Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.46 no.1-2 Córdoba ene./jun. 2011
ANATOMÍA Y MORFOLOGÍA
Leaf anatomy of Gaillardia cabrerae (Asteraceae): Basic plan and comparative study of two contrasting habitat populations
Laura Beinticinco1, Anibal Prina2 y Graciela Alfonso3
1 Facultad de Agronomía, Universidad Nacional de La Pampa. CONICET.
2 Facultad de Agronomía, Universidad Nacional de La Pampa.
3 Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa.
Summary: This study evaluates the leaf anatomy pattern of Gaillardia cabrerae Covas, an exclusive endemic camephyte from the Lihué Calel National Park, province of La Pampa, Argentina. Leaf cross sections and peelings of plants growing in two different populations were used to determine the basic leaf anatomy patterns and to estimate the influence of growth conditions in the two microhabitats.The analysis showed differences in epidermal cells area, stomata density and main diameter, lacunar parenchyma cells and central vascular bundle diameter and palisade parenchyma cells dimensions. These aspects might be reflecting environmental conditions of the populations, especially sun exposure and soil moisture. The results provide information on the relationship of the species to its environment, which could be used in the establishment of conservation policies.
Key words: Leaf anatomy; Endemism; Lihué Calel National Park.
Anatomía foliar de Gaillardia cabrerae (Asteraceae): plan básico y estudio comparativo de dos poblaciones de hábitats contrastantes.
Resumen:Gaillardia cabrerae Covas es un caméfito endémico del Parque Nacional Lihué Calel. En este estudio se evalúan características anatómicas de individuos de dos poblaciones provenientes de diferentes microhábitats de las Sierras de Lihué Calel. La información proporcionada es importante para entender las condiciones de vida de la especie y provee información inherente para establecer planes de conservación. Mediante cortes transversales y la técnica de peeling fue posible determinar el plan básico de la anatomía foliar y también verificar diferencias significativas en el área de células epidérmicas, densidad estomática, diámetro mayor de los estomas, diámetro de células del parénquima lagunar y del haz vascular central y en las dimensiones de las células del parénquima en empalizada. Las condiciones ambientales a las cuales estas poblaciones están sometidas, especialmente la exposición solar y la humedad del suelo son posibles factores que podrían explicar las diferencias detectadas en la anatomía foliar para ambas poblaciones.
Palabras clave: Anatomía foliar; Endemismo; Parque Nacional Lihué Calel
INTRODUCCIÓN
Gaillardia cabrerae Covas is one of the three endemic plant species of Lihué Calel National Park (NP), which is located in central-southern hilly regions of La Pampa province, Argentina (Fig. 1). The species belongs to the Asteraceae, subfamily Asteroideae, and it is known locally as the "margarita pampeana" or "margarita de la sierra".

Fig. 1. Location of Lihué Calel Nacional Park in central Argentina.
Morphologically it is a perennial shrub from 30 to 50 cm high with woody diffusely branched from the base (Fig. 2A). Leaves polymorphic, oblong or spatulated, from undivided to pinnatifid, shortly petiolate; involucrate heads radiate with dimorphic florets of 3,5-8 cm diameter, floral receptacle convex, long-peduncled, with dimorphic florets, marginal florets female, ligulate, yellow, with a truncate 3-lobed apex, disc center florets hermaphrodite, yellow-ocher colored, with a tubulose corolla and 5-lobate apex; achene densely hairy of 2,5-3,5mm long.
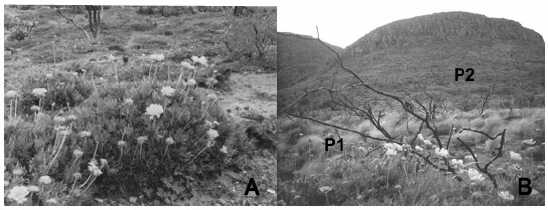
Fig. 2. Gaillardia cabrerae. A. Morphological aspect. B. Study populations on Lihué Calel National Park. Population 1 on the middle ladder (P1) and population 2 on high rocky ladder (P2).
