Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.48 no.2 Córdoba ago. 2013
MICOLOGIA
Effect of aqueous and alcohol extracts of Phytolacca tetramera (Phytolaccaceae) leaves on Colletotrichum gloeosporioides (Ascomycota)
Marcelo Hernández1, Mónica Murace2, Jorge Ringuelet3, Inés Petri4, Daniel Gallo4 And Ana Arambarri1
1Departamento de Ciencias Biológicas.
2Departamento de Recursos Naturales y Medio Ambiente.
3Departamento de Ciencias Exactas, Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata (UNLP), Calle 60 y 119, CC31 (1900) La Plata.
4Departamento de Biodiversidad, Fundación Biósfera. 16 Nº 1611 (1900) La Plata, Buenos Aires, Argentina.
Summary: Phytolacca tetramera Hauman ombusillo is an endemism of southeastern Buenos Aires province (Argentina). This species has fungicidal action against opportunistic pathogens of humans. In order to search natural alternatives for the control of diseases in plants caused by fungi, the objective was to evaluate the effects of aqueous and alcohol extracts of P. tetramera leaves on Colletotrichum gloeosporioides (Penz.) Sacc. This fungus has a wide distribution in different species with agricultural, forestry, and ornamental value. The antifungal activity of aqueous and ethanol leaf extracts was assessed in vitro against fungi. The fungus was subjected to two types of extracts already incorporated into the Potato Dextrose Agar (PDA) medium at 5-50% concentrations. The aqueous extract concentrations within the range 15-30% led to a decrease in the average diameter and speed of mycelium growth, while the range of 15-40% was the most effective in relation to a decrease in conidial production. Also, leaf alcohol extract inhibited the conidial production at concentrations of 5%, and had fungicidal action at concentrations of 15%. From ombusillo leaves a foam index of 250 was obtained. This high concentration of saponins would be at least one cause of the antifungal activity.
Key words: Antifungal activity in vitro, Biological control, Ombusillo, Phytolaccaceae, Saponins.
Resumen: Efecto de los extractos acuoso y alcohólico de la hoja de Phytolacca tetramera (Phytolaccaceae) sobre Colletotrichum gloeosporioides (Ascomycota). Phytolacca tetramera Hauman ombusillo es un endemismo del sudeste de la provincia de Buenos Aires (Argentina). Esta especie presenta acción fungicida contra patógenos oportunistas de humanos. Con el propósito de buscar alternativas naturales para el control de enfermedades en los vegetales, se planteó como objetivo evaluar el efecto de los extractos foliares acuoso y alcohólico de P. tetramera sobre el desarrollo del hongo Colletotrichum gloeosporioides (Penz.) Sacc., el cual tiene amplia distribución en especies de importancia agrícola, forestal y ornamental. El ensayo se realizó in vitro. El hongo fue cultivado en agar papa glucosado (APG), con aplicación del extracto en concentraciones del 5-50%. Las concentraciones del extracto acuoso del 15-30% produjeron una disminución del diámetro y velocidad media de crecimiento del micelio, mientras que las concentraciones del 15-40% fueron las más efectivas en el control de producción de conidios. El extracto alcohólico inhibió la producción de conidios con el 5% de concentración y con el 15% resultó fungicida. A partir de las hojas de ombusillo se obtuvo un índice de espuma de 250. Esta alta concentración de saponinas hace suponer que sería, al menos, una de las causas de la actividad antifúngica.
Palabras clave: Actividad antifúngica in vitro, Control biológico, Ombusillo, Phytolaccaceae, Saponinas.
