Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.45 no.3-4 Córdoba jul./dic. 2010
PALEOBOTANY AND PALYNOLOGY
Palynotaxonomy of Brazilian Viguiera (Asteraceae) Species
Mara Angelina Galvão Magenta1, Alessandra De Deus Nunes2, Cláudia Barbieri Ferreira Mendonça2 y Vânia Gonçalves-Esteves2
1 Universidade Santa Cecília, R. Oswaldo Cruz 266, Boqueirão, Santos, 11045-907, Brazil, maramagenta@ unisanta.br
2 Museu Nacional, Quinta da Boa Vista, São Cristóvão, Rio de Janeiro, 20940-040, Brazil.
Summary: With the aim of improving inter-specific delimitation of the genus Viguiera Kunth in Brazil, a palynological analysis was undertaken with 27 taxa, representing around 77% of all occurring species. Samples were obtained from herbarium specimens and the pollen grains were analyzed through light and scanning electron microscopy. Characteristics, including the shape of the pollen grains, polar and equatorial diameter, aperture measurements, surface ornamentation and exine thickness, were registered and compared. The pollen grains were medium-sized (25 - 50 mm), isopolar and oblate spheroidal (prolate spheroidal in V. aspilioides Baker). The observed values corroborated the synonymity of some species and also highlighted certain micro-morphological differences, such as polar diameter and the dimensions of the colpus and endoaperture, thus confirming the groups of species delimitation obtained by morphologic and phylogenetic analyses.
Kew words: Asteraceae; Brazil; Compositae; Pollen; Taxonomy; Viguiera.
Resumo: Palinotaxonomia de espécies brasileiras de Viguiera (Asteraceae-Heliantheae). Com a finalidade de obter subsídios para a delimitação interespecífica do gênero Viguiera Kunth no Brasil, foi efetuado um estudo palinológico com 27 táxons, representando cerca de 77% das espécies ocorrentes. As amostras foram obtidas de material herborizado e o grão de pólen foi analisado sob microscopias óptica e eletrônica de varredura. Os grãos de pólen foram caracterizados quanto à forma, às dimensões e tipo da abertura, à ornamentação da superfície e à espessura da exina. Os grãos de pólen são médios (25 - 50mm), isopolares, oblato-esferoidais (prolato-esferoidais em V. aspilioides Baker), tricolporados, endoabertura lalongada, sexina espinhosa. Os valores obtidos corroboraram a sinonimização de algumas espécies e diferenças micro-morfológicas, tais como os valores do diâmetro polar, as dimensões do colpo e da endoabertura confirmaram algumas delimitações de grupos de espécies obtidas em análises filogenéticas de morfologia.
Palavras-chave: Asteraceae; Compositae; Pólen; Taxonomia; Viguiera
INTRODUCTION
Viguiera Kunth sensu Blake (1918) is a Neotropical genus with more than 140 species belonging to the subtribe Helianthinae (Heliantheae - Asteraceae), with representatives occurring in an area stretching from southeastern North America to southern South America. In South America, the species of Viguiera are distributed from the equator to about 40º. In Brazil, the genus occurs mainly in the Brazilian Cerrado.
Over recent decades, several proposals to divide the genus have been put forward, mainly based on molecular data (Schilling & Jansen, 1989; Schilling & Panero, 1996a, b; Schilling et al., 2002). These authors agree that the Brazilian species form a sole cohesive group together with certain representatives from other South American countries. Nevertheless, problems in specific and even generic delimitation are still prevalent. Panero (2007) stated that without a doubt, when there were more available data, the classification of Viguiera will undergo modifica-tions regarding delimitation and phylogenetic relations with other genera. The proposed sinonimization of Viguiera in Rhysolepis, by Robinson & Moore (2004) has no significant base (Magenta, 2006; Magenta et al., 2010).