Although leaf anatomy studies are common, they are mostly focused on species with economic or taxonomic interest (Meicenheimer et al., 2008; Thorne et al., 2006; Rady et al., 1999; Zobayed et al., 1999; D´Ambrogio et al., 2000). This kind of researches is rarely done on endemic species even so a bibliographic search on works on leaf anatomy of species of the Lihué Calel National Park yielded no result. After the species was described by Covas (1969) the bibliographic records had been limited to the species citation (Pettenati & Ariza-Espinar, 1997; Troiani & Steibel, 1999). The present study is included in a comprehensive research program on reproductive biology of G. cabrerae (Prina & Alfonso, in prep.) and provides a basic plan of the leaf anatomy of Gaillardia cabrerae and a comparative analysis of leaf anatomy features in two populations developing in contrasting environments. Studies on basic aspects of G. cabrerae, such as its leaf anatomy, might help to detect adaptative characters to environmental conditions and consequently, to understand its strict condition.
MATERIALS AND METHODS
Study Area
The Lihué Calel NP, is located in the Monte phytogeographic region (Cabrera, 1971) in the center south region of La Pampa province (between 65º 39'- 65º 33'W and 37º 54'-38º 05'S) (Fig. 1). The landscape is mainly a group of hills of volcanic origin of 590 m above sea level (Llambías, 2008). It has continental type weather, with average temperatures of 7ºC and 25ºC and a large thermal daily fluctuation and typical semiarid region rainfall regime (300 to 400 mm annual). The Lihué Calel NP presents large environmental heterogeneity and a high biological diversity in which, besides G. cabrerae, two more endemic species are found: Adesmia lihuelensis Burkart and Grindelia covasii Bartoli & Tortosa. In Lihué Calel NP floristic composition is mainly determined by sun exposure and the soil type (Alfonso et al., 2001).
Plant material
All descriptions were made from specimens collected in two contiguous populations in the Lihué Calel NP during August 2004. Terminology in the descriptions of anatomy follow Metcalfe & Chalk (1979), Harris & Harris (1999) and Esau (1985). All voucher specimens mentioned in the Appendix are kept in SRFA (Thiers, 2010).
Two populations were defined as (1) Jarillal, consisting of a grassland with scattered Larrea divaricata shrubs in a gentle slope, and (2) an orophilous community on a rocky soil in a south facing slope with Grindelia covasii and pteridophytes (Fig. 2B). Both populations differ on their environmental conditions as to be considered as two different microhabitats: Pop. 2 receives lower levels of sun radiation than Pop. 1, develops on rocky soil and is exposed to lower humidity conditions.
Ten individuals were randomly selected from each of the above-mentioned populations and two leaves of each plant were taken. Fresh leaf material was fixed in FAA to make microscope slides. All slides were examined under light microscope (20X and 40X objective). Stomata, epidermal cells and epidermal hairs measurements were taken from single epidermal preparations, using fresh, no-fixed and no-stained material. In this case, ten leaves of each population were analyzed. For epidermal hairs and stomata density, three fields of view (area 0.95 mm2) were chosen. Measurements from leaf cross-section were based on permanent slides. The cuts were carried out with a rotation microtome of 12m width. Leaves were stained with triple coloration composed by hematoxiline, safranine and fast-green (Metcalfe& Chalk, 1979).
The samples were analyzed with an OLYMPUS BX40F-3 microscope. The pictures of the anatomical cuts were taken using a Kodak C340 digital camera with 100 and 200X.
Measurements were recorded in a spreadsheet and ANOVA, Tuckey test and analysis of variance were done with the software Statgraphics program (Manugistics inc.).
RESULTS
1. Anatomy
The leaf cross-sectional view presents a flat shape with prominent central vascular bundle and acute extremes.