IntroductIon
Diseases of cultivated crops is being considered as one important limitation to increase agricultural production. Therefore, protection of plants from pathogens remains a primary concern to agricultural scientists (Guleria & Kumar, 2006). Researchers have succeeded in controlling some devastating diseases since the very beginning of their appearance by using synthetic fungicides. On the other hand, the inappropriate use of such fungicides, expressed in terms of type, toxicity, number of applications and dosage have produced pollution that affects the agroecosystem, being the accumulation of waste potentially harmful to human and animal health (Bolivar et al., 2009). The use of plant origin pesticides has been suggested by some workers as natural alternatives to synthetic chemicals (Montes-Belmont, 2009). A series of recent studies have confrmed the effcacy of plant extracts in the control of fungal diseases (Farias Magalhães et al., 2003; Zapata et al., 2003; Sung Og et al., 2007; Bolivar et al., 2009; Pineda et al., 2010; Pérez et al., 2011). There is a history of the presence of active principles in the aqueous and alcohol extracts of the leaves and fruits of several species of the genus Phytolacca L., with analgesic, antiinfammatory, bactericidal, fungicidal, mitogenic and molluscicide action (Nickell, 1959; Parkhurst et al., 1973; Woo & Kang, 1975, 1976; Moreno & Rodríguez, 1981; Kang & Woo, 1987; Yang-Hua, 1989, 1990, 1992; Favel et al., 1994; Nielsen et al., 1995; Gattuso, 1996; Quiroga et al., 2001; Farias Magalhães et al., 2003; Delporte et al., 2009). Also, active principles have been found in fruit methanolic extracts of P. tetramera Hauman, which are a source of saponins with fungicidal action on opportunistic pathogens of humans by Escalante et al. (2002), Santecchia et al. (2002), and Zacchino (2004).
Phytolacca tetramera ombusillo is a shrub endemic of southeastern Buenos Aires, (Argentina). It grows in the districts of Magdalena (35º 05' lat. S-57º 31' long. O), Punta Indio (35º 16' lat. S-57º 13' long. O), Castelli (35° 55' 18.12 lat. S-57º 43' 16.19 long. O), and Chascomús (35º 30' lat. S-58º 30' long. O) (Hauman, 1913; Cabrera, 1949; Cabrera & Zardini, 1978; Guaglianone, 1987; Delucchi, 2006; Galup, 2006; Hernández et al., 2009; Petri et al., 2010) (Fig. 1 A-C).

Fig. 1. A: Phytolacca tetramera plant. B: Leaves and inforescence. C: Inforescences with maturation fruits. Scales: A: 1 m. B, C: 5 cm. Photographs were taken by M. P. Hernández.
Colletotrichum gloeosporioides (Penz.) Sacc. is a cosmopolitan fungus recognized to cause damage to the organs of many cultivated agricultural, forest and ornamental species (e.g., Carica papaya L., Cassia fstula L., Ceiba pentandra (L.) Gaertn., Ceiba speciosa (A. St.-Hil.) Ravenna, Codiaeum variegatum (L.) Rumph. ex A. Juss., Citrus sp., Liquidambar sp., Mangifera indica L., Olea europaea L., Populus spp., Quercus palustris Münch.) (Callan, 1998; Deschamps & Wright, 2000; Benyahia et al., 2003; Cabrera et al., 2004; Meireles Barguil et al., 2008; Sergeeva et al., 2008; Bolívar et al., 2009; Farr & Rossman in nt.ars-grin. gov/fungaldatabases, consulted in 2011).
The control of diseases caused by different species of Colletotrichum depends on the use of healthy seeds, seed treated with hot water, the selection of resistant varieties, and the crop rotation. The products management during and after harvest, and the treatment of packing house and containers are also important, as well as the treatment of plants and seeds in different growth stages, with application of synthetic fungicides (Agrios, 1997; Bolivar et al., 2009).
The aim of this investigation, therefore, is to assess the in vitro antifungal activity of aqueous and alcohol (ethanol) extracts of P. tetramera leaves. This plant species will be tested as potential source of biologically active natural substances against C. gloeosporioides.
materials and methods
Plant material
Fresh and healthy branches with leaves of Phytolacca tetramera were collected. Leaves were separated and washed thoroughly to remove dry dirt covering surfaces and used to perform the assays. The reference material was deposited at herbarium of Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata under the data: Hernández, M. P. 60-67, 22-II-2011 (LPAG).
Fungal agent used
Pure culture of Colletotrichum gloeosporioides was obtained from the Laboratory of Protección Forestal, Facultad de Ciencias Agrarias y Forestales, at Universidad Nacional de La Plata. The isolate was obtained from diseased samples of Ceiba speciosa. It was isolated on 2% Potato Dextrose Agar (PDA), purified and maintained at 4°C until use.
