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.45 no.3-4 Córdoba jul./dez. 2010
ANATOMÍA Y MORFOLOGÍA
Stomatal distribution, stomatal density and daily leaf movement in Acacia aroma (Leguminosae)
Marcelo P. Hernández1 y Ana M. ArambarriI2*
1 Cátedra de Sistemática Vegetal
2 Cátedra de Morfología Vegetal
1,2 Jardín Botánico y Arboretum ¨C. Spegazzini¨, Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, 60 y 119, C.C. 31 (1900) La Plata, Argentina
E-mail addresses: botgral@agro.unlp.edu.ar
* anaramba@yahoo.com.ar
Summary: Acacia aroma Gillies ex Hook. & Arn. grows in the Chacoan and Yungas Biogeographic Provinces, Argentina. It has numerous medicinal applications, sweet and edible fruits, and it may be used as forage. The objective of the present contribution was to analyse the stomatal distribution and stomatal density on the secondary leaflet surfaces, in different parts of the leaf, and at different tree crown levels, establishing the leaf movement and environmental condition relationships. The work was performed with fresh material and herbarium specimens, using conventional anatomical techniques. Stomatal distribution on the secondary leaflet surfaces was established, and differences in stomatal density among basal, medium and apical leaflets were found. A decrease in stomatal density from the lower level to the upper level of the tree crown would be connected with that. The stomatal distribution and density appear related to the secondary leaflet shape and its position on the secondary rachis, interacting with the daily secondary leaflets and leaf movement, and the weather conditions. It is interesting that the medium value of stomata density were found in the middle part of the leaf and at the middle level of the tree crown. Original illustrations are given.
Key words: Acacia aroma; Leaf movement; Leguminosae; Stomata.
Resumen: Distribución y densidad estomática y movimiento diario de la hoja en Acacia aroma (Leguminosae). Acacia aroma crece en las Provincias Biogeográficas Chaqueña y de las Yungas, Argentina. Este árbol posee numerosas aplicaciones en medicina popular, sus frutos son comestibles y puede ser usada como forraje. Los objetivos de la presente contribución fueron: establecer la distribución y densidad de los estomas en el folíolo secundario, en distintos folíolos secundarios de la misma hoja y en los folíolos secundarios de las hojas de la parte basal, media y superior de la copa del árbol, estableciendo relaciones con el movimiento diario de las hojas y condiciones ambientales. Para el estudio se utilizó material fresco y ejemplares de herbario empleando técnicas de anatomía convencionales. Se estableció la distribución de los estomas sobre las superficies adaxial y abaxial del folíolo secundario. Se encontraron diferencias en la densidad de estomas entre los folíolos secundarios de la parte basal, media y apical de la hoja que están relacionadas a la posición de éstas en la copa del árbol. Dentro de la copa del árbol se encontró que la densidad de estomas decrece desde la parte basal hasta la parte superior. La distribución y densidad estomática estarían relacionadas a la forma del folíolo secundario y posición de éstos sobre el raquis, al movimiento diario de los folíolos secundarios y de la hoja interactuando con los factores ambientales. Cabe destacar que el valor medio de densidad de estomas se halló en la parte media de la hoja y en la parte media de la copa del árbol. El trabajo se acompaña con ilustraciones originales.
Palabras clave: Acacia aroma; Leguminosae; Movimiento de las hojas; Estomas.
