Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.48 no.2 Córdoba ago. 2013
GENÉTICA Y EVOLUCIÓN
Karyotypic studies in wild species of Arachis (Leguminosae) belonging to sections Erectoides, Procumbentes and Rhizomatosae
Estudios cariotípicos en especies silvestres de Arachis (Leguminosae) pertenecientes a las secciones Erectoides, Procumbentes y Rhizomatosae
Alejandra M. Ortiz, María C. Silvestri and Graciela I. Lavia
Instituto de Botánica del Nordeste C.C. 209, 3400 Corrientes, Argentina. Facultad de Ciencias Exactas y Naturales y Agrimensura (UNNE), Corrientes, Argentina. lavia@agr.unne.edu.ar
Summary: Karyotypes of three diploid species belonging to sections Erectoides (Arachis hermannii), Procumbentes (A. rigonii) and Rhizomatosae (A. burkartii) were analyzed by Feulgen's technique. The karyotype formula was different in each of the taxa analyzed: 2n=2x=16m+4sm in A. hermannii, 2n=2x=18m+2sm in A. rigonii, and 2n=2x=20m in A. burkartii. All species had a pair of satellited chromosomes, which corresponded to type 2 in A. hermannii, type 9 in A. rigonii, and type 8 in A. burkartii. Arachis hermannii and A. rigonii presented chromosomal features similar to those of the other species included in their respective sections. However, A. burkartii showed chromosome characteristics different from those found in the rest of the species of section Rhizomatosae.
Key words: Arachis hermannii, A. rigonii, A. burkartii, Chromosomes, Phylogenetic relationships.
Resumen: Estudios cariotípicos en especies silvestres de Arachis (Leguminosae) pertenecientes a las secciones Erectoides, Procumbentes y Rhizomatosae. Los cariotipos de tres especies diploides pertenecientes a las secciones Erectoides (A. hermannii), Procumbentes (A. rigonii) y Rhizomatosae (A. burkartii) fueron analizados mediante la técnica de Feulgen. Las fórmulas cariotípicas obtenidas son diferentes en los taxones analizados, 2n=2x=16m+4sm en A. hermannii, 2n=2x=18m+2sm en A. rigonii, y 2n=2x=20m en A. burkartii. Las tres especies presentaron un par de cromosomas con satélite, en A. hermannii tipo 2, en A. rigonii tipo 9 y en A. burkartii tipo 8. Arachis hermannii y A. rigonii presentaron características cromosómicas similares a las especies incluidas en sus respectivas secciones. Sin embargo, A. burkartii no comparte características cromosómicas con el resto de las especies de la sección Rhizomatosae.
Palabras clave: Arachis hermannii, A. rigonii, A. burkartii, Cromosomas, Relaciones flogenéticas.
Introduction
Arachis is an exclusively South American genus presenting around eighty annual or perennial species (Krapovickas & Gregory, 1994; Valls & Simpson, 2005), distributed east of the Andes, from southern Amazon River to the north of the Plata River. They are organized in nine sections (Trierectoides, Erectoides, Extranervosae, Triseminatae, Heteranthae, Caulorrhizae, Procumbentes, Rhizomatosae and Arachis), according to their morphology, chromosome characteristics, and cross-compatibility relations (Krapovickas & Gregory, 1994).
Peanut (A. hypogaea L.) is one of the most important sources of dietary protein in the world. However, considering its productivity, this crop remains underexploited because of its susceptibility to pests and diseases. The main constraint to the genetic improvement of peanut is the narrow genetic base of the extant cultigen. Wild Arachis species, by contrast, are diverse and have the genetic variability and agronomically useful characters needed to improve the cultigen (Holbrook & Stalker, 2003) and constitute valuable resources for the genetic upgrading of peanut. In this sense, information on the cytogenetics and phylogenetic relationships among wild species and between these species and the cultigen is critical to the rational development of breeding programs and complete utilization of the wild materials.