Saponins assay
Determination of saponins was made according to Gallo (1979) methodology. In fact, one gram of fresh leaves with the addition of 100 mL of sterile distilled water was crushed. The resulting liquid of the filtered through cotton was placed to boil in water bath for 30 min. Once cold, it was completed to 100 mL with distilled water. Portions of 1 to 10 mL were taken by placing them in 16 cm x 16 mm test tubes. All were completed to 10 mL with distilled water (Table 1). The blocked tubes were waved in a longitudinal direction for 15 s. After 15 min the columns of foam were measured and the foam rate was obtained (maximum dilution of the sample, which maintains a 1 cm column of foam for more than 15 min).
Table 1. Dilution of aqueous extract (AE) with distilled water (DW).

Preparation of aqueous and alcohol extracts of P. tetramera leaves
One part of fresh leaf samples was exhaustively macerated with sterile distilled water and another identically part of leaves with 96% ethanol (1:1, w/v). The process was carried out at room temperature for 24 h according to Sharapin (2000). The mixtures were filtered and the solvent removed under vacuum in a rotary evaporator. Filtrates were preserved at 4oC. To avoid any prospective chemical alterations, the extracts were used within 3-4 days.
Antifungal bioassays
For each extract (aqueous and ethanol), eleven treatments were performed. Each treatment was replicated ten times. In fact, PDA medium was prepared and sterilized. The leaf extracts were thoroughly mixed with the medium, and poured in each sterilized Petri dishes of 9 cm diameter. Control (0), 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50% concentrations of leaf aqueous and alcohol extracts were used, respectively. After solidification, mycelial discs of 5 mm diameter were taken from 5-7 days old culture of C. gloeosporioides. They were placed in the center of each Petri dish. Dishes were incubated in an incubator at 25 ± 2ºC for 7 days. Aqueous and ethanol extract effects were evaluated by averaging measurements from each colony of the following parameters: colony average diameter growth (ADG), colony average speed growth (ASG), and conidia average production (CAP). The diameter of the mycelium growth rings were measured with a millimeter rule. The conidia count was made using a Nuebauer chamber by means of a Hokenn microscope. The photographs were taken with a Kodak easyshare C 653 digital camera (Fig. 8: A-C).
Statistical analysis
Treatments were arranged in a completely randomized design. All parameters evaluated (ADG, ASG, and CAP) were analyzed through ANOVA (to determine the statistical signifcance level) in statistical program (Statistica 7.0 for windows). To check signifcant differences between the levels of the main factor, Tukey comparison tests at 5% (P < 0.05), signifcance were applied (Figs. 2-7 and Tables 2-7).
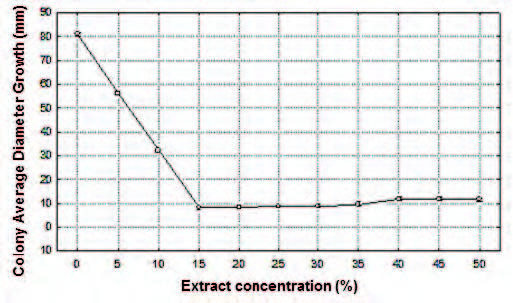
Fig. 2. Effect of aqueous extract of the P. tetramera leaves (%) on the colony average diameter growth (ADG) of C. gloeosporioides, in millimeters (mm).

Fig. 3. Effect of aqueous extract of P. tetramera leaves (%) on the colony average speed growth (ASG) of the C. gloeosporioides, in millimeters per day (mm/d).
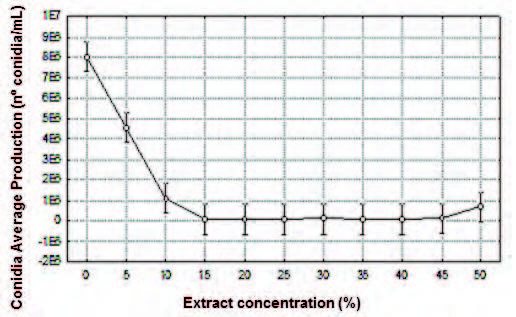
Fig. 4. Effect of aqueous extract of the P. tetramera leaves (%) on the conidia average production (CAP) expressed by number of conidia per milliliter (nº conidia/mL) of the C. gloeosporioides.