INTRODUCCIÓN
The genus Acacia Miller (Leguminosae, Mimosoideae, Acacieae), with 1200 species, occurs in the tropical and subtropical regions of America, Africa, Asia and Australia (Vassal 1981). In Argentina, it is represented by 21 species distributed in the northern and central provinces up to latitude 30° S (Cialdella, 1984, 1997; Zuloaga et al., 2008). Acacia aroma Gillies ex Hook. & Arn. grows in the Chacoan and Yungas Biogeographic Provinces; xerophilous forests are its typical habitat (Digilio & Legname, 1966). This tree is up to 4-6(-9) m tall; its leaves are pinnately compound, with thorn-like stipules. The petiole has a nectariferous sessile gland and other glands are present on the primary rachis on which pairs of pinnae are attached to. Each pinna is made up of the secondary rachis and the secondary leaflets with pulvini which facilitate nastic movements (Strasburger et al., 1994). The secondary leaflets are oblong and the presence of a submarginal midvein divides the leaf blade into one smaller acroscopic half and one larger basiscopic half; these halves tend to even out in the apical secondary leaflet (Cialdella, 1984). The flowers are in yellow heads, and the fruit is a moniliform legume (Burkart, 1952; Gunn, 1984; Digilio & Legname, 1996; Cialdella, 1997). This tree has numerous applications; its wood is used for rough structures, and also for fuel and coal production (del Valle Perea et al. 2007). In traditional medicine, twig and leaf infusion is used for gastritis, liver and stomach disorders, and as a digestive aid (Carrizo et al., 2005); its leaves are used as antiseptics, to treat canker sore, skin infections, conjunctivitis, and sore throat (Martínez Crovetto, 1981; Cialdella, 1984; Alonso & Desmarchelier, 2005; del Valle Perea et al., 2007). They may have antisyphilitic powers (Hieronymus, 1882; Rojas Acosta, 1907; Toursarkissian, 1980), and the dry powdered leaves are used as drying agents to enhance wound scar formation. The root has antiseptic and antiinflammatory properties (Barboza et al., 2006). The bark is antifungal (Martínez Crovetto, 1981). The oil from the flowers is used in cosmetics and, as infusions, for asthma and high blood pressure (Burkart, 1952; Peña-Chocarro et al., 2006). Its juice is used as a painkiller for earache (Martínez Crovetto, 1981) and the flue (Alonso & Desmarchelier, 2005). The fruits are sweet and edible (Demaio et.al., 2002), and the juice is astringent, though it should be used carefully as it has cyanogenetic compounds (Alonso & Desmarchelier, 2005). As regards its cultivation, it adapts to silvopastoral production systems since it can be used for winter forage with rapid growth (Demaio et al., 2002; del Valle Perea et al., 2007).
On examining the anatomy of tree leaves from the Yungas Biogeographic Province (Arambarri et al., 2009), we have observed that in Acacia aroma the stomata were unevenly distributed both among the adaxial and abaxial sides of the blade and in the acroscopic and basiscopic semi-blades in the secondary leaflets. It is known that stomatal density is affected by the stage of leaf development, and it is different in several parts of the blade, and also among different leaves of the same plant (Stace, 1965; Poole et al., 1996). Stomatal density is also influenced by the plant species and environmental factors such as light brightness (Gay & Hurd, 1975), water availability, temperature and sun radiation (Ma et al., 2004), relative humidity (Bravo & Grau, 1992), drought and high salinity concentrations (Salas et al., 2001), nutrient availability and atmospheric carbon dioxide concentration (Woodward, 1987; Woodward & Bazzaz, 1988; Royer, 2001; Ma et al., 2004). Our first hypothesis was that the uneven stomatal distribution is a result of the light differential impact on secondary leaflets. We propose to study the stomatal distribution and stomatal density: (1) on both surfaces of the secondary leaflet; (2) in pinnae located in different parts of the leaf; (3) in leaves from different tree crown levels. We also studied the daily leaf movement in order to establish a possible relationship with the stomata distribution and density.