Palynological analysis represents an efficient tool in taxonomical studies of the family Asteraceae (Wodehouse, 1926, 1928; Wells, 1971; Tomb et al., 1974; Feuer & Tomb, 1977; Vezey et al., 1994; Perveen, 1999; Qreshi et al., 2002; Wortley et al., 2007). In the tribe Heliantheae, the pollen grains present a structural pattern that has been named Helianthoid (Skvarla & Larson, 1965a, b; Skvarla & Turner, 1966, 1969; Skvarla et al., 1977). Such a pattern is also found in the tribes Eupatorieae, Astereae, Helenieae and Calenduleae, as well as in certain taxa from Inuleae senso lato, Senecioneae and Anthemideae, and is characterized by caveate exines and an internal foramen. Several works on pollinic morphology have presented relevant results for Heliantheae taxonomy (Wodehouse, 1928; Felippe & Salgado-Labouriau, 1964; Wells, 1971; Horner & Pearson, 1978; Melhem et al., 1979; Gonçalves-Esteves & Esteves, 1989; Meo & Khan, 2006). However, it is necessary to take into consideration which pollinic feature is best suited for use when analyzing the systematics of each group. For example, when working with spiny pollen grains, Felippe & Salgado-Labouriau (1964) proposed employing the presence or absence of perforations in the spines in order to differentiate species. Nevertheless, as the authors themselves noted, Bidens gardneri Baker is capable of presenting multiple types, as outlined in the work: some pollen grains have only one cavity, and others have two, whereas several have solid spines. Because of this, Skvarla et al. (1977) and Bolick et al. (1984) rejected the use of this feature in the Heliantheae tribe. Even though it is necessary to undertake isolated studies for the diverse taxa, palynological characteristics not only furnish additional information but also further systematic analyses (Qreshi et al., 2002).
The only analysis of groups of Brazilian species of Viguiera was done by Gonçalves-Esteves & Esteves (1989), who examined 10 species. The authors situated the pollen in the 'Aspilia' type, a term that was adopted by Salgado-Labouriau (1973) to designate the morphology of the pollen of V. robusta Gardner, which is similar to that of Aspilia Thouars (Ecliptinae). The pattern is defined by pollen grains that are oblate spheroidal to prolate spheroidal and tricolporate, with nexine and sexine that are separated by a cavity and joined only at the edges of the openings, and sexine tegillate.
The aim of this study is to establish the palynological differences between species and the similarities between groups of species belonging to the genus Viguiera in Brazil.
MATERIALS AND METHODS
A palynological analysis was undertaken with 27 taxa (Appendix), which represent around 77% of all Viguiera species occurring in Brazil. The other species present in Brazil were not analyzed because it was not possible to find samples with mature anthers. Fertile anthers from flowers in anthesis and/or well developed flower-buds were removed from sheets deposited in the following herbaria (acronyms according to Holmgren et al.,1990): BR, CPAP, D, HAS, HASU, HEPH, HUEPG, HUFU, IBGE, ICN, IPA, K, MBM, P, R, RB, SP, SPF, SPFR, SPSF, TENN, TEX, UB, UEC, US.
Wherever possible, an attempt was made to analyze pollen grains from three specimens of the same species. One of these was chosen as the standard (material that has withstood the process of acetolysis and provided sufficient quantity in the histological slide - indicated in the examined material by means of an asterisk) for measurements, pollinic descriptions and illustrations. The remaining specimens were used for comparing results. Pollen grains were prepared according to the Erdtman (1952) acetolythic method. Acetolyzed pollinic material was used for obtaining photomicrographs. For scanning electron microscopy (SEM) studies, non-acetolysed and acetolysed (see legends) pollen samples were mounted on stubs and coated with gold-platinum. The analysis was done by using a JSM-5310 microscope at the Hertha Meyer Cell Ultrastructure Laboratory at the Institute of Biophysics (Universidade Federal do Rio de Janeiro).
For most species, twenty-five pollen grains from standard materials were divided into equatorial sections (polar diameter = PD and equatorial diameter = ED) and subjected to measurements. The results were analyzed statistically, using a calculator, in order to calculate the arithmetic mean (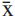 ), standard deviation of the sample (s), standard deviation of the mean (sx), coefficient of variability (CV%) and confidence interval (CI) to 95%. For measurements of the remaining characteristics, such as the equatorial diameter in polar view (EDPV), the apocolpium side (AS), the polar area index (PAI), the openings and the exine, the arithmetic means of 10 measurements were calculated. This was also performed for measurements of the diameters of pollen grains of the comparison material.
), standard deviation of the sample (s), standard deviation of the mean (sx), coefficient of variability (CV%) and confidence interval (CI) to 95%. For measurements of the remaining characteristics, such as the equatorial diameter in polar view (EDPV), the apocolpium side (AS), the polar area index (PAI), the openings and the exine, the arithmetic means of 10 measurements were calculated. This was also performed for measurements of the diameters of pollen grains of the comparison material.