Chromosomal data have played a pivotal role in accelerating crop improvement (Jauhar, 2006) and our understanding of the phylogenetic relationships between wild and cultivated species (Cao, 2003). On the other hand, cytological data are of great importance in the study of plant evolution and diversifcation (Stebbins, 1950; 1971; Hong, 1990; Stace, 2000). Moreover, the analysis of karyotype characteristics has contributed valuable data for inferring evolutionary trends within particular plant groups and analyzing traits such as changes in chromosome numbers (Mercado-Ruaro & Delgado-Salinas, 1998), karyotype symmetry, and chromosome length (Poggio et al., 2007).
The most comprehensive work on cytogenetics in Arachis was carried out by Fernández & Krapovickas (1994), who analyzed species belonging to different sections. Chromosome numbers are currently known for 95% species of Arachis. Most of them are diploids based on x=10 (67), few (4) are diploids based on x=9, and some (5) are tetraploids with x=10 as base number (Peñaloza et al., 1996; Lavia, 1998; 2000; Peñaloza & Valls, 2005). All diploids with x=18 belong to section Arachis (A. decora, A. palustris and A. praecox), except A. porphyrocalyx, which is included in section Erectoides.
Phylogenetic relationships among them have been investigated by molecular analysis, crossability experiments and cytogenetic studies (Lavia et al., 2008 and references therein; Lavia et al., 2009). However, the chromosome characteristics of each Arachis species are not yet described. Therefore, to increase the cytogenetic knowledge of this genus, this work presents the karyotype characteristics of the three diploid species belonging to the sections
Erectoides (A. hermannii Krapov. & W.C. Gregory), Procumbentes (A. rigonii Krapov. & W.C. Gregory) and Rhizomatosae (A. burkartii Handro).
materIals and methods
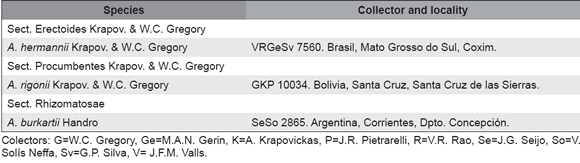
The material studied is presented in Table 1. Voucher specimens were deposited in the herbaria of the Instituto de Botánica del Nordeste (CTES), Corrientes, Argentina, and of the Centro Nacional do Recursos Genéticos e Biotenología (CEN), Brasilia, Brazil.
Table 1. Studied material.

Colectors: G=W.C. Gregory, Ge=M.A.N. Gerin, K=A. Krapovickas, P=J.R. Pietrarelli, R=V.R. Rao, Se=J.G. Seijo, So=V. Solís Neffa, Sv=G.P. Silva, V= J.F.M. Valls.
The material used for this study was provided by the Instituto de Botánica del Nordeste, Corrientes, Argentina. Mitotic preparations were obtained from root tips. After a pretreatment of 3 h in 0.002 M 8-hydroxyquinoline solution at room temperature, the material was fxed in ethanol:acetic acid (3:1), stained following Feulgen`s technique, and then squashed in a drop of 2% acetic orcein.
At least three plants per species and fve metaphase plates per individual were used for chromosome measurements using the free version of the MicroMeasure 3.3 program (http://www.colostate.edu/Depts/Biology/MicroMeasure/).
For the numerical characterization of the karyotypes, the following parameters were calculated: total chromosome length (TCL), mean length of the chromosomes (ML), mean centromeric index (CI), intrachromosomal asymmetry index (A1) and interchromosomal asymmetry index (A2) (Romero Zarco, 1986). SAT chromosomes were classifed according to Fernández & Krapovickas (1994) and Lavia (2000).
The nomenclature followed for karyotype description was proposed by Levan et al. (1964).
Chromosome morphology was determined using the centromeric index (short arm x 100/total length). Accordingly, chromosomes were classified as metacentric (m) = 50-37.5 and submetacentric (sm) = 37.5-25. Mean karyotype values for each species were represented as haploid complements in the idiograms. Chromosomes were ordered primarily by morphology and then by decreasing size.