MATERIAL AND METHODS
Plant materials studied
The study was performed by using adult leaves of Acacia aroma collected from trees located in the area of the Arboretum and Botanical Garden "C. Spegazzini", Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de la Plata, situated at 21 m a.s.l.; the reference material was deposited at LPAG herbarium. Samples from the herbarium (LP), Facultad de Ciencias Naturales y Museo de La Plata, with the purpose to corroborate the stomatal distribution were also examined. ARGENTINA. Buenos Aires province: Pdo. La Plata, La Plata, 14-I-2009, Arambarri & Bayón 275 (LPAG). Catamarca province: Dpto. Belén, Cercos, 27-II-1929, Cabrera 1145 (LP); Dpto. Belén, Londres de Quimiril, 22-V-1991, Capparelli 2 (LP). Chaco province:Dpto. San Fernando, Resistencia, 25-IX-1961, Fabris & Hunziker 7493 (LP). Córdoba province: Valle de Punilla, Carlos Paz, 28-XI-1938, Bridarolli 552 (LP). Jujuy province: Dpto. Ledesma, between Bananal and Aguas Negras, 17-III-1973, Cabrera et al., 23308 (LP); Dpto. San Pedro, San Lucas, 11-13-1973, Cabrera et al. 24041 (LP). La Rioja province: Dpto. Chilecito, Sierra Velazco, XI-1945 Morello 5054, (LP). Salta province: Dpto. Guachipas, Guachipas, I-1937, Job 1476 (LP); Dpto. La Viña, between La Viña and Talapampa, 29-XI-1996, Delucchi 1366 (LP). San Juan province: Dpto. Ullún, cerros de Ullún, XI-1941, Rodrigo 2828 (LP). Santa Fe province: Dpto. General Obligado, El Rabón, 27-XI-1939, Birabén 106 (LP). Santiago del Estero province: Dpto. Robles, Beltrán, 28-XI-1940, Maldonado B 523, (LP).
Techniques
The fresh material of adult leaves was collected from the lower, middle and upper crown level and above the middle part of branches facing north, where they were exposed to ample sun. Leaf samples were fixed in formalin aceto-alcohol solution (FAA) for further study. The herbarium specimen leaves were reconstituted by immersion in water with a drop of detergent in an oven at 30-35°C, for 48 hours, previous to its FAA fixation. Semi-permanent preparations were made from the basal, medium and apical pinnae and also from the lower, middle and upper tree crown level. In order to achieve transparent leaves two clarification techniques were applied. The first technique of Dizeo de Strittmatter (1973) and the second technique is called cold clarification, which we are testing in the laboratory of Morfología Vegetal, at Facultad de Ciencias Agrarias y Forestales, UNLP, where the work has been carried out. This last technique involved placing a mixture of equal parts of 5% sodium hydroxide and 5% sodium hypochlorite in a glass container, where the pinnae were soaked for 4-5 days, the material was removed, washed and bleached with 50% sodium hypocholorite, and then washed and immersed in choral hydrate for 24 hours. The advantage of the cold method was that it allowed to mount the whole pinna and observe the secondary leaflets from the basal, medium and apical parts of the pinna. The cleared pinnae were stained with 80% alcoholsafranin and mounted in gelatin-glycerin. Stomatal density values are averages of 10 counts on each semi-blade surface, and are expressed in stomata number/mm-2. In order to examine the leaf movements, photographs were taken with a Kodak Easyshare digital camera, m1033, 10 Mpx, every two hours since before dawn until it was already dark, when they remained closed. To that effect, a camera tripod was set up and focused on the leaves located on the branches facing to north and at the middle crown level. The leaf morphology was analysed and photographs were taken with a stereoscopic microscope (Wild M8). A light microscope (Leitz SM Lux) with a camera lucida was used in order to study the anatomy and to draw the stomatal distribution diagrams. A color PAL CCD camera attached to a microscope Gemalux allowed to capture the images digitalized by means of Hyper Media Center software. The leaf movement was photograph on 01-6-2009. The pictures were interpreted in light of the data recorded by the weather station located at 200 m from the Botanical Garden and provided by the staff of the Weather Information Office at Facultad de Ciencias Astronómicas y Geofísicas, Universidad Nacional de La Plata (Chart 1).