Adopted terminology and pollinic descriptions were applied according to criteria defined by Barth& Melhem (1988) and Punt et al. (2007) to take into consideration size, shape, number of openings, and sexine ornamental pattern. Denomination and size of the polar area and the size of the aperture are in accordance with the classification established by Faegri & Iversen (1966) for the polar area index.
The decision to keep the name Viguiera instead Rhysolepis, as proposed by Robinson & Moore (2004) for the species in Brazil, is based on the results of cladistic analysis using morphological features of Magenta (2006). In these analisys, Rhysolepis and Viguiera species from Brazil emerged in different clades.
The species full names are cited in the appendix.
RESULTS
The pollen grains were medium-sized (25 - 50 mm), isopolar, oblate spheroidal, (prolate spheroidal in the case of Viguiera aspilioides) (Table 1), and tricolporate with a small (0.26 - 0.32 mm) to very small (0.21 - 0.25 mm) polar area (Table 2), subcircular amb and spiny sexine.
Table 1: Measurements (in m) of pollen grains of Viguiera, in equatorial section (n = 25)1

Table 2 - Means (in m) of pollen grains from Viguiera species, in polar section (n = 10)2

The lowest values of the confidence interval of the polar diameter in equatorial section (Table 1) were found in Viguiera veredensis (32.3 - 33.1 mm), and the highest values were found in V. macrorhiza and V. oblongifolia (41.1 - 42.0 mm).
The colpi were long to very long, narrow with acute extremities and had a notably elongated endoape. The extremities were acute (Fig. 2 D, M) and obtuse only in V. aspilioides (Fig. 1 K), V. bracteata (Fig. 1 P) and V. pilosa (Fig. 3 B). The longest colpus (ca. 18.0 mm) was found in V. filifolia, and the shortest (ca. 11.3 mm) was found in V. aspilioides (Table 3). The presence of a granulated membrane was observed in colpi in SEM (Figs. 1 C, 2 N, 3 N).

Fig. 1. Photomicrographs and electromicrographs of Viguiera species. A-C: V. amphychlora. A: Polar view, optical section. B, C: Equatorial view, aperture and surface. D-F: V. anchusifolia (*). D: Polar view, optical section. E, F: Equatorial view, aperture and surface. G-I: V. arenaria. G: Polar view, optical section. H: Equatorial view, optical section. I: Equatorial view, surface detail. J-L: V. aspilioides (*). J: Polar view, optical section. K: Equatorial view, optical section. L: Equatorial view, aperture and surface. M, N: V. bakeriana (*). M: Polar view, optical section. N: Equatorial view, aperture and surface. O, P: V. bracteata. O: Polar view, optical section. P: Equatorial view, optical section. Q, R: V. corumbensis (*). Q: Polar view, optical section. R: Equatorial view, aperture and surface. S, T: V. gardneri. S: Polar view, optical section. T : E quatorial view, optical section. Scale bars. Photomicrographs = 10 µm; electromicrographs = 5 µm. (*) acetolized for SEM.

Fig. 2. Photomicrographs and electromicrographs of Viguiera species. A, B: V. grandiflora (*). A: Polar view, optical section. B: Equatorial view, aperture and surface. C, D: V. hispida. C: Polar view, optical section. D: Equatorial view, aperture. E-H: V. kunthiana (*). E: Polar view, optical section. F: Polar view, surface at the region of the apocolpium. G: Equatorial view, aperture. H: Equatorial view, detail of the surface and aperture. I-K: V. macrorhiza. I: Polar view, optical section. J: Equatorial view, aperture. K: Equatorial view, aperture and surface. L-O: V. megapotamica. L: Polar view, optical section. M: Equatorial view, aperture. N: Equatorial view, aperture and surface. O: Equatorial view, detail of the surface. P, Q: V. nudibasilaris. P: Polar view, optical section. Q: Equatorial view, aperture. R-T: V. paranensis. R: Polar view, optical section. S: Equatorial view, aperture. T: Equatorial view, detail of the surface. Scale bars. Photomicrographs = 10 µm; electromicrographs = 5 µm. (*) acetolized for SEM.