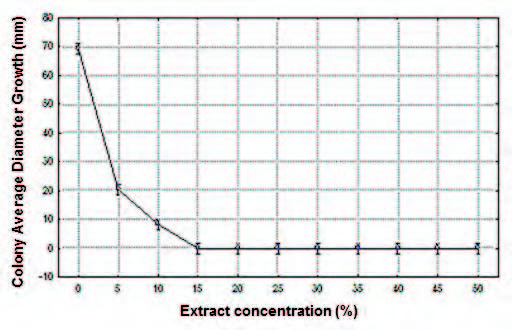
Fig. 5. Effect of ethanol extract of the P. tetramera leaves (%) on the colony average diameter growth (ADG) of C. gloeosporioides, in millimeters (mm).

Fig. 6. Effect of ethanol extract of the P. tetramera leaves (%) on the colony average speed growth (ASG) of C. gloeosporioides, in millimeters per day (mm/d).
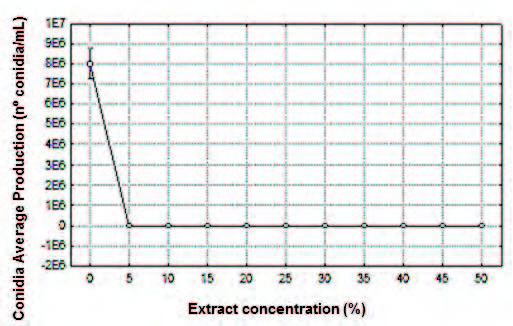
Fig. 7. Effect of ethanol extract of the P. tetramera leaves (%) on the conida average production (CAP) expressed by number of conidia per milliliter (nº conidia/mL) of the C. gloeosporioides.

Fig. 8: Colletotrichum gloeosporioides mycelium growth. A: Control. B: Treatment at alcohol extract concentration of 5%. C: Treatment at alcohol extract concentration of 15%.
results
Saponins assays
The dilution of 4 mL of aqueous extract in 6 mL of distilled water led to obtain 1 cm column of foam for more than 15 min. Foam index = 250.

Effect of aqueous extract on C. gloeosporioides
The aqueous extract of the P. tretramera leaves signifcantly decreased the average of the analyzed parameters (ADG, ASG and CAP) in relation to the control (Figs. 2, 3 and 4; Tables 2, 3 and 4).
Colony Average Diameter Growth (ADG). No signifcant differences were found between the mean values of ADG obtained with extract concentrations in the ranges 15-35% and 40-50%. Within the concentration range of 15-35% mycelium development was not observed. Significant differences were found at extract concentrations of 5%, 10% and respect to the ranges 15-35%, and 40-50%. Contrary to expectations, the extract concentration range of 15-35% exhibited means values signifcantly lower than those obtained within the range 40-50%. The highest average values of ADG were obtained at aqueous extract concentration of 5% and 10%, with signifcant differences between them, and with the rest of the treatments. The highest was at 5%. The lowest average value was obtained at extract concentration of 15%, proving to be the most effective concentration (Fig. 2; Table 2).
Table 2. Multiple Range Test (Tukey, p<0.05) for colony average diameter growth (mm) per extract concentration (%).
| Treatments | Average | Homogeneous groups | ||
| 15 | 8.175 | X | ||
| 20 | 8.500 | X | ||
| 25 | 8.925 | X | ||
| 30 | 9.050 | X | ||
| 35 | 9.500 | X | ||
| 50 | 11.500 | X | ||
| 40 | 11.850 | X | ||
| 45 | 11.865 | X | ||
| 10 | 33.19 | X | ||
| 5 | 56.38 | X | ||
| (control) 0 | 81.050 | X | ||
Table 3. Multiple Range Test (Tukey, p<0.05) for colony average speed growth per day (mm/d) per extract concentration (%).