Statistical analysis
The data obtained were averaged and processed according to the analysis of variance (ANOVA) in order to determine the level of statistical significance of the differences in stomatal density among lower, middle and upper crown. Tests of media comparison according to Tukey were carried out, at 5% level of probability (P < 0.05). The significant differences detected in stomatal density for the three crown levels were studied by means of the multiple range test. The analyses were performed by using Statistics 7.0 software for windows (Tables 1-4 and Graphs 1-4).
RESULTS
Morphology of the leaf
As it is observed in Fig. 1 A, the leaf is bipinnately compound; two rows of 10-25 pairs of opposite pinnae are located on the primary rachis. In Fig. 1 B, it is observed that every pinna has a secondary rachis with an adaxial rib where the bending pulvini, which give movement to the secondary leaflets, are attached to small cavities or pits. This arrangement of petiolules on the adaxial surface of the secondary rachis makes secondary leaflets close upwards. Each secondary leaflet is asymmetric with a smaller acroscopic semi-blade and a larger basiscopic semi-blade.

Fig. 1. Acacia aroma. Leaf morphology. A, leaf showing a pinna formed by secondary leaflets attached to the secondary rachis. B, detail of adaxial view of the secondary rachis with the two rows of secondary leaflets: ac, acroscopic semi-blade of the secondary leaflet; ba, basiscopic semi-blade of the secondary leaflet; lea, secondary leaflet; pi, pit where the pulvinus of the secondary leaflet is attached to; ri, adaxial rib of the secondary rachis; pu, pulvini. Simple eglandular trichomes may be seen on the secondary leaflet margin. Scale bar: A, 1 cm; B, 1 mm.
Stomatal distribution and density in the secondary leaflet, foliole or pinnule
Figure 2 Adx, represents the adaxial surface of the secondary leaflet: on the acroscopic semi-blade (ac), a few stomata are present in the basal part and the number increases gradually in the medium part, achieving the highest density in its apical end. On the basiscopic semi-blade (ba), stomata are nearly absent in the basal part. Also, the number of stomata in the medium part is very limited; while the number of stomata increases towards the apical end. Figure 2 Abx, corresponds to the abaxial surface of the secondary leaflet: on the acroscopic semi-blade (ac), the stomatal density is similar in the basal and the apical part of it, but the largest number is located in the medium part. On the basiscopic semi-blade (ba), the largest stomatal concentration is located in the basal part of the secondary leaflet and reduces gradually to the apical end.

Fig. 2. Acacia aroma. Secondary leaflets. Adx, adaxial surface; Abx, abaxial surface. ac, acroscopic semi-blade of the secondary leaflet; ba, basiscopic semi-blade of the secondary leaflet. The largest stomatal concentration is represented by the darkest shade which lightens until it is white where stomata are absent.
Stomatal density per unit area (s n° / mm-2) in the surfaces of the secondary leaflet located in the basal, medium and apical pinna of the leaf, and in the leaves situated at lower, middle and upper tree crown level
On the adaxial surface the highest stomatal density was found in the medium pinna of the leaf, in every tree crown level. On the abaxial surface stomatal density varies according to the location of the leaf in the crown. Thus, at lower crown level (LL), the leaf medium pinna has the highest stomatal density; at middle crown level (ML), the stomatal density is higher in the apical pinna, and at the upper crown level (UL), the highest stomatal density corresponds to the basal pinna (Chart 2).

Statistical analysis of the stomatal density per unit of area (s n° / mm-2), in the semi-blade surfaces per tree crown level (LL = lower level; ML = middle level; UL = upper level).
1. Stomatal density of the acroscopic Adaxial semi-blade surface (SdacAdx) per tree crown level.

Graph 1. Mean stomatal density (s n° / mm-2) per tree crown level. It is clearly observed that mean stomatal density value decreases while the crown level increases. No significant differences between the mean values obtained for the middle (ML) and upper (UL) levels were found, but they were significantly different from the lower level (LL) (Table 1, Graph 1).