A cluster analysis of the karyotype data was carried out to examine karyotype similarity among species and sections. A data matrix of 16 operational taxonomic units (OTUs) x 2 variables (the mean chromosome length and type of SAT chromosome, Fig. 3B) was constructed using data obtained in this paper and those obtained by Fernández & Krapovickas (1994) and Lavia (2001). Clustering was performed using the unweighted pair-group method (UPGMA) with the software InfoStat (2008). The cophenetic correlation was 0.93 indicating a good ft between the cophenetic value matrix and the mean taxonomic distance matrix.
results and discussion
Mitotic chromosomes and idiograms are shown in Figs. 1 and 2. Karyotype formula and the parameters analyzed for the species are summarized in Table 2. Firstly, we describe general chromosome characteristics and secondly we discuss each particular species.

Fig. 1. Mitotic chromosomes of Arachis species. A: A. hermannii. B: A. rígonii. C: A. burkartii. SAT chromosomes: ► arm 1 + proximal segment, ► satellite. Bar = 5 pm.

Fig. 2. Idiograms of Arachis species. A: A. hermannii (Section Erectoides). B: A. rígonii (Section Procumbentes). C: A. burkartii (Section Rhizomatosae). H Satellite. Bar = 2 pm.
Table 2. Karyotype parameters for Arachis species. Karyotype, SAT chromosome type (SAT), total chromosome length (TCL), mean chromosome length (ML), size range, centromeric index (CI), intrachromosomal asymmetry index (A1), interchromosomal asymmetry index (A2). TCL, ML and size range in µm.
The karyotypes of A. hermannii and A. burkartii are reported for the frst time here, while the karyotype of A. rigonii was presented previously in Cai et al. (1987). As a whole, the karyotypes were symmetrical, and most chromosomes were metacentric. However, the karyotype formula was different in the three entities: 2n=20=16m+4sm in A. hermannii (Figs. 1A and 2), 2n=20=18m+2sm in A. rigonii (Figs. 1B and 2) and 2n=20=20m in A. burkartii (Figs. 1C and 2). Previously, Cai et al. (1987) found 12m+6sm+2st in A. rigonii. However, the same accession was used in both studies since this species is known only from the type locality, Santa Cruz de la Sierra in Bolivia. Therefore, the higher number of submetacentric chromosomes and even the presence of subtelocentric chromosomes found by Cai et al. (1987) could be due to the different conditions of the root pretreatment. In this study, roots were pretreated for 3 h at 25ºC, whereas in other work, roots were pretreated for 4-6 h at 0-4ºC. These different pretreatments may be responsible for differential chromosome condensation resulting in different chromosome morphology.
All the chromosomes analyzed belong to the small category according to Lima de Faria (1980), because the average of chromosome length varied from 1.56 to 2.94 µm. Among these species, A. hermannii presented the longest chromosome length (51.77 µm), while A. rigonii (46.82 µm) and A. burkartii (45.56 µm) presented intermediate chromosome lengths.
Arachis hermannii presented a mean chromosome
length of 2.59 µm, and a pair of SAT chromosomes type 2 (Figs. 1A and 2). This type of SAT chromosomes has also been found in other species of section Erectoides and suggested to be one of the most primitive of the genus (Fernández & Krapovickas, 1994; Lavia, 2001). Inside section Erectoides it has been observed that the species could be included in two groups with different morphological (Fernández & Krapovickas, 1994) and cytogenetic (Lavia, 2001) characteristics: one with shorter chromosomes (between 2.11 and 2.34 µm) and a pair of SAT chromosomes type 3 or 4, and the other with larger chromosomes (between 2.80 and 2.96 µm) and SAT chromosomes type 2 (Fig. 3A). Therefore, A. hermannii should be included in the second group (Fig. 3A). However, since only 28.57 % of species of section Erectoides have been karyotypically analyzed, it would be necessary to study a higher number of species of the section to clarify the intrasectional relationships.