| Treatments | Average 0.530 | Homogeneous groups X | |
| 20 | |||
| 25 15 | 0.550 0.569 | X X | |
| 30 | 0.570 | X | |
| 35 | 0.585 | XX | |
| 50 | 0.715 | XX | |
| 40 | 0.725 | X | |
| 45 | 0.731 | X | |
| 10 | 2.272 | X | |
| 5 (control) 0 | 3.541 5.789 | X | X |
Table 4. Multiple Range Test (Tukey, p<0.05) for conidia average production (CAP) expressed in number of conidia per milliliter (nº conidia/mL) per extract concentration (%).

Table 5. Multiple Range Test (Tukey, p<0.05) for colony average diameter growth (mm) per extract concentration (%).

Table 6. Multiple Range Test (Tukey, p<0.05) for colony average speed growth per day (mm/d) per extract concentration (%).

Colony Average Speed Growth (ASG). No signifcant differences were found between the mean values of ASG obtained with extract concentrations in the range 15-35%. Contrary to expectations, there was a lower effect within the extract concentration range of 40-50% and no signifcant differences were found in the values obtained. As expected, the highest average values of ASG were obtained at aqueous extract concentrations of 5% and 10% with signifcant differences between them, and with the rest of the treatments. The highest was at 5%. The lowest average value was obtained at extract concentration of 20%, proving to be the most effective concentration (Fig. 3; Table 3).
Conidia Average Production (CAP). A signifcant difference was found between the mean values of conidia production at extract concentration of 5% respect to the range of 10-50%. No signifcant differences were obtained at the extract concentration in the range 10-50%, however the most effective in relation to a decrease in conidial production was the range 15-40%. The lowest average value was obtained at extract concentration of 15%, proving to be the most effective concentration (Fig. 4, Table 4).
Effect of alcohol extract on C. gloeosporioides
The alcohol extract of the P. tretramera leaves signifcantly decreased the average of the analyzed parameters (ADG, ASG and CAP) in relation to the control (Figs. 5, 6 and 7; Tables 5, 6 and 7).
Colony Average Diameter Growth (ADG). No signifcant differences were found between the mean values of ADG obtained with extract concentrations in the ranges 15-50%. Within the concentration range of 15-50% mycelium development was not observed. Signifcant differences were found at extract concentration of 5%, 10% and respect to the range 15-50%. The highest average values of ADG were obtained at alcohol extract concentrations of 5% and 10%, with signifcant differences between them, and with the rest of the treatments. The highest was at 5 %. The lowest average value was obtained at extract concentration of 15 %, proving to be the most effective concentration (Fig. 5; Table 5).
Colony Average Speed Growth (ASG). No signifcant differences were found between the mean values of ASG obtained with extract concentrations in the ranges 15-50%. Within the concentration range 15-50% mycelium development was not observed. Signifcant differences were found at concentrations of 5%, 10% and respect to the range 15-50%. The highest average values of ASG were obtained at alcohol extract concentration of 5% and 10%, with signifcant differences between them, and with the rest of the treatments. The highest was at 5%. The lowest average value was obtained at extract concentration of 15%, proving to be the most effective concentration (Fig. 6; Table 6).
Conidia Average Production (CAP). Leaf alcohol extract concentration at 5% inhibited conidia production (Figure 3). A signifcant difference was found between the mean values of CAP within the concentration range of 5-50% in relation to the control. The average value obtained at extract concentration of 5% proved to be the most effective (Fig. 7, Table 7).
