Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.45 no.3-4 Córdoba jul./dic. 2010
PALEOBOTANY AND PALYNOLOGY
Pollen deposition in tauber traps and surface soil samples in the Mar Chiquita coastal lagoon area, pampa grasslands (Argentina)
Fabiana Latorre1, Claudio F. Pérez2, Silvina Stutz1 y Susana Pastorino1
1Departamento de Biología, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Funes 3250, (7600) Mar del Plata, Argentina. T.E. (+54 223) 475-2426 ext. 444, FAX (+54 223) 475-3150. fabianalatorre@yahoo.com.ar
2Departamento de Ciencias de la Atmósfera y los Océanos, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. Pabellón II, 2º piso, Ciudad Universitaria, (1428), Buenos Aires, Argentina.
Summary: Pollen deposition in Taubertraps and surface soil samples in the Mar Chiquita coastal lagoon area, pampa grasslands (Argentina). Estimations of airborne pollen loadings deposited in Tauber traps were studied in a coastal lagoon from south-eastern Pampa grasslands, Argentina, in order to assess their relationship with surface samples and to interpret the representativeness of local, regional and extraregional vegetation. Three different environments were considered: a coastal dune barrier with a psammophytic community, a salt marsh with a halophytic community in Mar Chiquita lagoon, and a freshwater community at Hinojales freshwater lake. Based on a record of surface samples taken from a previous paper, a parametric model was built to classify Tauber samples gathered from the natural vegetation communities of the study area. Results revealed that just like their surface counterparts, Tauber trap records qualitatively reflect the predominant vegetation types, although ecological groups feature different quantitative representations depending on the record type. Pollen loadings showed that airborne pollen transport was predominantly of local range, in accordance with previous results from the same study area. Airborne - surface samples relationships enrich our knowledge of the present environment that could be useful to improve paleoecological interpretations of the area.
Key words: Modern pollen deposition; Discriminant analysis; Spatio-temporal scales.
Resumen: Depósito polínico en trampas Tauber y muestras de superficie en el área de la laguna costera de Mar Chiquita, pastizales pampeanos (Argentina). Se estimó el depósito polínico atmosférico de trampas Tauber en una laguna costera del sudeste de la estepa pampeana argentina, con el objetivo de analizar su relación con muestras de polen superficial e interpretar la representatividad de la vegetación local, regional y extraregional. Se consideraron tres ambientes diferentes: una barrera costera de dunas con vegetación psamofítica, la marisma de la laguna costera Mar Chiquita, con vegetación halofítica, y la laguna continental Hinojales, con vegetación hidrofítica. En base a las muestras de superficie y análisis de un trabajo previo, se construyó un modelo paramétrico para clasificar las muestras Tauber tomadas en la vegetación natural del área de estudio. Los resultados revelan que como su contraparte en suelo superficial, los registros polínicos Tauber reflejan cualitativamente los tipos de vegetación predominante, aunque los tipos ecológicos difieren cuantitativamente su representación, según el tipo de registro observado. Los datos de polen muestran que el transporte atmosférico de polen es predominantemente local, de acuerdo con resultados previos en la misma área. Las relaciones entre muestras aéreas y de superficie enriquecen nuestro conocimiento del ambiente actual el cual puede ser útil para mejorar las interpretaciones paleoecológicas en el área.
Palabras clave: Depósito polen actual; Análisis discriminante; Escalas espacio-temporales.
INTRODUCTION
When it comes to answering significant questions about ecological changes from the past, interpreting fossil records is crucial. Presently to attain such reconstruction, methods require refined knowledge of the relationships between patterns and processes, which can only be acquired from modern pollen-vegetation analyses at different spatiotemporal scales. Nevertheless, it is known that surface samples may contain several years of pollen accumulation, able to mask seasonal and annual variation. However, experiments have not been able to determine the number of years represented by such pollen content.
Tauber traps have been used in North America and Europe to characterize daily, seasonal and annual pollen dispersal and deposition from grasslands to forests (Tauber, 1977; Markgraf, 1980; Hall, 1990). Moreover, the need of such information has promoted the development of the Pollen Monitoring Program in a clear attempt to refine and standardize techniques to get comparable results of yearly pollen deposition (Hicks et al., 1996, 2001). Studies of such nature are still scarce in South America. In Argentina, available examples are the use of Tauber traps for the construction of pollen calendars of urban or natural areas in different environments (Cuadrado, 1978, 1979; Borromei & Quattrocchio, 1990; García de Albano, 1991; Majas & Romero, 1992; Naab, 1999; Latorre & Caccavari, 2006). Some of them rely on the calibration of the pollen-vegetation relationships on coastal areas (Fontana et al., 2001; Fontana, 2003; Pérez et al., 2009), becoming great contributors to the environmental reconstruction of the Holocene sea level fluctuations (e.g. Stutz et al., 2006; Vilanova et al., 2006). For instance, Stutz & Prieto (2003) found similarities between modern pollen spectra of soil samples from different environments in Mar Chiquita coastal lagoon area (Pampa grasslands, Argentina) and fossil pollen assemblages from a core obtained nearby, which allowed interpreting the area vegetation history. Subsequent vegetation and deposition relationships analyzed using Tauber traps enabled to recognize the contribution of local, regional and extraregional sources as well as the role that seasonal phenology, transport and re-deposition play as significant processes, thereby providing additional information to such interpretation (Pérez et al., 2009). Nevertheless, the progressive development of pollen analysis is also dependent on advancing our understanding of the way in which pollen reaches and incorporates into the sediment. An area of focus in this paper is the distinctive features of the relationship between airborne, soil samples and the vegetation from Mar Chiquita coastal lagoon area. Its ultimate aim is to enrich paleoecological interpretation by seeking an enhanced understanding of the present environment in the southeastern coast of the Pampa grasslands.