Fig. 3. A) Phenogram of unwighted-pair group method (UPGMA) based on the mean chromosome length (ML) and type of SAT chromosome for eleven Arachis diploid species belonging to sections Erectoides (^■), Procumbentes () and Rhizomatosae (*). B) Valúes of the mean chromosome length (ML) in um and type of SAT chromosome of these species. Abbreviations: Aher = A. hermannii, Amaj = A. major, Adou = A. douradiana, App = A. paraguariensis subsp. paraguariensis, Ast = A. stenophylla, Apc = A. paraguariensis subsp. capibariensis, Abur = A. burkartii, Arig = A. rígonii, Alig = A. lignosa, Asub = A. subcoríacea, Akret = A. kretschmerí, Aappr = A. appressipila. 1 Fernández & Krapovickas (1994), 2 Lavia (2001).
Arachis rigonii presented a mean chromosome length of 2.34 µm and a pair of SAT chromosomes type 9 (Figs. 1B and 2). This type of satellite and mean chromosome lengths between 2.30 and 2.38 µm have been proposed as differential chromosomal characteristics of species of section Procumbentes (Fernández & Krapovickas, 1994; Lavia, 2001); therefore, A. rigonii shares the characteristics of this section (Fig. 3). On the other hand, A. rigonii can produce hybrids with other species of section Procumbentes (Krapovickas & Gregory, 1994) and presents low values of asymmetry indices (A1=0.12 and A2=0.13). These two features suggest that this species could be considered as one of the most primitive of the section Procumbentes.
Arachis burkartii presented a pair of SAT chromosomes type 8 (Figs. 1C and 2). However, this type of SAT is not found in the rest of the species of section Rhizomatosae, which are tetraploids mand have SAT chromosomes type 3 (Peñaloza & Valls, 2005; Ortiz, 2012). Previous studies have found genetic differentiation between species with different ploidy level of section Rhizomatosae, since A. burkartii (2x) clusters in a group different from that of 4x species (Bechara et al., 2010; Wang et al., 2011). Therefore, it would be necessary to carry out more cytogenetic studies to bring light over the phylogenetic relationships between the species of this section.
Acknowledgements
This study was supported with funds from Consejo Nacional de Investigaciones Científcas y Tecnológicas –CONICET, PIP 6265-, Secretaría General de Ciencia y Técnica de la Universidad Nacional del Nordeste –SGCyT UNNE, PI 038-2008- and Agencia Nacional de Promoción Científca y Tecnológica –ANPCyT, PICTO 2007-00099.
bibliography
- BECHARA, M.D., M.C. MORETzSOHN, D.A. PALMIERI, J.P. MONTEIRO, M. BACCI JR., J. MARTINS JR., J.F.M. VALLS, C.R. LOPES & M.A. GIMENES. 2010. Phylogenetics relationships in genus Arachis based on ITS and 5.8S rDNA sequences. BMC Plant Biol. 10: 255.
- CAI, Q., S. LU & C.C. CHINNAPPA. 1987. Analysis of karyotypes and Giemsa C-banding patterns in eight species of Arachis. Genome 29: 187-194.
- CAO, W. 2003. Cytogenetic and molecular genetic evidence on evolution of genus Triticum. In: Sharma, A.K. & Sharma A. (eds.) Plant Genome. Biodiversity and Evolution. Vol. 1A: Phanerogam – Angiosperm. pp 223-247. Enfeld (NH), USA, Science Publishers.
- FERNÁNDEz, A. & A. KRAPOVICKAS. 1994. Cromosomas y Evolución en Arachis (Leguminosae). Bonplandia 8: 187-220.
- HOLBROOK, C.C. & H.T. STALKER. 2003. Peanut breeding and genetic resources. In: Janick J. (ed.). Plant Breeding Reviews. Volume 22. pp 297-356, John Wiley & Sons.
- HONG, D.Y. 1990. Plant Cytotaxonomy. Science Press, Beijing.