discussion
There is an ample interest to develop environment-friendly alternatives to synthetic fungicides for the control of fungal plant diseases. In fact, there are a large number of contributions about this theme. In this study we compared the in vitro antifungal activities of aqueous and alcohol P. tetramera leaf extracts. Our results demonstrated alcohol extract provided the best fungus control. These results are in agreement with previous references on antifungal activities of different species and substance tested, such as, from essential oils extracted from different plants (Sung Og et al., 2007), saponins from Fabaceae (Farias Magalhaes et al., 2003) to fungicide property of propolis (Pineda et al., 2010). Also, accord with similar determination on Colletotrichum gloeosporioides using ethanol extracts of leaf from different species, which were rich in essential oils (Bolivar et al., 2009). Our test of persistent foam in dilute aqueous solution proved that P. tetramera leaves are rich in saponins. It is knowledge that the presence of saponins in the genus Phytolacca was determined by the frst time by Dominguez (1928) in P. dioica L. Later, many saponins were isolated from different species of Phytolacca (Woo & Kang, 1975, 1976; Kang & Woo, 1987; Yang-Hua, 1989, 1990, 1992; Nielsen et al., 1995; Santecchia et al., 2002). In 1996, Gattuso (1996) referred the presence of the triterpenoid saponins given them a chemotaxonomic significance to the subfamily Phytolaccoideae. On the other hand, it is known that saponins have antifungal activity (Moreno & Rodríguez, 1981; Farias Magalhaes et al., 2003), because their capacity to form complexes with membrane sterols and producing membrane disintegration (Glauert et al., 1962; Montes-Belmont, 2009). Also, the inhibitory effect against human pathogenic fungi activities of saponins isolated from P. tetramera fruits were reported by Escalante et al. (2002), and Zacchino (2004). In this paper, we agree with previous authors and according to their and our results we attributed to this chemical compound the antifungal activity of aqueous and alcohol extracts of P. tetramera leaves.
conclusion
Aqueous and alcohol extract of the P. tretramera leaves signifcantly decreased the average of the analyzed parameters (colony average diameter growth, colony average speed growth, and conidia average production) in relation to the control. However, the alcohol extract was more effective because with a concentration of 5% reduced the mycelium growth of Colletotrichum gloeosporioides and inhibited conidia production, and at a concentration of 15% had fungicidal action. A foam index of 250 was found in P. tetramera leaves. In fact, we attributed to this chemical compound the effect against C. gloeosporioides.
acknowledgements
We are indebted to Maria Alejandra Migoya who helped us to prepare fgures, and Romina Ywatany for valuable suggestions upon the English text. We thank to the reviewers because their suggestions helped to improve the manuscript.
Bibliography
- AGRIOS, G. N. 1997. Plant Pathology. 4th ed. Academic Press, San Diego, California.
- BENYAHIA, H., A. JRIFI, C. SMAIL, M. AFELLAH & L. TIMMER. 2003. Primer informe de Colletotrichum gloeosporioides causando withertip en las ramas y rotura mancha en la fruta de los cítricos en Marruecos. Plant Pathol. 52: 728.
- BOLIVAR, K. M. E., D. SANABRIA, M. DE CAMACARO RODRÍGUEZ, D. ULACIO, L. J. CUMANA & O. CRESCENTE. 2009. Potencial efecto fungicida de extractos vegetales en el desarrollo in vitro del hongo Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. y de la antracnosis en frutos de mango. Revista UDO Agrícola 9: 175-181.
- CALLAN, B. E. 1998. Diseases of Populus in British Columbia: A diagnostic Manual. Natural Resources Canada, Canadian Forest Service, Victoria.
- CABRERA, A. L. 1949. Las comunidades vegetales de los alrededores de La Plata Provincia de Buenos Aires, República Argentina. Lilloa 20: 269-274.
- CABRERA, A. L. & E. M. ZARDINI. 1978. Manual de la fora de los alrededores de Buenos Aires. 2 ed., Acme, Buenos Aires.
- CABRERA, M. G., N. T. SOSA DE CASTRO, R. E. ALVAREZ & A. LOPEZ. 2004. Identifcación de patógenos fúngicos causantes del atizonamiento en lluvia de oro (Cassia fstula L.) en Corrientes, Argentina. Agricultura Técnica 64: 213-219.
- DELUCCHI, G. 2006. Las especies vegetales amenazadas de la Provincia de Buenos Aires: una actualización. APRONA, Bol. Cientif. 39: 19-31. [ Links ]
- DELPORTE, V., G. MIRANDA & O. ASENCIO. 2009. Evaluación de la actividad analgésica aguda y crónica de Phytolacca dioica. Tesis de Grado, Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile.