Geomorphology, climate and vegetation of the study area
Mar Chiquita coastal lagoon (37º43'S; 57º24'W) and Hinojales freshwater shallow lake (37º34'S, 57º27'W) are located in the SE of Pampa grasslands (Buenos Aires Province, Argentina) (Fig. 1-2). The area features a smooth topography of different geomorphologic origin, which comprises late Pleistocene deflation basins and paleodunes, and Holocene deposition landforms originated during the last sea level fluctuations, ca. 6000 years BP (Schnack et al., 1982; Fasano, 1991; Violante, 1992). From the Atlantic Ocean to inland, three main landforms are recognized: the coastal barrier of sandy dunes and adjacent beaches, the marginal flats whose Holocene deposition process gives rise to the Mar Chiquita coastal lagoon and associated salt marsh, and the Pampa plain with numerous deflation basins, occupied by freshwater bodies like Hinojales lagoon, and associated paleodunes (Stutz & Prieto, 2003).

Fig. 1. Phytogeographic classification system of Argentina: a) based on "Regiones fitogeograficas Argentinas" (Cabrera, 1976, 1994), excluding the Argentine Antarctic Sector (Antarctic Province, Antarctic Domain, Antarctic Region); b) northeast fraction based in a previous version (Cabrera, 1958); c) southeast fraction according to Cabrera and Willink (1973, 1980). All maps are accurately recreated from the originals.
The climate is temperate with annual mean temperature and precipitation of 13.8ºC and 940.6 mm respectively (Mar del Plata Aero-meteorological station, 37º33.6'S, 57º21'W, Servicio Meteorológico Nacional, unpublished). Mean temperature varies from 20ºC in January to 7.3ºC in July. Rainfall occurs mainly from spring to autumn, with a maximum of 112.3 mm in February and a minimum of 45.3 mm in August. During summer, winds are typically from the N, NE and E turning to NW to SW in winter.
Several authors have described and classified the vegetation of the area (Vervoorst, 1967; Cabrera, 1976; León, 1991; Stutz, 2001; Federman, 2003). In the early 20th century, natural vegetation was significantly affected by human settlements, being displaced by agriculture and cattle breeding and by their attendant flora of weeds. Natural vegetation persists in freshwater shallow lakes, in marginal salty flat, and in the coastal barrier of sandy dunes where soils cannot be cultivated. Freshwater lake communities are characterized by emergent, floating-leaf and submerged macrophytes. The dominant species is Schoenoplectus californicus (C.A. Mey.), which grows in a patchy distribution towards the lake centre. Other emergents like Zizaniopsis bonariensis (Balansse and Poitr.), Hydrocotyle bonariensis Lam., H. ranunculoides L.f., Alternanthera philloxeroides (Mart.) Griseb. f. philoxeroides, Buddlegia elegans Cham. & Schltdl. ssp. elegans, Solanum glaucophyllum Desf., Bacopa monnieri (L.) Pennell, Polygonum punctatum Elliott, and Ranunculus apiifolius Pers. grow interspersed within Schoenoplectus patches in the shallowest zones. Near the shore, Ricciocarpus natans (L.), Azolla filiculoides Lam., Lemna valdiviana Phil., and Wolffiella lingulata (Hegelm.) Hegelm. form a dense carpet. Myrioplyllun elatinoides Gaudich. and Ceratophyllum demersum L. develop not only in the deepest parts but also in the shore where no other vegetation exists (S. Stutz & M. González-Sagrario, personal observations). On silty dunes surrounding the lakes, monospecific woodlands of Celtis tala L. constitute the only native arboreal vegetation. On the marginal flat, surrounding Mar Chiquita lagoon, the halophytes Spartina densiflora Brong. and Sarcocornia perennis (Mill.) A.J.Scott, are the main components of an extended halophytic community. Surrounding this zone, Distichlis scoparia (Kunth) and D. spicata (L.) co-dominate, accompanied by Atriplex montevidensis