Fig. 3. Photomicrographs and electromicrographs of Viguiera species. A, B: V. pilosa. A: Polar view, optical section. B: Equatorial view, optical section and aperture. C, D: V. robusta. C: Polar view, optical section. D: Equatorial view, aperture. E-G: V. rubra. E: Polar view, optical section. F: Equatorial view, aperture. G: Equatorial view, aperture and surface. H, I: I. V. santacatarinensis. H: Polar view, optical section. I: Equatorial view, aperture. J, K: V. squalida. J: Polar view, optical section. K: Equatorial view, optical section. L, M: V. tenuifolia. L: Polar view, optical section. M: Equatorial view, aperture and surface. N, O: V. trichophylla (*). N: Polar view, optical section. O: Equatorial view, aperture and surface. P-R: V. tuberosa. P: Polar view, optical section. Q: Polar view, surface at the region of the apocolpium. R: Equatorial view, optical section. S, T: V. veredensis. S: Polar view, optical section. T: Equatorial view, aperture. Scale bars. Photomicrographs = 10 µm; electromicrographs = 5 µm. (*) acetolized for SEM.
Table 3 - Means (in m) of pollen grain apertures and exine of Viguiera species (n = 10);
* total exine including spine

The exine were thick, contained a cavity [the widest cavity (ca. 1.5 mm) was found in Viguiera nudibasilaris and the narrowest (ca. 0.8 mm) in V. grandiflora], simplicolumellate, and had long and narrow spines presenting perforations at the base. The distance between the spines was ca. 8.0 mm (Fig. 2 H, Q, T). Both the layer of columellae and the cavity were pronounced. The sexine was always thicker than the nexine.
The spines were conical (Figs. 1 L, 2 E, 3 I) or present as projections at the base (Figs. 1 F, 2 A, 3 G). The longest, widest (ca. 11.0 x 6.1 mm) and farthest apart (ca. 10.4 mm) were encountered in Viguiera oblongifolia (Table 3). The shortest spines (ca. 5.0 mm) were found in V. veredensis. The narrowest (ca. 2.5 mm) was found in V. rubra, and V. amphychlora had spines that were the closest together (ca. 7.1 mm) (Table 3).
When analyzing the results obtained for comparison to the respective standard material (Table 4), it was evident that both the shape and size of the pollen grains were constant except in the case of Viguiera aspilioides, which presented a variation in form from prolate spheroidal (standard material) to oblate spheroidal (comparison material).
Table 4 - Means (in m) of the diameters of pollen grains in equatorial section (polar and equatorial diameter), polar view (equatorial diameter, side of the apocolpium), relationship of polar and equatorial diameters and the shape of material for comparison from Viguiera species (n = 10)3

By using pollinic characteristics, it was possible to separate species into four groups when applying confidence interval values to 95% of the polar diameter. Most species are included in the fourth group and this one was divided into sub-groups as shown below.
Identification key for groups of species
I - CI 95% polar diameter = 41.1 - 42.0 mm: V. macrorhiza, V. oblongifolia
II- CI. 95% polar diameter = 39.3 - 40.6 mm: V. aspilioides, V. paranensis, V. pilosa, V. santacatarinensis
III-CI 95% polar diameter = 32.3 - 33.1 mm: V. veredensis
IV - CI 95% polar diameter = 34.0 - 39.2 mm
IV.1- length of colpus = 11.3 - 14.9 mm
a- width of endoaperture = 5.5 mm: V. discolor
b- width of endoaperture = 10.3 - 11.7 mm
b.1- small polar area: V. bracteata (0.27), V. gardneri (0.28), V. nudibasilaris (0.25), V. tuberosa (0.26)
b.2-very small polar area: V. bakeriana (0.21), V. megapotamica (0.24)
c- width of endoaperture = 12.0 - 14.0 mm
c.1- small polar area: V. corumbensis (0.28), V. tenuifolia (0.28)
c.2- very small polar area: V. arenaria (0.23) V. rubra (0.24)
IV.2- length of colpus = 15.8 - 18.0 mm
a- width of endoaperture = 11.3 - 11.7 mm
a.1- length of spine = 6.0 mm: V. grandiflora
a.2- length of spine = 7.4 mm: V. trichophylla
b- width of endoaperture = 12.0 - 13.2 mm: V. amphychlora (0.26), V. anchusifolia (0.29), V. filifolia (0.29), V. hispida (0.25), V. kunthiana (0.32), V. robusta (0.30), V. squalida (0.29)
DISCUSSION AND CONCLUSIONS
Pollinic data on Viguiera are sparse in the literature. Based on the results obtained in the present study, the genus can be characterized by the presence of medium-sized, oblate-spheroidal and tricolporate pollen grains with a small to very small polar area, long to very long colpi, notably elongated endoapertures in most species and spiny exine with a pronounced cavity. Even though there were discrepancies between the groups that were obtained based on their pollinic characteristics and those based on similarities in the external phenotype, variations in the size of the pollen grains (expressed in confidence interval values), polar area and aperture sizes (colpus and endoaperture) were found to contribute to our understanding of the taxonomy of the genus and have proved to be extremely useful in the delimitation of various species.