There is a strong trend to isolaterality (Fig. 3) in which the adaxial and abaxial faces present a well developed palisade parenchyma composed by two or three cell layers. Lacunose parenchyma is scarce and is limited to the surroundings of the collenchymatous tissue rings that protect the vascular bundles.

Fig. 3. Cross section cut of G. cabrerae leaves. A. Tendency to isolaterality. In the adaxial face (AD) exists a well developed palisade parenchyma (PC) while the abaxial face (AB) is occupied by a not arranged tissue with intermedium type cells (IC). B. Central vascular bundle (CVB) surrounded by a ring of lacunose colenquimatous cells (CC).
The vascular bundle is vertical transcurrent type (Metcalfe & Chalk, 1979) the fact that it is surrounded by a conspicuous lacunose collenchymatous tissue ring, which communicates both leaf faces (Fig. 3B). Also the collenchymatous tissue exists surrounding the secondary vascular bundles but less developed in this case. These cells represent the only support tissue, since no typical support cells, as sclerenchymatic ones, were found.
Epidermal tissue constitutes a single layer on both surfaces (Fig. 3A). The cuticle is 2m thick. The epidermal cells are variable in size and shape with regular outlines (Fig. 4).

Fig. 4. Leaf surface. A. Paradermal view showing epidermal cells (EC), random orientate stomata (S) and an epidermal hair base (EHB). B. Cross-section view of a stomata cavity (SC) surrounded by palisade cells (PC). EC=Epidermal cells.
Leaves are amphistomatic, while the stomata are anomocytic (Fig. 4A), with subsidiary cells very similar to adjacent epidermic cells (Metcalfe & Chalk, 1979), they do not follow a fixed distribution pattern and they orientate in all directions.
Epidermal hairs are pluricelled and not branched. Hairs constituted by two, three and even four cells had been found (Fig. 5).

Fig. 5. Epidermal hairs. A. Surface view showing a four-cell hair. B. Different hair types of G. cabrerae, constituted by 2, 3 or 4 cells. C. Cross-section view of 2-cell hairs.
The genus Gaillardia is well known by having essential oils (Adams et al., 2008; Duschatzky et al., 2005; Bohlmann et al., 1969) which are produced by sessile glands located in depressions of the epidermic tissue (Fig. 6).

Fig. 6. Cross-section view of an essential oil gland arranged in a slight epidermal depression from epidermal surface.
2. Statistic Analysis
Mean and standard deviation were calculated for the different variables here considered and then they were statistically compared through variance analysis. Tuckey test was applied for the comparisons between both populations and leaf faces according to the different variables.
Means and standard deviations of the obtained data by cross sections are summarized in Table 1 and those from epidermal surfaces in Table 2.
Table 1. Mean and standard deviation calculated for characters from both populations obtain by crosssection cuts. n=10.
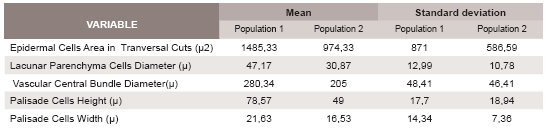
Table 2. Mean and standard deviation calculated for adaxial and abaxial epidermis obtain by peeling from both populations. n=10.

The comparison of the analyzed variables is summarized in Table 3. The statistic analyses indicate significant differences in the following variables: epidermal cells area; stomata density; stomata main diameter; lacunar parenchyma cells diameter; central vascular bundle diameter and palisade parenchyma cells dimensions. The last three variables show significant differences between the two populations for both leaf surfaces.
Table 3. Variance analysis (ANOVA) between both leaf faces and between populations. df=free degree. *=statistically significant differences p≤0.01.