Spreng., Spartina alterniflora, Grindelia discoidea Hook. & Arn., and Limonium brasiliense (Boiss.) Kuntze, among the most important species. In the highest zone, patches of Juncus acutus Guss. also occur with Ambrosia tenuifolia Spreng., Paspalum vaginatum Sw., Hydrocotyle bonariensis, Scirpus cernuus Vahl., Apium sellowianum H. Wolff and Samolus valerandi L.. The coastal barrier is mainly distinguished by psammophytic open vegetation composed of Poaceae, Cyperaceae, and Asteraceae species. Patches of Spartina coarctata Trin. with Calycera crassifolia (Miers) Hicken, Senecio crassiflorus (Poir.) DC. var. crassiflorus and Cakile maritima Scop., as associated species, characterize recently formed dunes facing the beach. Mobile dunes exhibit patches of the pioneer grass Panicum racemosum (P. Beauv.) Spreng. while further inland, on fixed dunes, Adesmia incana Vogel var. incana and Poa lanuginosa Poir. dominate, accompanied by Poa barrosiana Parodi, Hydrocotyle bonariensis, Margyricarpus pinnatus(Lam.) Kuntze, Solidago chilensis Meyen var. chilensis, Oenothera mollissima L., Polygala cyparissias A. St.-Hil. & Moq., Senecio crassiflorus, Ambrosia tenuifolia, Baccharis juncea (Lehm.) Desf., B. Microcephala (Less.) DC., Gnaphalium cheiranthifolium Lam., Daucus montevidensis Link ex Spreng., Androtrichium tryginum (Spreng.) H. Pfeiff., and the adventitious Centaurium pulchellum (Sw.) Druce, Blackstonia perfoliata (L.) Huds., Medicago lupulina L., Melilotus indicus (L.) All. and M. albus Desr. On slightly humid soils Androtrichium tryginum and Tessaria absinthioides (Hook. & Arn.) DC. are dominant, frequently associated with Cortaderia selloana (Schult. & Schult. f.) Asch. & Graebn. which forms almost mono-specific populations. Inter-dune depressions present a coarser vegetation cover, often linked to high water tables quite similar to the freshwater lake communities described above, though with less species. Their main constituents are: Typha angustifolia L., T. latifolia L., Schoenoplectus californicus (C.A. Mey.), Scirpus maritimus L., Carex extensa Gooden. var. vixdentata Kük., Eleocharis montevidensis Kunth and several species of Juncus. Exotic trees (Pinus, Cedrus, Cupressus, Eucalyptus, Populus, and Acacia) were introduced in the area at the beginning of the 20th century in public parks and gardens. On the coastal barrier, plantations of Pinus, Eucalyptus and Acacia are common.
MATERIALS AND METHODS
A combination of atmospheric trapping and surface samples was used to investigate the dynamics of pollen deposition in the study area. Exotic trees were excluded in order to centre solely on natural vegetation. Only pollen types found in both records were reported and used for the classification analysis. Pollen sums and percentages were recalculated based on the shared taxa.
Correspondence between pollen assemblages of surface samples with plant communities, established by Stutz & Prieto (2003) (Fig. 3a), were used to build a parametric model, afterwards employed to classify the airborne pollen samples collected from traps at each vegetation sub-area. The selected technique was a stepwise discriminant analysis (McLachlan, 1992), which extracts a subset of variables into a set of functions that provide the best separation of a priori established groups. Other authors have successfully applied this statistical technique to similar designs, though to different vegetation types (Xu et al., 2009). The statistical method allows the model to be validated by reclassifying the same set of surface samples and by checking its performance against the already known assignment of each sample. Pollen diagrams were created with TGView 2.0.2 (Grimm, 2004).
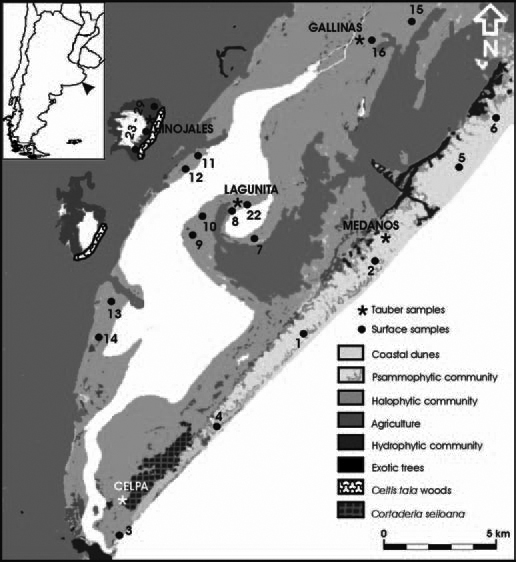
Fig. 2. Vegetation map of the study área and location of surface and Tauber trap samples.