Viguiera hilairei was originally regarded as a valid species (Blake, 1918). In a taxonomic study undertaken by Magenta (2006) for re-delimitation of Brazilian species, it was noted that V. hilairei should be considered synonymous with V. bracteata. The pollinic analysis elaborated herein corroborated this position.
Viguiera anchusifolia can present a vegetative portion similar in shape to that of V. pilosa. Furthermore, it is apparent that both species form hybrids, thereby complicating their delimitation. Based on the results found here, we have demonstrated that the taxa are pollinically different, with V. anchusifolia (R.I. 95% of polar diameter = 34.8 - 35.2 mm) remaining in the IV.2.b.2 group, and V. pilosa (R.I. 95% of polar diam-eter = 39.3 - 39.7 µm) can be placed in group II (pollinic groups herein established).
Certain species are very similar morphologically and their separation only becomes possible after detailed observation of reproductive characteristics. In herbaria, Viguiera arenaria, V. gardneri and V. rubra are often confused with V. robusta but can also be distinguished by taking into consideration pollinic characteristics related to polar diameter and width of the endoaperture. The width of the endoaperture of pollen grains has provided a new measure for delimiting several of these taxa, as shown in the following cases.
In group IV.1 (length of the colpus = 11.3 - 14.9 mm), Viguiera discolor is placed in subgroup a (width of the endoaperture = 5.5 mm) and V. bakeriana (width of the endoaperture within the range 10.3 - 11.7 mm) in subgroup b.
In group IV.2 (length of the colpus = 15.8 - 18.0 mm), Viguiera grandiflora is allocated to subgroup a (width of the endoaperture = 11.3 - 11.7 mm) and V. squalida to subgroup b (width of the endoaperture = 12.0 - 13.2 mm). In this same group, V. trichophylla is placed in subgroup a, and V. filifolia in sub-group b. Each of these pairs of species is very similar morphologically.
Other species of difficult delimitation are: Viguiera tenuifolia (group IV.1) and V. kunthiana (group IV.2); V. megapotamica (group IV.1) and V. anchusifolia (group IV.2); V. aspilioides (group II) and V. tuberosa (group IV.1). As revealed in the present study, these taxa present marked morphopollinic characteristics.
Felippe & Salgado-Labouriau (1964) and Salgado-Labouriau (1973) dealt with Heliantheae (Asteraceae) species from the Cerrado, including Viguiera arenaria and V. robusta, which fell into the 'Aspilia' type. According to the authors, this type is common throughout the Heliantheae tribe and is basically characterized by the shape of the pollen grains (oblate spheroidal to prolate spheroidal) as well as by the amb with around 12 spines, the type of aperture (tricolporate) and by the spiny exine with a cavity. Results presented here describing these species are similar to those reported by the above-mentioned authors; the only difference is the values of the diameters of pollen grains.
When evaluating systematic implications through the use of transmission electron microscopy in studies on Asteraceae, Skvarla & Turner (1966) made use of 184 species from 11 tribes, including Viguiera dentata (Cav.) Spreng., to which the genus-type belongs. According to these authors, in the Heliantheae tribe the basal layer is always very thin and the presence of the cavity in all species is common. These characteristics were demonstrated in the current study.
Gonçalves-Esteves & Esteves (1989) considered the pollen grains of these species to be homogeneously medium to large-sized with conical spines or spines possessing base projections. The spines were described as tegillum types that are 'hollow', meaning they have a perforation in the base, or 'simple', meaning that they are solid. The present study presents different results regarding pollen grain size and the reporting of the existence of the cavity. However, there is agreement with respect to the 'Aspilia' pollinic type for all of the analyzed Viguiera species.