2- Stomatal density of the basiscopic Adaxial semi-blade surface (SdbaAdx) per tree crown level.
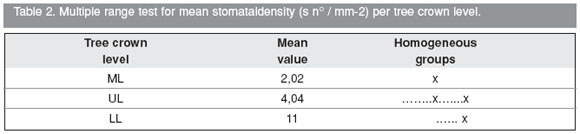
Graph 2. Mean stomatal density (s n° / mm-2) per tree crown level. It is clearly observed that mean stomatal density values decrease from lower level (LL) to upper level (UL) and then middle level (ML). Although the lowest mean stomatal density value corresponds to the middle level, this value is larger at the upper level even increasing more at the lower level, the mean stomatal density value at (LL) obtained is not significantly different from the values found at (ML) and (UL), at the same time there is a significant difference between the values found at (LL) and (ML) (Table 2, Graph 2). The lowest stomatal density at the middle crown level is due to the fact that the basiscopic adaxial semi-blade is characterized by the almost absency of stomata (See Fig. 2 Adx, ba).

3 - Stomatal density of the acroscopic Abaxial semi-blade surface (SdacAbx) per tree crown level.


Graph 3. Mean stomatal density (s n° / mm-2) per tree crown level. It is clearly observed that the mean stomatal density value decrease while the crown level increase. No significant differences between the mean values obtained for the lower (LL) and middle levels (ML) were found, but they were significantly different from the upper level (UL) (Table 3, Graph 3).
4 - Stomatal density of the basiscopic Abaxial semi-blade surface (SdbaAbx) per tree crown level.


Graph 4. Mean stomatal density (s nº / mm-2) per tree crown level. It is clearly observed that the mean stomatal density value decrease while the crown level increase. No significant differences between the mean values obtained for the lower (LL) and middle levels (LL) were found but they were significantly different from the upper level (UP) (Table 4, Graph 4).
Leaf movement
While there is darkness (Fig. 3 A), the leaflets are closed upwards, leaving only the apical end of the abaxial surface exposed. When day brightness increases, the pinnae are separated from the primary rachis and the two oppositing secondary leaflets rows also separate from the secondary rachis. This happens while the light is increasing until the two secondary leaflets rows form an angle nearly to 180º with complete brightness (Fig. 3 B). This means that these move to a flat level. Between 11 am and 3 pm (Fig. 3 C and D), in coincidence with the highest temperature and level of brightness, more intense winds, low relative humidity and high atmospheric pressure, and all dehydrating elements (Chart 1), the pinnae are oriented towards the leaf distal end. At the same time, the two secondary leaflets rows reduce their opening angle, forming a right angle first, and then an acute angle, with a slight movement towards the pinna apical end. They start becoming imbricated. This movement exhibit only the adaxial basiscopic semi-blade surface exposed, where the stomata are just absent (Fig. 2 Adx, ba). It has been observed that in this species the secondary leaflets are easily covered one another, and it is due to the fact that the distance between the pulvini over the secondary rachis is 0.5-0.8 mm in length, being the secondary leaflets very close to each other. If the above mentioned environmental conditions are maintained, the two leaflets rows keep forming an acute angle between them (Fig. 3 E) until these close completely at dusk when these adopt again the "circadian rhythm" position (Fig. 3 F).

Fig. 3. Acacia aroma. Leaves showing the pinnae and the parallel rows of secondary leaflets movement: A, at 7 am; B, at 9 am; C, at 11 am; D, at 3 pm; E, at 7 pm; F, at 9 pm. Scale bar: 1 cm.