Tauber samples
Tauber traps (Tauber, 1974) were used to assess pollen loading in each area. The traps were placed at five sites corresponding to the main vegetation communities, i.e., Hinojales (freshwater community), Lagunita and Gallinas (halophytic community), Médanos and CELPA (psammophytic community) (Fig. 2). Sampling was carried out at about 1.5 m above ground level in order to enhance the collection of regional pollen (Hicks & Hyvärinen, 1986) and to avoid surrounding vegetation interference that could result in percentages overestimation. The sampling period ranged from November 1994 to September 1996, when it was interrupted due to logistic problems. Whenever feasible, traps were emptied on a monthly basis so as to avoid trap loss due to vandalism or any other unexpected circumstances that could arise after longer exposure periods. Notwithstanding the precautions taken, several traps were lost as a result of cattle or inhabitants destruction (CELPA: 10/95, 12/95, 1/96; Médanos: 11/95, 12/95, 8/96; Hinojales: 12/94, 11/95, 12/95 and Lagunita: 7/96).
Particulate matter entering the trap orifice was collected in10 ml of fluid comprising 1% phenol in glycerol. Collected residues were processed following standard procedures: sieving through a 250 μ mesh, HF and acetolisis (Faegri & Iversen, 1992). Two Lycopodium clavatum tablets were added to each sample before treatment for subsequent calculation of pollen loadings (Stockmarr, 1971). A minimum of 300 grains was counted on each sample. The available literature (Heusser, 1971; Markgraf & D'Antoni, 1978; Erdtman, 1971) and the pollen reference collection of the Laboratory of Palaeoecology and Palynology, Universidad Nacional de Mar del Plata, served to conduct the identification analysis.
Pollen types were grouped into ecological groups in line with Stutz & Prieto (2003). Caryophyllaceae, Rosaceae (eg. Margyricarpus pinnatus (Lam.) Kuntze), Rutaceae, Calycera crassifolia Hicken, and Adesmia incana Vogel were included as psammophytic types whereas, Solanaceae (eg. Solanum glaucophyllumDesf.), Typha, Alternanthera philoxeroides, Myriophyllum, Azolla, Triglochin, and Ranunculus were taken as hydrophytic types. Cyperaceae included representatives from the hydrophytic and psammophytic communities such as S. californicus and Androtrichum tryginum respectively, which could not be unambiguously identified. Therefore, it was excluded from the hydrophytic as well as from the psammophytic pollen type categories. Extraregional types encompassed: Nothofagus, Ephedra, and Schinus, whose main sources are located in the Sub-Antarctic forests and in Monte province, circa 1,100 and 500 km away from the study area (Morello, 1958; Cabrera, 1976). Exotic tree types like Cupressus, Populus, Eucalyptus, Pinus, Ulmus, Acacia, Fraxinus, Morus, Tamarix, Alnus, Quercus, Castanea among others, were reported as "Other trees", and not included in further analyses, while Scrophullariaceae, Monocotyledoneae, Rosaceae, and Lythraceae were added and reported as "Other taxa". The results to be compared with surface data were the percentages of the cumulative sums corresponding to the entire 23-month sampling period, considering only those taxa shared by Tauber and surface records.
Different pollen source areas were termed "local", "extralocal", "regional", and "extraregional", according to Prentice (1985).
Surface samples
Data from 24 surface soil samples published in a previous work (Stutz & Prieto, 2003) were selected from the main plant communities (Fig. 1). The same excluding and joining criteria explained above for airborne samples were used to create synthetic pollen variables (Other taxa, psammophytic, hydrophytic, and extraregional types) in the surface samples subset. Data were expressed as percentages of the shared taxa as detailed earlier. The numbering of surface samples was in accordance with Stutz & Prieto (2003). Samples 17 to 21 from the coastal lagoon were not considered in this work.
RESULTS
The comparison between surface soil and trap samples indicates that they feature similar compositions. They had fifty one percent of the pollen types in common. Fifteen pollen types were exclusively found in surface samples while 13 were only present in pollen traps, most of them trees. Nonetheless, the shared taxa were the main contributors of both pollen assemblages.
Trap records evidenced great differences in pollen composition among sites, although a few ones that exceeded 50% of the total sum dominated most of the traps. An extreme case is CELPA with a 48% corresponding only to Poaceae, followed by Apiaceae (10%) and Asteroideae (8%). Chenopodiaceae dominates Lagunita and Gallinas with 33 and 30%, respectively. Poaceae and Asteroideae are also important taxa with 24 and 11% at Lagunita, and 14 and 18% at Gallinas. Poaceae, Ambrosia/Xanthium, Cyperaceae, and Asteroideae represent the main taxa detected in the trap at Médanos, with 24, 16, 13, and 14%, respectively. Hinojales greatly differs from the other sites. The contribution of C. tala, the unique native arboreal taxon of the area, reaches 18% at this site, followed by Poaceae (19%) and Asteroideae (13%). The contribution of other taxa with 16% of the total sum (Fig. 3b) is also particularly worth of mention.