The pollen grain, when considered together with its attributes, revealed itself to be an important diagnostic feature. This was mainly true at the specific level where, besides classes of size of the confidence interval of the polar diameter and polar area type, characteristics like the shape and size of the apertures could be used to identify species and corroborate many of the results presented in the Magenta (2006) taxonomic studies.
ACKNOWLEDGMENTS
Our gratitude goes to the Cell Ultrastructure Laboratory at the Institute of Biophysics, Rio de Janeiro Federal University (UFRJ) and with special thanks to Noêmia Rodrigues Gonçalves for his technical support with the scanning electron microscopy. Furthermore, we wish to thank FAPERJ (proc. E-26/171.071/03) and CNPq (proc. 481595/2004-0) for the third author's productivity scholarship and CAPES for the first author's doctorate scholarship.

BIBLIOGRAPHY
1. BARTH, O. M. & T. S. MELHEM. 1988. Glossário ilustrado de palinologia. Unicamp, Campinas. [ Links ]
2. BLAKE, S. F. 1918. A revision of the genus Viguiera. Contr. Gray Herb. 54: 1-205. [ Links ]
3. BOLICK, M. R., J. J. SKVARLA & B. L. TURNER. 1984. On cavities in spines of Compositae pollen - A taxonomic per-spective. Taxon 33: 289-295. [ Links ]
4. ERDTMAN, G. 1952. Pollen morphology and plant taxonomy. Angiosperms. Almquist & Wiksell, Stockholm. [ Links ]
5. FAEGRI, K. & J. IVERSEN. 1966. Textbook of modern pollen analysis. 2nd ed. Scandinavian University Books, Copenhagen. [ Links ]
6. FELIPPE, G. M. & B. L. SALGADO-LABOURIAU. 1964. Pollen Grains of the "Cerrado" - VI - Compositae - Tribus Heliantheae. Anais Acad. Brasil. Ci. 36: 85-101. [ Links ]
7. FEUER, S. & A. S. TOMB. 1977. Pollen morphology and detailed structure of family Compositae, tribe Cichorieae II. Subtribe Microseridinae. Amer. J. Bot. 64: 230-245. [ Links ]
8. GONÇALVES-ESTEVES, V. & R. L. ESTEVES. 1989. Contribuição ao estudo polínico da tribo Heliantheae (Compositae) VI. Bol. Mus. Nac. Rio de Janeiro, Bot. 80: 1-11. [ Links ]
9. HOLMGREN, P. K., B. L. HOLMGREN & L. G BAINETT. 1990. Index Herbariorum. Part. 1. The Herbaria of the world. New York Botanical Garden, New York. [ Links ]
10. HORNER, H. T. JR. & C. B. PEARSON 1978. Pollen wall and aperture development in Helianthus annus (Compositae -Heliantheae). Amer. J. Bot. 65: 293-309. [ Links ]
11. MAGENTA, M. A. G. 2006. Viguiera Kunth (Asteraceae -Heliantheae) na América do Sul e sistemática das espécies do Brasil. PHd Thesis, Instituto de Biociências, Universidade de São Paulo, Brazil (unpublished). [ Links ]
12. MAGENTA, M. A. G., J. R. PIRANI & C. A. MONDIN. 2010. Novos táxons e combinações de Viguiera Kunth (Asteraceae - Heliantheae) no Brasil. Rodriguesia. 61: 01-11. [ Links ]
13. MELHEM, T. S., M. S. F. SILVESTRE & H. MAKINO. 1979. Grãos de pólen de plantas alergógenas. Hoehnea 8: 73-100. [ Links ]
14. MEO, A. A. & M. A. KHAN. 2006. Pollen morphology as an aid to identification of Crhysanthemum species (Compositae -Anthemideae) from Pakistan. Pak. J. Bot. 38: 29-41. [ Links ]
15. PANERO, J. L. 2007. Compositae: Tribe Heliantheae. In: KADEREIT, J.W. & C. JEFFREY (eds.), Families and genera of vascular plants, vol. 8, pp. 440-477. Springer-Verlag, Berlin, Heidelberg. [ Links ]
16. PERVEEN, A. 1999. Contributions to the pollen morphology of the family Compositae. Turk. J. Biol. 23: 523-535. [ Links ]