DISCUSSION
The tree of Acacia aroma has xeromorphic characteristics such as small leaves, tiny secondary leaflets, with glabrous adaxial surface and thornylike stipules. The bicompound leaf has a ventral rib on the secondary rachis which only allows an upwards secondary leaflet movement when these are closed. According to the results, to the alternation of day and night which would correspond to the autonomous turgor movement (Strasburger et al., 1994), the secondary leaflets movements (and leaf pinnae) shall be added along the day and with weather conditions, produced by the pulvini reaction which would be thermal and photonastically sensitive, performing opening and closure movements, which are called nictinastic (Esau, 1982; Strasburger et al., 1994). Acacia aroma leaves are amphistomatic, the epidermal cells have periclinal walls with a cuticle covered by remarkable epicuticular waxes, and slightly curved anticlinal cell walls (Arambarri et al., 2009). Thus, this would be adapted to high brightness levels in coincidence with what has been exposed by (Gay & Hurd, 1975; González, 1992). The stomata are anomocytic and paracytic types in coincidence with Metcalfe & Chalk (1979). The presence of more than one type of stomata is frequent in the Leguminosae (Stenglein et al., 2003, 2005; Arambarri et al., 2006). The stomatal density on the secondary leaflet surface is high on the abaxial and reduced on the adaxial sides, and they are infrequent on the adaxial basiscopic semi-blade surface. The stomatal distribution and frequency has been constant in every analyzed specimens. This distribution would be connected to secondary leaflets shape and position on the secondary rachis, interacting with daily leaf movement and environmental factors. The leaf movement interacting with the stomatal distribution and density would be contributing to reduce the water loss.
The variability found in the stomatal density among the basal, medium and apical pinnae of the leaves in relation to the placement at different levels of the tree crown show us that at lower and upper levels of tree crown where the leaves are more exposed to the environmental factors, the highest density of stomata is found in the basal and medium parts of the pinnae, whereas at the middle level of the tree crown where the leaves are more protected, the highest stomata density is found in the apical pinnae. As regards the placement in the tree crown, the stomatal density is high at the lower level of the crown reduced towards the upper level of it, in coincidence with the variability mentioned in the stomatal density at different crown levels by Salisbury (1927), and more recently, by the results obtained in leaves from different parts of the stem by Barrientos Priego et al. (2003). However, it shall be emphasised that stomatal density mean values are always in the secondary leaflet middle part and in the middle part of the tree crown.
ACKNOWLEDGEMENTS
We appreciate Liliana Katinas´s critical reading of the first draft. We would like to thank María Alejandra Migoya for preparing figure 2. Romina Ywatani for the English translation. We also thank staff at the Weather Information Office at Facultad de Ciencias Astronómicas y Geofísicas, Universidad Nacional de La Plata. We are also very grateful to curators of LP and LPAG herbaria for loaning the specimens. This work was supported by the Comisión de Incentivos a los docentes-investigadores, Decreto 2427/93, Secretaría de Política Universitaria del Ministerio de Educación de la Nación Argentina.