- INFOSTAT. 2008. InfoStat version 2008. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
- JAUHAR, P.P. 2006. Modern biotechnology as an integral supplement to conventional plant breeding: the prospects and challenges. Crop. Sci. 46: 1841-1859. [ Links ]
- KRAPOVICKAS, A. & W.C. GREGORY. 1994. Taxonomía del género Arachis (Leguminosae). Bonplandia 8: 1-186.
- LAVIA, G.I. 1998. Karyotypes of Arachis palustris and A. praecox (section Arachis), two species with basic chromosome number x = 9. Cytologia 63: 177–181. [ Links ]
- LAVIA, G.I. 2000. Chromosomes studies in wild Arachis (Leguminosae). Caryologia 53: 277-281. [ Links ]
- LAVIA, G.I. 2001. Chromosomal characterization of germplasm of wild species of Arachis L. belonging to sections Trierectoides, Erectoides and Procumbentes. Caryologia 54: 115-119.
- LAVIA, G.I., A. FERNÁNDEz & J.G. SEIJO. 2008. Cytogenetic and molecular evidences on the evolutionary relationships among Arachis species. In: Sharma, A.K. & Sharma A. (eds.). Plant genome. Biodiversity and evolution V 1E, pp 101–134. Calcutta, Science Publishers.
- LAVIA, G.I., A.M. ORTIz & A. FERNÁNDEz. 2009. Karyotypic studies in wild germplasm of Arachis (Leguminosae). Genetic resources and crop evolution. 56: 755-764.
- LEVAN, A., K. FREDGA & A.A. SANDBERG .1964. Nomenclature for centromeric position on chromosomes. Hereditas 52: 201-220.
- LIMA DE FARíA, A. 1980. Classifcation of genes, rearrangements and chromosomes according to the chromosome feld. Hereditas 93: 1-46. [ Links ]
- MERCADO-RUARO, P. & A. DELGADO-SALINAS. 1998. Karyotypic studies on species of Phaseolus (Fabaceae: Phaseolinae). Amer. J. Bot. 85: 1-9.
- ORTIZ, A. 2012. Análisis de la constitución genómica de las especies de la sección Rhizomatosae del género Arachis mediante citogenética clásica y molecular. Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Argentina. Tesis doctoral.
- PEñALOZA A, MT POzzOBON, JFM VALLS. 1996. Cytogenetic fndings in wild species of Arachis (Leguminosae). 42° Congresso Nacional de Genética, Caxambu, MG, Brasil.
- PEñALOzA, A. & J.F.M. VALLS. 2005. Chromosome number and satellite chromosome morphology of eleven species of Arachis (Leguminosae). Bonplandia 14: 65–72.
- POGGIO, L., G. GONzÁLEz & C.A. NARANJO. 2007. Chromosome studies in Hippeastrum (Amaryllidaceae): variation in genome size. Bot. J. Linn. Soc. 155: 171-178.
- ROMERO zARCO, C. 1986. A new method for estimating karyotype asymmetry. Taxon 35: 526-530. [ Links ]
- STACE, C.A. 2000. Cytology and Cytogenetics as a Fundamental Taxonomic Resource for the 20th and 21st Centuries. Taxon 49: 451-477. [ Links ]
- STEBBINS, G.L. 1950. Polyploidy I: Occurrence and nature of polyploid types. En: Variation and evolution in plants. pp 298-341, New York, Columbia University Press.
- STEBBINS, G.L. 1971. Chromosomal evolution in higher plants. Addison-Wesley, London.
- VALLS, J.F.M. & C.E. SIMPSON. 2005. New species of Arachis (Leguminosae) from Brazil, Paraguay and Bolivia. Bonplandia 14: 35-64.
- WANG, C.T., X.z. WANG, Y.Y. TANG, D.X. CHEN, F.G. GUI, J.C. zHANG, S.L. YU. 2011. Phylogeny of Arachis based on internal transcribed spacer sequences. Genet. Resour. Crop Evol. 58: 311-319.
Recibido el 10 de mayo de 2012, aceptado el 25 de septiembre de 2012.