17. PUNT, W., S. BLACKMORE, S. NILSSON & A. LE THOMAS. 2007. Glossary of pollen and spore terminology. Rev. Paleobot. Palynol. 143: 1-81. [ Links ]
18. QRESHI, S. J., A. G. AWAN, M. A. KHAN & S. BANO. 2002. Palynological study of the genus Sonchus from Pakistan. Pakistan J. Biol. Sci. 2: 98-105. [ Links ]
19. ROBINSON, H. & A. J. MOORE 2004. New species and new combinations in Rhysolepis (Heliantheae - Asteraceae). Proc. Biol. Soc. Washington 117: 423-446. [ Links ]
20. SALGADO-LABOURIAU, M. L. 1973. Contribuição à palinologia dos cerrados. Academia Brasileira de Ciências, Rio de Janeiro. [ Links ]
21. SCHILLING, E. E. & R. K. JANSEN. 1989. Restriction fragment analysis of chloroplast DNA and the systematics of Viguiera and related genera (Asteraceae-Heliantheae). Amer. J. Bot. 76: 1769-1778. [ Links ]
22. SCHILLING, E. E., R. K. JANSEN & J. L. PANERO. 2002. A revised classification of subtribe Helianthinae (Asteraceae-Heliantheae). I. Basal lineages. Bot. J. Linn. Soc. 140: 65-76. [ Links ]
23. SCHILLING, E. E. & J. L. PANERO. 1996a. Relationships in Heliantheae subtribe Helianthinae based on chloroplast DNA restriction site analysis. In: HIND, D. J. & H. J. BEENTJE (eds.), Compositae: systematics proceedings of the international Compositae conference, vol. 1, pp 361-376. Royal Botanic Gardens, Kew. [ Links ]
24. SCHILLING, E. E., & J. L. PANERO. 1996b. Phylogenetic reticulation in subtribe Helianthinae. Amer. J. Bot. 83: 939-948. [ Links ]
25. SKVARLA, J. J. & D. A. LARSON. 1965a. An electron microscopy study of pollen morphology in the Compositae with special reference to Ambrosiinae. Grana Palynol. 9: 50-62. [ Links ]
26. SKVARLA, J. J. & D. A. LARSON. 1965b. Interbedded exine components in some Compositae. Southw. Naturalist 10: 65-68. [ Links ]
27. SKVARLA, J. J. & B. L. TURNER. 1966. Systematic implications from electron microscope studies of Compositae pollen - a review. Ann. Missouri Bot. Gard. 3: 220-256. [ Links ]
28. SKVARLA, J. J. & B. L. TURNER. 1969. Fine structure of Petrobinae (Compositae-Heliantheae) pollen walls. Amer. J. Bot. 56: 418-419. [ Links ]
29. SKVARLA, J. J., B. L. TURNER & V. C. PATEL. 1977. Pollen morphology in the Compositae and morphologically related families. In: HEYWOOD V. H., J. B. BARBONE & B. L. TURNER (eds.), The biology and chemistry of the Compositae, pp. 141-217. Academic Press, London. [ Links ]
30. TOMB, A. S., D. A. LARSON & J. J. SKVARLA. 1974. Pollen morphology and detailed structure of family Compositae, tribe Cichorieae I. Subtribe Stephanomeriinae. Amer. J. Bot. 61: 486-498. [ Links ]
31. VEZEY, E. L., L. E. WATSON & J. J. SKVARLA. 1994. Plesiomorphic and apomorphic pollen structure characteristics of Anthemideae (Asteroideae: Asteraceae). Amer. J. Bot. 81: 648-657. [ Links ]
32. WELLS, J. R. 1971. Variations in Polymnia pollen. Amer. J. Bot. 58: 124-130. [ Links ]
33. WODEHOUSE, R. P. 1926. Pollen grain morphology in classification of Anthemidae. Bull. Torrey Bot. Club. 53: 479-485. [ Links ]
34. WODERHOUSE, R. P. 1928. Pollen grains in the identification and classification of plants II. Barnadesia. Bull. Torrey Bot. Club. 55: 449-462. [ Links ]
35. WORTLEY, A. H., V. A. FUNK, H. ROBINSON, J. SKVARLA & S. BLACKMORE. 2007. A search for pollen morphological synapomorphies to classify rogue genera in Compositae (Asteraceae). Rev. Paleobot. Palynol 146: 169-181. [ Links ]
Recibido el 31 de marzo de 2009
Aceptado el 19 de julio de 2010.