Classification results
The Stepwise Discriminant analysis included 9 variables and yielded the best separation among the three vegetation categories (Table 1). Partial Wilks' Lambda clearly demonstrated that the variable that contributes the most to the overall group discrimination is Chenopodiaceae, followed by C. tala, Ambrosia/Xanthium and Psammophytic types. Ninety three percent of the cumulative proportion of the total variance corresponded to the first discriminant function that accounted for a negative association between Chenopodiaceae and Cyperaceae (Table 2 and 3). The second function positively correlated to Psammophytic types, Apiaceae, Extraregional types and C. tala, and negatively to Cyperaceae and Hydrophytic types (Table 3). Nevertheless, statistics confirmed that they make no contribution to group discrimination, as the second function is not significant (Table 2).
Table 1. Summary of discriminant function analysis. Selected variables from 24 surface samples categorized into three groups: Psamophytic, Hidrophytic and Halophytic communities. Wilks' Lambda = 0.2718, approx. F (18, 26) = 7.3167, p < 0.0001.

Table 2. Eigenvalues, cumulative proportion of variance and Chi square test (a = 0.05) for the first and second discriminant functions.
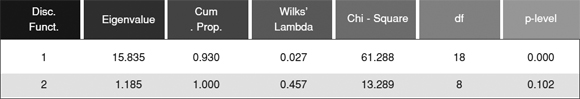
Table 3. Discriminant loadings and classification functions coefficients.

Figure 4 summarizes the model application to surface (validation) and airborne samples. Results indicate that 87.5 % of the surface samples were properly re-classified; however, performance differed in each group depending on each variable contribution to the model. Consequently, 100 % of the halophytic community group cases were properly re-classified, while 66.6 % of the psammophytic community group and 85.7 % of the hydrophytic community group were. Miss-classified samples were 1 and 5 from the coastal barrier, assigned to the freshwater community group, and 27 from Hinojales freshwater lake, assigned to the psammophytic community group (Fig. 4).
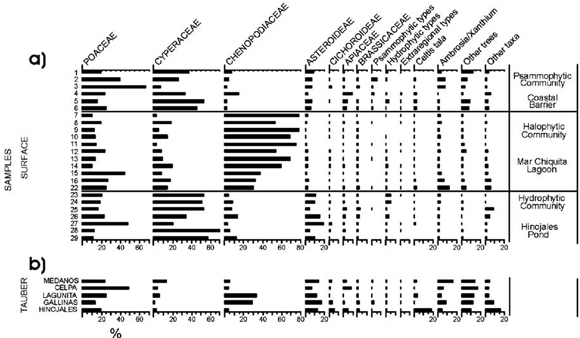
Fig. 3. Surface (a) and Tauber (b) percentages of pollen contribution to each site. Zonation of surface samples was taken from Stutz & Prieto (2003). "Other trees" were excluded for further analysis.
Considering the airborne samples, Hinojales and Lagunita were assigned to the halophytic community, Médanos and Gallinas to the psammophytic community, and CELPA to the freshwater community (Fig. 4).

Fig. 4. Calculated scores for the first discriminant function. The dots shapes show the actual group ownership of surface samples. PST: psammophytic community group, HAT: halophytic community group, HYT: hydrophytic community group. AS: corresponds to airborne samples from Tauber traps. Ellipses represent pos hoc model assignment of surface and trap samples to halophytic (hat), psammophytic (pst) and hydrophytic (hyt) groups.
DISCUSSION
Generally speaking trap record and surface samples bear similarities, as they share half of their pollen types, which are also those characteristic of the different types of vegetation in the area. The only exceptions are surface and trap samples at Hinojales. Surprisingly, Celtis and Cyperaceae, which would be expected to be relevant taxa at Hinojales shallow lake, were not always well represented in both types of records. Trap samples from Hinojales displayed extremely low values of Cyperaceae considering its proximity to extensive patches of Schoenoplectus. According to Federman (2003), mean Schoenoplectus cover at Hinojales freshwater lake reached 16.9%, while the mean percentage cover of total emergent species amounted to 22.6 %. The low percentages of trap samples could be attributed to the fact that sampling was interrupted before the onset of Schoenoplectus flowering during the second year of survey. Being an anemophilous species, Celtis also yielded somewhat low percentages maybe due to the trap loss in November 1995, when the maximum Celtis air concentration takes place (Latorre, 1999). On the other hand, Celtis also exhibits low percentage values in surface samples collected near the lakeshore. As stated by Vilanova et al. (2006), Celtis woodlands are characterised by surface pollen percentages ranging from 30% in their northern distribution to 70% in the localities closed to the study area. The results obtained cannot be clearly explained, but the perturbed flows induced by the paleodune could play a part. Nevertheless, micro-meteorological records from this area should be available to support this hypothesis.