DISCUSSION AND CONCLUSIONS
The basic plan of G. cabrerae leaf anatomy fits to certain common characteristics defined for different Asteraceae species (Metcalfe & Chalk, 1979; Pyykko, 1966). Among those characteristics amphistomatic leaves type, anomocytic stomata, stomata distributed all over epidermal surface without a determinate pattern, the presence of uniseriated hairs and isolateral compact mesophile can be mentioned. Furthermore, isolateral leaf anatomy is a property to be a xeromorphic feature and its development exclusively depends on solar radiation (Pyykko, 1966).
Generally, dicotyledonous leaves develop collenchymatous tissue in the subepidermic zone. It acts as a mechanical tissue due to the thickness of its cell walls reinforces the leaf structure avoiding the damage caused by the wind and at the same time adds to the desiccation resistance of the cells (Venning, 1949). In this way, collenchyma is the typical support tissue in this group of plants. On the other hand, it can cover the vascular bundle completely like a sheath. In those cases, it is called collenchymatous parenchyma (Esau, 1985).
Referring to stomata size, this is a very variable parameter that is why its diagnostic utility is restricted (Metcalfe & Chalk, 1979). G. cabrerae has small stomata (guardian cells smaller than 15m long). Smaller stomata are generally associated to high densities while bigger stomata are found at lower densities. In G. cabrerae the bigger stomata are present in population 1, where these epidermal structures are in lower densities than population 2, and where consequently, stomata densities are higher. Epidermal cells area might be in close relation to this pattern. Regarding to stomata localization, G. cabrerae fixes to the tendency that exists in isolateral leaves that they have not got a basic pattern, they are randomly orientated (Metcalfe & Chalk, 1979).
The secretions structures observed in some epidermal zones have not got the typical complexity of those described by trichomes or true glands (Esau, 1985). Its specific analysis it was not part of our objectives in this research. So that, we consider that further and detailed studies of this structures joined with epidermal hairs might be necessary to describe and identify them correctly.
The considered populations in the present study are exposed to contrasting conditions regarding soil, moisture and light characteristics, typical of the different microenvironments found in the area. The significant differences found in the leaf-anatomy features would evidence certain adaptation to determined environmental conditions. For example, strong development of palisade parenchyma is a well-known change in the internal structure of leaves of Dycotiledoneae plants as a response to xeromorphic environments. This differential development of palisade tissue occurs in decrement of the lacunar tissue.
Humidity and sun radiation are the main factors that could determinate the palisade tissue development (Pyykko, 1966; Esau, 1985). Some studies suggest that the development of this tissue cells is higher in leaves that grow at a higher light exposure than those of the same species, even of the same individual, which develop under shade (Pyykko, 1966; Esau, 1985). This phenomenon is evident only in these cells length but not in a greater number of cell layers that constitute the leaf. These arguments could explain the greater length leaves cells of population 1 which receives more sun radiation exposure than leaves of population 2, where sun radiation is significantly lower due to its south orientation.
In conclusion, the comparative study of anatomical structures between plants of contrasting environmental conditions offers evidence of possible adaptative strategies of plants. This information will be joined with ongoing population genetic studies and will be used as tools in the implementation of conservation policies.