BIBLIOGRAPHY
1. ALONSO J. & C. DESMARCHELIER. 2005. Plantas medicinales autóctonas de la Argentina. Bases científicas para su aplicación en atención primaria de la salud Ed. L.O.L.A., Buenos Aires. Págs. 136-139. [ Links ]
2. ARAMBARRI A. M., S. E. FREIRE, M. N. COLARES, N. D. BAYÓN, M. C. NOVOA, C. MONTI & S. A. STENGLEIN. 2006. Leaf anatomy of medicinal shrubs and trees from Gallery forests of the paranaense province (Argentina). Part 1. Bol. Soc. Argent. Bot. 41: 233-268. [ Links ]
3. ARAMBARRI A. M., S. E. FREIRE, N. D. BAYÓN, M. N. COLARES, C. MONTI, M. C. NOVOA & M. P. HERNÁNDEZ. 2009. Morfoanatomía foliar de árboles medicinales de la Provincia Biogeográfica de las Yungas (Argentina). Bol. Latinoam. Caribe Plant. Med. Aromat. 8: 342-379. [ Links ]
4. BARBOZA G. E., J. J. CANTERO, C. O. NÚÑEZ & L. ARIZA ESPINAR (eds.). 2006. Flora medicinal de la provincia de Córdoba (Argentina). Museo Botánico de Córdoba. Gráficamente ediciones, Córdoba. Págs. 683-684. [ Links ]
5. BARRIENTOS PRIEGO A. F., M. W. BORYS, C. TREJO & L. LÓPEZ. 2003. Índice y densidad estomática foliar en plántulas de tres razas de aguacatero. Rev Fitotecnia Mexicana 26: 285-290. [ Links ]
6. BRAVO S. J. & A. GRAU 1992. Variaciones de la densidad estomática en poblaciones de Alnus acuminata en un gradiente altitudinal. Lilloa 38: 39-45. [ Links ]
7. BURKART A. 1952. Las leguminosas argentinas. Acme S.A.C.I., Buenos Aires. Pág. 102. [ Links ]
8. CARRIZO E. DEL V., M. O. PALACIO & L. D. ROIC (ex aequo). 2005. Uso medicinal de algunas especies nativas en Santiago del Estero (República Argentina). Dominguezia 21: 25-32. [ Links ]
9. CIALDELLAA. M. 1984. El género Acacia (Leguminosae) en la Argentina. Darwiniana 25: 59-111. [ Links ]
10. CIALDELLA A. M. 1997. Acacia. En: A. T. HUNZIKER (ed.), Flora fanerogámica Argentina. Vol. 35: 1-21., ProFlora-CONICET, Argentina. [ Links ]
11. DEL VALLE PEREA M., G. PEDRAZA & J. DEL VALLE LUCEROS. 2007. Relevamiento de la flora arbóreaautóctona en la provincia de Catamarca. Consejo Federal de Inversiones, Buenos Aires. Págs. 38-40. [ Links ]
12. DEMAIO P., U. O. KARLIN & M. MEDINA. 2002. Árboles nativos del centro de Argentina, Ed. L.O.L.A., Buenos Aires. Págs. 1-210. [ Links ]
13. DIGILIO A. P. & P. LEGNAME. 1966. Los árboles indígenas de la provincia de Tucumán. Fundación Miguel Lillo, Tucumán. Opera Lilloana 15: 27. [ Links ]
14. DIZEO DE STRITTMATTER C. 1973. Nueva técnica de diafanización. Bol. Soc. Argent. Bot. 15: 126-129. [ Links ]
15. ESAU K. 1982. Anatomía de las plantas con semilla. Ed. Hemisferio Sur, Buenos Aires. Págs. 339-341. [ Links ]
16. GAYA. P. & R. G. HURD. 1975. The influence of light on stomatal density in the tomato. New Phytol. 75: 37-46. [ Links ]
17. GONZÁLEZ J. A. 1992. Influencia de la intensidad de la luz sobre la distribución de materia orgánica y características morfológicas en una especie de alta montaña: Oenothera nana Griseb. Lilloa 38: 47-54. [ Links ]
18. GUNN C. R. 1984. Fruits and seeds of genera in the subfamily Mimosoideae (Fabaceae) USDA, Maryland. Techn. Bull. 1681: 194. [ Links ]
19. HIERONYMUS J. 1882. Plantae diaphoricae florae argentinae. Bol. Acad. Cienc. Córdoba 4: 199-598. [ Links ]