- DESCHAMPS, J. R. & J. E. WRIGHT. 2000. Micosis de importancia forestal en el cono sur de América. Bol. Soc. Micol. Madrid 25: 127-144.
- DOMÍNGUEZ, J. A. 1928. Contribuciones a la materia médica Argentina. Trabajos del Instituto de Botánica y Farmacología 44: 1- 433. Peuser, Buenos Aires.
- ESCALANTE, A. M., C. B. SANTECCHIA, S. N. LÓPEZ, M. A. GATTUSO, A. GUTIÉRREZ, F. DELLE MONACHE, M. GONZÁLEZ SIERRA & S. A. ZACCHINO. 2002. Isolation of antifungal saponins from Phytolacca tetramera, an Argentinean species in critic risk. J. Ethnopharmacol. 1: 29-34.
- FARIAS MAGALHÃES, A., A. M. GOULART DE AZEVEDO TOZZI, C. CAPARICA SANTOS, D. R. SERRANO, E. M. ZANOTTI-MAGALHÃES, E. GONÇALVES MAGALHÃES & L. A. MAGALHÃES. 2003. Saponins from Swartzia langsdorffi: biological activities. Mem. Inst. Oswaldo Cruz 98: 713-718.
- FARR, D. F. & A.Y. ROSSMAN. 2011. Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA, http://nt.ars-grin.gov/fungaldatabases/ (July 2011).
- FAVEL, A., M. D. STEINMETZ, P. REGELI, E. VIDAL-OLLIVIER, R. ELÍA & G. BALANSARD. 1994. In vitro antifungal activity of triterpenoid saponins. Planta Med. 60: 50-53.
- GALUP, A. 2006. El ombusillo, una fgura emblemática. En: MÉRIDA E. & J. ATHOR (eds.), Talares bonaerenses y su conservación, pp. 244-245. Fundación de Historia Natural Félix de Azara, Universidad Maimónides, Buenos Aires.
- GALLO, G. G. 1979. Plantas tóxicas para el ganado en el cono sur de América, Eudeba, Buenos Aires.
- GATTUSO, M. A. 1996. Estudio anatómico, ultraestructural y ftoquímico de las Phytolaccaceae de la Argentina. Tesis Doctoral, Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario.
- GLAUERT, A. M., J. T. DINGLE & J. A. LUCY. 1962. Action of saponin on biological cell membranes. Nature 196: 953-955.
- GUAGLIANONE, E. R. 1987. Phytolaccaceae. En: BURKART, A. (ed.), Flora ilustrada de Entre Ríos 6: 209-232. Colec. Cientif. INTA, Buenos Aires.
- GULERIA, S. & A. KUMAR. 2006. Antifungal activity of some Himalayan medicinal plants using direct bioautography. J. Cell Mol. Biol. 5: 95-98.
- HAUMAN, L. 1913. Notes Sur les Phytolaccacées argentines. Anal. Mus. Nac. Hist. Nat. Buenos Aires 24: 471-516. [ Links ]
- HERNÁNDEZ, M. P., D. A. GALLO & D. A. FERNÁNDEZ. 2009. Conserving ombusillo, an endangered plant from the province of Buenos Aires, Argentinian. Revista Colomb. Biotecnol. 10: 132-142.
- KANG, S. S. & W. S. WOO. 1987. Two new saponins from Phytolacca americana. Planta Med. 53: 338-340.
- MEIRELES BARGUIL, B., J. E. AGUIAR BESERRA JR. & S. M. ALVES DE OLIVEIRA. 2008. Leaf spots on Codiaeum variegatum caused by Colletotrichum gloeosporioides. Summa Phytopathol. 34: 289-289.
- MONTES-BELMONT, R. 2009. Diversidad de compuestos químicos producidos por las plantas contra hongos ftopatógenos. Revista Mex. Micol. 29: 73-82. [ Links ]
- MORENO, M. & V. M. RODRIGUEZ. 1981. Yiamoloside B, a fungistatic saponin of Phytolacca octandra. Phytochemistry 20: 1446-1447.