APPENDIX
Studied Specimens:
ARGENTINA, Prov. La Pampa: Dpto. Lihuel Calel, Parque Nacional Lihué Calel: 38º 1' 17.8"S - 65º 35' 38.7"W, 23/02/09, Beinticinco, Prina & Alfonso 0001; 38º 1' 17.8"S - 65º 35' 38.8"W, 23/02/09, Beinticinco, Prina & Alfonso 0002; 38º 1' 19.1"S - 65º 35' 38.8"W, 23/02/09, Beinticinco, Prina & Alfonso 0003; 38º 1' 21.1"S - 65º 35' 33.6"W, 23/02/09, Beinticinco, Prina & Alfonso 0004; 38º 1' 21.4"S - 65º 35' 32.2"W, 23/02/09, Beinticinco, Prina & Alfonso 0005, 38º 1' 20.7"S - 65º 35' 29.8"W, 23/02/09, Beinticinco, Prina & Alfonso 0006; 38º 1' 21"S - 65º 35' 30"W, 23/02/09, Beinticinco, Prina & Alfonso 0007; 38º 1' 20.7"S - 65º 35' 29"W, 23/02/09, Beinticinco, Prina & Alfonso 0008; 38º 1' 23.1"S - 65º 35' 32"W, 23/02/09, Beinticinco, Prina & Alfonso 0009; 38º 1' 22.4"S - 65º 35' 32.2"W, 23/02/09, Beinticinco, Prina & Alfonso 0010; 38º 1' 13.2"S - 65º 35' 34.5"W, 23/02/09, Beinticinco, Prina & Alfonso 0019; 38º 1' 13.5"S - 65º 35' 33.9"W, 23/02/09, Beinticinco, Prina & Alfonso 0020; 38º 1' 14.2"S - 65º 35' 33.5"W, 23/02/09, Beinticinco, Prina & Alfonso 0021; 38º 1' 14.0"S - 65º 35' 32.8"W, 23/02/09, Beinticinco, Prina & Alfonso 0022; 38º 1' 13.5"S - 65º 35' 32.1"W, 23/02/09, Beinticinco, Prina & Alfonso 0023; 38º 1' 13.8"S - 65º 35' 30.9"W, 23/02/09, Beinticinco, Prina & Alfonso 0024; 38º 1' 13.7"S - 65º 35' 30.9"W, 23/02/09, Beinticinco, Prina & Alfonso 0025; 38º 1' 13.8"S - 65º 35' 29.9"W, 23/02/09, Beinticinco, Prina & Alfonso 0026; 38º 1' 13.3"S - 65º 35' 29.5"W, 23/02/09, Beinticinco, Prina & Alfonso 0027; 38º 1' 14.7"S - 65º 35' 30.6"W, 23/02/09, Beinticinco, Prina & Alfonso 0028; 38º 1' 14.0"S - 65º 35' 31.4"W, 23/02/09, Beinticinco, Prina & Alfonso 0029 (SRFA).
ACKNOWLEDGEMENTS
We especially thank Ernesto Morici and Ofelia Nabb† (Facultad de Agronomía, Universidad Nacional de La Pampa) and to Nilda Dottori and the team of Cátedra de Morfología y Anatomía Vegetal of Facultad de Ciencias Exactas, Físicas y Naturales of Universidad Nacional de Córdoba for providing helpful tools for carry out this work. We are grateful to Jorge Chiapella (IMBIV) for the review of the paper.
This research was possible thanks to Facultad de Agronomía and Facultad de Ciencias Exactas y Naturales (Universidad Nacional de La Pampa). We also thank to Administración de Parques Nacionales (APN) that gave us permission to access Lihué Calel National Park. This research was supported by Universidad Nacional de La Pampa, project API nº 25.