By analysing Tauber samples, it can be ascertained that pollen grains have two distinctive depositional patterns in this complex area, depending on the location of their pollen sources. Scarce inter annual and inter site variation in extraregional pollen loadings lead to infer the existence of a regular contribution from distant sources. Small quantities of pollen types such as Nothofagus, Schinus and Ephedra are present after the dilution of plumes transported over large distances at high altitudes with atmospheric perturbations being unaffected by local circulation (Gassmann & Pérez, 2006). The same hypothesis is held for Schinus and Ephedra despite the fact that they have been reported closer to the study area like the dune systems at Monte Hermoso, riverbanks of Paraná river, Austral ranges and Celtis woodlands of Buenos Aires Province (Vervoorst, 1967; Fontana, 2005; G. Sottile, personal communication). Even so, all these types are not main contributors to the pollen spectra. On the other hand, the other pollen types showed high variability in time and space. In these cases, patterns could be tied to the predominant vegetation composition with minor differences ascribed to the pollination syndrome or to the presence of insect remains inside the traps (Pérez et al., 2009). Therefore, pollen loadings proved that the airborne pollen transport is predominantly local, which is consistent with the results previously described for surface and trap samples (Stutz & Prieto, 2003; Pérez et al., 2009). In some cases, dispersion and transport can be attributed to local circulation systems like the sea-land breeze already described for coastal locations on close localities as already described for Poaceae and Celtis (Gassmann et al., 2002; Gassmann & Pérez, 2006).
Discriminant analysis and classification of Tauber trap samples
According to Stutz & Prieto (2003), the psammophytic community is characterized by Poaceae and Cyperaceae presence as well as by psammophytic species. Maximum values of Chenopodiaceae, and the freshwater community, in turn, distinguish the halophytic community, by maximum values of Cyperaceae and hydrophytic types. Chenopodiaceae and Cyperaceae types could be considered the main contributing variables to vegetation communities' distinction. Particularly the first function showed a negative association between Chenopodiaceae and Cyperaceae, thus halophytic community is the best distinguished from hydrophytic and psammophytic groups. Therefore, the model seems to miss-classify surface samples from the psammophytic community, identifying them as those from the freshwater community (associated probabilities of 33.4 and 12.5% respectively). With regard to their pollen assemblages, it is clear that there is a strong resemblance based on their main representatives, Poaceae and Cyperaceae. Morphological reasons led some hydrophytic and psammophytic species such as S. californicus and A. tryginum (hydrophytic and psammophytic Cyperaceae respectively) or Hordeum murinum L. ssp. murinum and P. racemosum (hydrophytic and psammophytic Poaceae) to be included in the same taxon, thereby diluting the differences between both communities. Yet there are some representatives present in both communities such as Schoenoplectus, growing at Hinojales banks and at the interdune depressions that contribute to the similarities.
The classification of Tauber samples showed that only Lagunita and Médanos were correctly assigned to the vegetation communities. Despite the high percentages of Chenopodiaceae pollen, explained by its location within the salt marsh, Gallinas was possibly assigned to the Psammophytic community given the unexpectedly high values of Ambrosia and psammophytic pollen types. The later registered an over-representation of Caryophyllaceae, due to the presence of insect remains in one of the traps (Pérez et al., 2009). Despite CELPAbeing located within the psammophytic vegetation, the classification assigned it to the hydrophytic community. The presence of interdune depressions with abundant Schenoplectus, Carex and Eleocharis (Stutz & Prieto, 2003), and a nearby monospecific stand of C. dioica, contributes to high percentages of Cyperaceae and Poaceae, which is also distinctive of the hydrophytic community. Hinojales was assigned to the halophytic community regardless of the very low percentages of Chenopodiaceae and the high values of C. tala, Asteroideae and Poaceae. Nevertheless, it seems that this sample is poorly represented by the first discriminant function but best related to the second, which is not statistically significant.
The first discriminant function describes a pattern of vegetation from positive scores for the hydrophytic community to negative ones for the halophytic community with the psammophytic community lying in between. These results seem to represent a gradient of salinity: the more negative the scores the more salty the soils, in agreement with previous results (Stutz & Prieto, 2003). The uneven results derived from the classification of Tauber samples are not clear enough to determine whether trap samples follow this salinity gradient detected in surface samples or not. More research and longer monitoring periods are necessary to be conclusive about a record providing faster responses to weather and phenology rather than to plant populations and vegetation communities.
CONCLUSIONS
The main pollen types at Mar Chiquita costal lagoon area shared by Tauber and surface records which qualitatively reflect the predominant vegetation types. Differences were mainly observed at the hydrophytic community (Hinojales freshwater lake) where C. tala and Cyperaceae, the main characteristic types of the local vegetation, were not always well represented on both records. Also, pollen transport was observed on both records as predominantly local.
Most surface samples were properly reclassified after discriminant validation, but classification of Tauber traps yielded regular results in accordance with the particular features of each site. The halophytic community was the easiest to recognize according to the discriminant function that yielded a negative association between Chenopodiaceae and Cyperaceae, probably connected to a salinity gradient. Nevertheless, morphological features did not allow identifying characteristic psammophytic from hydrophytic pollen types which yielded poor classification results for the communities. Further and extensive research efforts would be necessary to raise the level of understanding on the way in which pollen reaches and incorporates in the sediment in this area.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Dr. F.I. Isla for his valuable help in the field and to encourage this work. Also thanks to R. and M. Arbelaiz and A. Romano who allowed us working in their premises. This survey was supported by the Universidad Nacional de Mar del Plata, Universidad Autónoma de Entre Ríos and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) with the following grants: EXA 462/09, PID 550/08 and PIP 6235/06. C.F. Pérez, F. Latorre and S. Stutz are members of the Scientific Researcher Career (CONICET).