20. MA Q. W., C. S. LI, F. L. LI & S. V. VICKULIN. 2004. Epidermal structures and stomatal parameters of Chinese endemic Glyptostrobus pensilis (Taxodiaceae). Bot. J. Linn. Soc. 146: 153-162. [ Links ]
21. MARTÍNEZ CROVETTO R. 1981. Las plantas utilizadas en medicina popular en el noroeste de Corrientes. Fundación Miguel Lillo. Miscelánea 69: 7-139. [ Links ]
22. METCALFE C. R. & L. CHALK. 1979. Anatomy of the Dicotyledons. Vol 1. Clarendon Press, Oxford. Págs. 97-117. [ Links ]
23. PEÑA-CHOCARRO M. C., J. DE EGEA JUVINEL, M. VERA, H. MATURO & S. KNAPP. 2006. Guía de árboles y arbustos del Chaco húmedo. The Natural History Museum, Guyra Paraguay, Fundación Moisés Bertoni y Fundación Hábitat y Desarrollo: Asunción. Págs. 222-223. [ Links ]
24. POOLE I., J. D. B. WEYERS, T. LAWSON & J. A. RAVEN. 1996. Variations in stomatal density and index: implications for palaeoclimatic reconstructions. Pl. Cell Environm. 19: 705-712. [ Links ]
25. ROJAS ACOSTA N. 1907. Catálogo de las plantas medicinales del Chaco austral. Tipografía de P Gadola. Buenos Aires, pp. 20. [ Links ]
26. ROYER D. L. 2001. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 114: 1-28. [ Links ]
27. SALAS J. A., M. E. SANABRIA & R. PIRE. 2001. Variación en el índice y densidad estomática en plantas de tomate (Lycopersicon esculentum Mill.) sometidas a tratamientos salinos. Bioagro 13: 99-104. [ Links ]
28. SALISBURY E. 1927. On the causes and ecological significance of stomatal frequency with special refence to the woodland flora. Phil. Trans. R. Soc. Lond., Ser B 216: 1-65. [ Links ]
29. STACE C. A. 1965. Cuticular studies as an aid to plant anatomy. Bull. Br. Mus. (Nat. Hist.) Bot. 4: 1-78. [ Links ]
30. STENGLEIN S. A., A. M. ARAMBARRI, M. N. COLARES, M. C. NOVOA & C. E. VIZCAÍNO. 2003. Leaf epidermal characteristics of Lotus subgenus Acmispon (Fabaceae: Loteae) and a numerical taxonomic evaluation. Canad. J. Bot. 81: 933-944. [ Links ]
31. STENGLEIN S. A., A. M. ARAMBARRI, M. C. MENÉNDEZ SEVILLANO & P. A. BALATTI 2005. Leaf epidermal characters related with plant´s passive resistance to pathogens vary among accessions of wild beans Phaseolus vulgaris var. aborigineus (Fabaceae-Phaseoleae). Flora 200: 285-295. [ Links ]
32. STRASBURGER E., F. NOLL, H. SCHENCK & A. F. W. SCHIMPER. 1994. Tratado de Botánica. (Sitte P., Ziegles H., Ehrendorfer F., Bresinsky A., eds.) Ed. Omega, Barcelona. Págs. 477-487. [ Links ]
33. TOURSARKISSIAN M. 1980. Plantas medicinales de la Argentina. Ed. Hemisferio Sur, Buenos Aires. Pág. 66. [ Links ]
34. VASSAL J. 1981. Tribe 4. Acacieae Benth. (1842), nom. conserve. prop. In: R. M. Pholhill & P. H. Raven (eds.), Advances in legume systematic Part 1: 169-171. Royal Botanic Gardens, Kew, London. [ Links ]
35. WOODWARD F. I. 1987. Stomatal numbers are sensitive to increases in CO2 from preindustrial levels. Nature 327: 617-618. [ Links ]
36. WOODWARD F. I. & F. A. BAZZAZ. 1988. The responses of stomatal density to CO2 partial pressure. J. Exp. Bot. 39: 1771-1781. [ Links ]
37. ZULOAGA F. O., O. MORRONE & M. J. BELGRANO (eds.). 2008. Catálogo de las plantas vasculares del Cono Sur (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). II. Monogr. Syst. Bot. Missouri Bot. Gard. 107: 1905-1908. [ Links ]
Recibido el 23 de junio de 2010
Aceptado el 25 de octubre de 2010.