BIBLIOGRAPHY
1. ADAMS, A., M.A. ROSELLA, E.D. SPEGAZZINI, S.L. DEBENEDETTI & N. DE KIMPE. 2008. Composition of essential oils of Gaillardia megapotamica and Gaillardia cabrerae from Argentina. J. Essen.t Oil Res. 20(6): 521-524. [ Links ]
2. ALFONSO, G., A. PRINA, J. MARTÍNEZ & C. CASCÓN. 2001. Excursión al Parque Nacional Lihué Calel. Publicación miscelánea para las XXXVIIIº Jornadas Argentinas de Botánica. SAB. UNLPam. [ Links ]
3. BOHLMANN, F., U. NIEDBALLA & J. SCHULZ. 1969. Über einige Thymolderivate aus Gaillardiaund Helenium-Arten. Chem. Ber. 102 (3): 864–871. [ Links ]
4. CABRERA, A.L. 1971. Fitogeografía de la República Argentina. Bol. Soc. Argent. Bot. 14: 1-42. [ Links ]
5. COVAS, G. 1969. Nueva especie de Gaillardia (Compositae) de la flora argentina. Bol. Soc. Argent. Bot. 11(4): 262-264 [ Links ]
6. D'AMBROGIO, A., S. FERNANDEZ, E. GONZALEZ, I. FURLAN & N. FRAYSSINET. 2000. Estudios morfoanatómicos y citológicos en Atriplex saggitifolia (Chenopodiaceae). Bol. Soc. Argent. Bot. 35(3-4): 215-226. [ Links ]
7. DUSCHATZKY, C.B., M.L. POSSETTO, L.B. TALARICO, C.C. GARCÍA, F. MICHIS, N.V. ALMEIDA, M.P. DE LAMPASONA, C. SCHUFF & E.B. DAMONTE. 2005. Evaluation of chemical and antiviral properties of essential oils from South American plants. Antivir. Chem. & Chemother. 16: 247–251. [ Links ]
8. ESAU, K. 1985. Anatomía Vegetal. Ed. Omega S.A., Barcelona. [ Links ]
9. HARRIS, J.G. & M.W. HARRIS. 1999. Plant Identification Terminology. An illustrated glossary. Spring Lake publishing, Spring Lake, Utah. [ Links ]
10.LLAMBÍAS, E. J. 2008. "La Sierra de Lihuel Calel". In: CSIGA (Eds.), Sitios de Interés Geológico de la República Argentina. Pp. 537-550. Instituto de Geología y Recursos Minerales. Servicio Geológico Minero Argentino. Anales 46 II. Buenos Aires. [ Links ]
11. MEICENHEIMER, R.D., D.W. COFFIN & E.M. CHAPMAN. 2008. Anatomical basis for biophysical differences between Pinus nigra and P. resinosa (Pinaceae) leaves. Amer. J. Bot. 95: 1191-1198. [ Links ]
12. METCALFE, C. R. & L. CHALK. 1979. Anatomy of Dicotyledons. Vol. I. 2nd Ed. Clarendon Press, Oxford. [ Links ]
13. PETENATTI, E. M. & L. ARIZA-ESPINAR. 1997. Asteraceae. In: A. T. HUNZIKER (ed.) Flora Fanerogámica Argentina 45 (280): 1-35. [ Links ]
14. PYYKKO, M. 1966. The leaf anatomy of east patagonian xeromorphic plants. Ann. Bot. Fenn. 3: 453-622. [ Links ]
15. RADY, M.R. & Z.A. ALI. 1999. Comparison of physiology and anatomy of seedlings and regenerants of sugar beet. Biol. Pl. 42 (1): 39-48. [ Links ]
16. THIERS, B. 2010. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium, [continuously updated]. http://sweetgum.nybg.org/ih/ [ Links ]
17. THORNE, E.T., B.M. YOUNG, G.M. YOUNG, J.F. STEVENSON, J.M. LABAVITCH, M.A. MATTTHEWA & T.L. ROST. 2006. The structure of xylem vessels in grapevine (Vitaceae) and a possible passive mechanism for the systemic spread of bacterial disease. Amer. J. Bot. 93: 497-504. [ Links ]
18. TROIANI, H. & P. STEIBEL. 1999. Sinopsis de las compuestas (Compositae Giseke) de la Provincia de La Pampa. Rev. Fac. Agronomía, UNLPam. 10 (1): 1-86. [ Links ]
19. VENNING, F.D. 1949. Stimulation by wind motion of colenchyma formation in celery petioles. Bot. Gaz. 110:511-514. [ Links ]
20. ZOBAYED, S.M.A, F. AFREEN-ZOBAYED, C. KUBOTA & T. KOZAI. 1999. Stomatal characteristics and leaf anatomy of potato plantlets cultured in vitro under photoautotrophic and photomixotrophic conditions. In Vitro Cell. Dev. Biol. Pl. 35 (3): 183-188. [ Links ]
Recibido el 28 de abril de 2010
Aceptado el 11 de noviembre de 2010.