Bibliography
1. BORROMEI, A. M. & M. QUATTROCCHIO. 1990. Dispersión del polen actual en el área de Bahía Blanca (Buenos Aires, Argentina). Anales Asoc. Palinól. Lengua Esp. 5: 39 - 52. [ Links ]
2. CABRERA, A. L. 1976. Regiones Fitogeográficas Argentinas. Enciclopedia Argentina de Agricultura y Jardinería. ACME. T. II. Buenos Aires. [ Links ]
3. CUADRADO, G. A. 1978. Polen atmosférico de la ciudad de Corrientes (Argentina). Facena 3: 55 - 68. [ Links ]
4. CUADRADO, G. A. 1979. Calendario polínico preliminar para Corrientes (Argentina) y sus alrededores. Facena 3: 65 - 83. [ Links ]
5. ERDTMAN, G. 1971. Pollen morphology and plant taxonomy, Angiosperms. Hafner Publishing Company. New York. USA. [ Links ]
6. FASANO, J. L. 1991. Geología y geomorfología. Región III. Faro Querandí - Mar de Cobo. Provincia de Buenos Aires. Informe Final. Convenio de Cooperación Horizontal CFI y UNMdP. Universidad Nacional de Mar del Plata. [ Links ]
7. FAEGRI, K. & J. IVERSEN. 1992. Textbook of pollen analysis (4th ed.). En: K. Faegri, P. E. Kaland & K. Krzywinski (eds.). John Willey & Sons. Chichester, New York, Brisbane, Toronto, Singapore. [ Links ]
8. FEDERMAN, M. 2003. Mapeo y caracterización de la comunidad de macrófitas en tres lagos someros del sudeste bonaerense. Tesis de grado. Universidad Nacional de Mar del Plata. Mar del Plata. [ Links ]
9. FONTANA, S. L. 2003. Pollen deposition in coastal dunes, south Buenos Aires Province, Argentina. Rev. Palaeobot. Palynol. 126: 17 - 37. [ Links ]
10. FONTANA, S. L. 2005. Coastal dune vegetation and pollen representation in south Buenos Aires Province, Argentina. J. Biogeogr. 32: 719735. [ Links ]
11. FONTANA, S. L., C. A. FERNÁNDEZ. & A. C. DEDOMENICI. 2001. Calendario polínico preliminar del área de Monte Hermoso, Buenos Aires, Argentina. En: Fombella Blanco, M.A., Fernández González, D., Valencia Barrera, R.M. (Eds.), Palinología: Diversidad y Aplicaciones, pp. 227 - 233. Universidad de león, León, España. [ Links ]
12. GARCÍA DE ALBANO, M. E. 1991. Aeropalinología de Santiago del Estero. Arch. Argent. Alergia Inmunol. Clín. 22: 6 - 12. [ Links ]
13. GASSMANN, M. I. & C. F. PÉREZ. 2006. Trajectories associated to regional and extra-regional pollen transport in the southeast of Buenos Aires Province, Mar del Plata (Argentina). Int. J. Biometeorol. 50: 280 - 291. [ Links ]
14. GASSMANN, M. I., C. F. PÉREZ & J. M. GARDIOL. 2002. Sea-land breeze in a coastal city and its effect in the pollen transport. Int. J. Biometeorol. 46: 118 - 125. [ Links ]
15. HALL, S. A. 1990. Pollen deposition and vegetation in the southern Rocky Mountains and southwest Plains, USA. Grana 29: 47 - 52. [ Links ]
16. HEUSSER, C. 1971. Pollen et Spores of Chile. Modern types of Pteridophyta, Gimnospermae and Angiospermae. The University of Arizona Press, Tucson. [ Links ]
17. HICKS, S. & V. P. HYVÄRINEN. 1986. Sampling modern pollen deposition by means of "Tauber traps": Some considerations. Pollen & Spores 28: 219 - 242. [ Links ]
18. HICKS, S., B. AMMANN, M. LATALOWA, H. PARDOE, H. TINSLEY (Eds.). 1996. European Pollen Monitoring Programme: project description and guidelines. University of Oulu, Oulu. [ Links ]
19. HICKS, S., H. TINSLEY, A. HUUSKO, C. JENSEN, M. HÄTTESTRAND, A. GERASIMIDES & E. KVAVADZE. 2001. Some comments on spatial variation in arboreal pollen deposition: first records from the Pollen Monitoring Programme (PMP). Rev. Palaeobot. Palynol. 117: 183 -194. [ Links ]
20. LATORRE, F. 1999. El polen atmosférico como indicador de la vegetación y de su fenología floral. Tesis de Doctorado. Universidad de Buenos Aires. Buenos Aires. [ Links ]