- NICKELL, L. 1959. Antimicrobial activity of vascular plants. Econ. Bot. 13: 281-318. [ Links ]
- NIELSEN, S. E., U. ANTHONY, C. CHRISTOPHERS & C. CORNETT. 1995. Triterpenoid saponins from Phytolacca rivinoides and Phytolacca bogotensis. Phytochemistry 39: 625-630.
- PARKHURST, R. M., D. W. THOMAS & W. A. SKINNER. 1973. Molluscicidal saponins of Phytolacca dodecandra. Phytochemistry 12: 1437-1442.
- PÉREZ, C. A., S. J. ROJAS, A. L. CHAMORRO & P. K. PÉREZ. 2011. Evaluación de la actividad antifúngica de Melia azederach sobre aislados de Colletotrichum spp. Revista Colomb. Ci. Anim. 3: 309-320.
- PETRI, I. M., D. J. GALLO & F. M. OLLIER. 2010. Primera área protegida, en el partido de Magdalena para la preservación del Ombusillo (Phytolacca tetramera Hauman), in situ. Revista Colomb. Biotecnol. 12: 259-261.
- PINEDA, J., J. PRINCIPAL, C. BARRIOS, D. MILLA, Y. SOLANO & E. GIL. 2010. Propiedad fungistática in vitro de propóleos sobre tres aislamientos de Colletotrichum gloeosporioides. Zootec. Trop. 28: 83-91.
- QUIROGA, E. N., A. R. SAMPIETRO & M. A. VATTUONE. 2001. Screening antifungal activities of selected Medicinal plants. J. Ethnopharmacol. 74: 89-96.
- SANTECCHIA, C., A. ESCALANTE, M. GATTUSO, M. ZACCHINO, A. GUTIERREZ RAVELO, F. DELLE MONACHE & F. GONZALEZ SIERRA. 2002. Phytolacca tetrámera, una fuente de saponinas triterpenoides. Revista Lat. Am. Quim. 28: 246-247.
- SERGEEVA, V., N. G. NAIR & R. SPOONER-HART. 2008. Evidence of early fower infection in olive (Olea europaea) by Colletotrichum acutatum and C. gloeosporioides causing anthracnose disease. Australas. Plant Dis. 3: 81-82.
- SHARAPIN, N. 2000. Fundamentos de tecnología de productos ftoterapéuticos, CYTED, Bogotá.
- SUN OG, L., G. J. CHOI, K. SOO JANG, H. KYOUNG LIM, K. YUN CHO & JIN-CHEOL KIM. 2007. Antifungal activity of fve plant essential oils as fumigant against postharvest and soilborne plant pathogenic fungi. Plant Pathol. J. 23: 97-102.
- WOO, W. S. & S. S. KANG. 1975. The occurrence and chemistry of Phytolacca triterpenoids. J. Pharm. Soc. Korea 19: 189-208.
- WOO, W. S. & S. S. KANG. 1976. Phytolaccoside B: triterpene glucoside from Phytolacca americana. Phytochemistry 15: 1315-1317.
- YANG-HUA, Y. 1989. A new active saponin from Phytolacca esculenta. Planta Med. 55: 551-552.
- YANG-HUA, Y. 1990. Sculentoside L and K: two news saponins from Phytolacca esculenta. Planta Med. 56: 301-303.
- YANG-HUA, Y. 1992. A triterpenoid saponin from Phytolacca esculenta. Phytochemistry 31: 2552-2554.
- ZACCHINO, S. A. 2004. Productos naturales y análogos sintéticos con propiedades inhibitorias de hongos ftopatogénicos y oportunistas humanos. Estudios de mecanismos de acción. Revista Soc. Quím. México 1: 48.
- ZAPATA, R., M. E. SANABRIA & D. RODRÍGUEZ. 2003. Reducción del desarrollo de hongos ftopatógenos con extracto de cardón lefaria (Cereus defciens Otto & Diert). Interciencia 28: 302-307.
Recibido el 10 de mayo de 2012, aceptado el 3 de julio de 2012.