21. LATORRE, F. & M. A. CACCAVARI. 2006. Depositación polínica anual en el Parque Nacional Pre-Delta (Entre Ríos, Argentina). Rev. Mus. Argent. Ci. Nat. Bernardino Rivadavia 8: 195 - 200. [ Links ]
22. LEÓN, R.J.C. 1991. Vegetación. En: R.T. Coupland (ed.). Ecosystems of the World 8A. Natural Grasslands. Introduction and Western Hemisphere, pp. 380 - 387. Elsevier, New York. [ Links ]
23. MAJAS, F. D. & E. J. ROMERO. 1992. Aeropalynological research in the northeast of Buenos Aires Province, Argentina. Grana 31: 143 - 156. [ Links ]
24. MARKGRAF, V. & H. L. D' ANTONI. 1978. Pollen flora of Argentina. Modern spore and pollen types of Pteridophyta, Gimnospermae and Angiospermae. The University of Arizona Press, Tucson. [ Links ]
25. MARKGRAF, V. 1980. Pollen dispersal in a mountain area. Grana 19: 127 - 146. [ Links ]
26. MCLACHLAN, G. J. 1992. Discriminant analysis and statistical pattern recognition. John Wiley & Sons, New York. [ Links ]
27. MORELLO, J. 1958. La Provincia Fitogeográfica del Monte. Opera Lilloana 2: 5 - 115. [ Links ]
28. NAAB, O. A. 1999. Lluvia polínica actual en el Parque Nacional Lihuel-Calel, La Pampa, Argentina. Asoc. Paleontol. Argent. Publ. Espec. 6: 85 - 89. [ Links ]
29. PÉREZ, C. F., F. LATORRE, S. STUTZ & S. PASTORINO. 2009. A two year report of pollen influx into Tauber traps in Mar Chiquita coastal lagoon, Buenos Aires, Argentina. Aerobiologia 25: 167-181. DOI: 10.1007/s10453-009- 9122-x [ Links ]
30. PRENTICE, C. 1985. Pollen representation, source area and basis size: unified theory of pollen analysis. Quatern. Res. 23: 76-86. [ Links ]
31. SCHNACK, E. J., J. L. FASANO & F. I ISLA. 1982. The evolution of Mar Chiquita Lagoon coast, Buenos Aires Province, Argentina. Holocene Sea Level Fluctuation, magnitude and causes. I.G.P.C., 61 Annual Meeting, South Carolina, USA, pp. 143-155. [ Links ]
32. STOCKMARR, J. 1971. Tablets with spores used in absolute pollen. Pollen & Spores 13: 615-621. [ Links ]
33. STUTZ, S. 2001. Vegetación del área de la laguna de Mar Chiquita. En: Iribarne, O. (Ed.), Reserva de Biósfera Mar Chiquita: Características físicas, biológicas y ecológicas, pp. 75 - 78. Editorial Martín, Mar del Plata. [ Links ]
34. STUTZ, S. & A. R. PRIETO. 2003. Modern pollen and vegetation relationships in Mar Chiquita coastal lagoon area, southeastern Pampa grasslands, Argentina. Rev. Palaeobot. Palynol. 126: 183 - 195. [ Links ]
35. STUTZ, S., A. R. PRIETO & F. I. ISLA. 2006. Holocene evolution of the Mar Chiquita coastal lagoon area, Argentina, indicated by pollen analysis. J. Quatern. Sci. 21: 17-28. [ Links ]
36. TAUBER, H. 1974. A static non-overload pollen collector. New Phytol. 73: 359-369. [ Links ]
37. TAUBER, H. 1977. Investigations of aerial pollen transport in a forested area. Dansk Bot. Ark. 32: 1 - 121. [ Links ]
38. VERVOORST, F. 1967. La vegetación de la República Argentina VII. Las comunidades de la depresión del Salado (Provincia de Buenos Aires). INTA, Serie Fitogeográfica 7. Buenos Aires. [ Links ]
39. VILANOVA, I., A. R. PRIETO & S. STUTZ. 2006. Historia de la vegetación de las llanuras costeras del este de la provincia de Buenos Aires durante el Holoceno. Ameghiniana 43: 147- 159. [ Links ]
40. VIOLANTE, R. A. 1992. Ambientes sedimentarios asociados a un sistema de barrera litoral del Holoceno en la llanura costera al sur de Villa Gesell, Provincia de Buenos Aires. Rev. Asoc. Geol. Argent. 47: 201-214. [ Links ]
41. XU, Q.H., Y. C. Li, F. TIAN, X. Y. CAO & X. I. YAN. 2009. Pollen assemblages of tauber traps and surface soil samples in steppe areas of China and their relationships with vegetation and climate. Rev. Palaeobot. Palynol. 153: 85 - 101. [ Links ]
Recibido el 11 de mayo de 2010
Aceptado el 23 de octubre del 2010.














