Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.50 no.1 Córdoba mar. 2015
ECOLOGÍA Y FITOGEOGRAFÍA
Interactions between Eriosyce villicumensis (Cactaceae) and shrubs: a study case in the hyper arid Monte desert of Argentina
Martín Guillermo Almirón1 and Eduardo Martínez Carretero2
1 National Council of Scientific and Technical Research. Department of Biology, Faculty of Exact, Physical and Natural Sciences, National University of San Juan; Av. Ignacio de la Roza (Oeste) 599, J5402DCS Rivadavia, San Juan, Argentina. martinalmiron2000@hotmail.com
2 National Council of Scientific and Technical Research. Geobotany and Phytogeography, Argentine Institute for Arid Zone Research, CC 507, 5500 Mendoza, Argentina. mcarrete@mendoza-conicet.gob.ar
Summary
The spatial intraspecific and interspecific distribution of Eriosyce villicumensis (Rausch) Katt. was studied in three physiographic units and at micro-scale under shrubs. The influence of shrubs on photosynthetically active radiation and on soil temperature was analyzed. The intraspecific distribution of E. villicumensis was random. The interspecific distribution was highly associated with Larrea cuneifolia and/or Zuccagnia punctata, observing decreasing association from the centre toward the edge of shrub canopies, coinciding with both the light and temperature gradients detected under those shrubs. In desert environments, the interaction between species is a strategy that allows survival of several plants.
Key words: Wiegand-Moloney O-ring statistics; Specific plant association; Cactaceae.
Resumen
Interacciones entre Eriosyce villicumensis (Cactaceae) y arbustos: Un caso de estudio en el desierto hiperárido del Monte de Argentina. Se estudió la distribución espacial intraespecífica e interespecífica de Eriosyce villicumensis (Rausch) Katt. en tres unidades fisiográficas y a microescala bajo los arbustos. Se analizó la influencia de los arbustos en la radiación fotosintéticamente activa y temperatura del suelo. La distribución intraespecífica de E. villicumensis resultó aleatoria. La distribución interespecífica estuvo asociada con Larrea cuneifolia y/o Zuccagnia punctata, observándose disminución de asociación desde el centro al borde de los arbustos, coincidiendo con los gradientes de luz y temperatura detectados bajo los arbustos. En ambientes desérticos, la interacción entre especies es una estrategia que permite la supervivencia de varias especies.
Palabras clave: Estadística Wiegand-Moloney O-ring; Asociación específica de plantas; Cactaceae.
Introduction
The importance of intra and interspecific plantplant interactions is nowadays widely accepted. Both positive (Callaway and Walker, 1997; Hacker and Gaines, 1997) and negative interactions (Yeaton & Cody, 1976; Yeaton, 1978; Connell, 1983; Schoener, 1983) coexist simultaneously, resulting in a dynamic balance (Callaway & Walker, 1997; Holmgren et al., 1997; Tielbörger & Kadmon, 2000) which depends also on the life stages of intervening plants (Valiente-Baunet et al., 1991; Flores-Martínez et al., 1994) and on environmental conditions (Tielbörger & Kadmon, 2000; Armas & Pugnaire, 2005). Apparently, facilitation increases in situations of stress, whereas negative interactions prevail under more beneficial conditions (Bertness & Callaway, 1994; Callaway & Walker, 1997; Tewksbury & Lloyd, 2001).
In desert environments, some cactus species have been reported to associate with shrubs that attenuate high solar radiation and soil temperature (Franco & Nobel, 1989; Valiente-Baunet & Ezcurra, 1991; Valiente-Baunet et al., 1991; Leirana- Alcocer & Parra-Tabla, 1999; Carrillo-Garcia et al., 2000; Mandujano et al., 2002, Almirón & Martínez Carretero, 2010), to improve moisture and nutrient content (Carrillo-Garcia et al., 2000; Mendez et al., 2004), to protect seedlings against herbivory (Valiente-Baunet & Ezcurra, 1991). Some shrubs could provide preferential sites for seed deposition. In this sense, De Viana et al. (2000) suggest that Larrea cuneifolia and Prosopis ferox are used like perch by some frugivorous bird species. On the other hand, allelopathic effects were suggested (De Viana et al., 2000). Cazón et al. (2002) showed inhibitory effects on the germination of Trichocereus pasacana seeds by chloroformic extract of flavonoids from Baccharis boliviensis. Also were detected competition for resources (McAuliffe, 1984; Franco & Nobel, 1989; Flores-Martínez et al., 1994) between shrubs and cacti. Godinez-Alvarez et al. (2003) suggest that majority observed distribution patterns is aggregated, sometimes related to patch distribution of environmental conditions such as environmental improvement under nurse plants, and/or patch distribution of seeds on the soil. In the literature about cacti ecology, scarce works take in account both intra and interspecific interactions (Almirón & Martínez Carretero, 2010, 2013a), normally they consider interspecific interactions (De Viana, 2000; Mendez et al., 2004, and others cited above) due to the statistical tests applied (Chi2, Test G, among others).
The different modes of reproduction (sexual and/or clonal) employed by beneficiary cacti could be determinant in their relationship with potential nurse shrubs. Columnar and globose cacti can only reproduce sexually through seed germination, while opuntioid cacti are able to reproduce both sexually (by seed germination) and asexually (by rooting of cladodes). Cladodes are clonal reproduction units which are more tolerant than seedlings to extreme bare soil conditions. This hypothesis is reported by some authors (Mendez et al., 2004; Lopez & Valdivia, 2007; Almirón & Martínez Carretero, 2010, 2013b), suggesting that opuntioid cacti can develop both under shrubs and in open areas, while columnar and globose cacti grow exclusively under shrubs. The interaction between cacti of the same species (intraspecific interaction) could be influenced by density-dependent processes, considering physiographic units (at any scale, landscape with homogeneous geomorphology, plant physiognomy and environmental conditions) and by the environmental variability associated with each of these units (Almirón & Martínez Carretero, 2013a). On this account, the present study was directed to determine both associations (intraspecific and interspecific), using the spatially explicit technique proposed by Wiegand & Moloney (2004).
In order to advance in the knowledge of the ecology of interactions between cacti and shrubs, the objective of this work was to analyze the spatial distribution patterns of E. villicumensis in relation to other individuals of its population (intraspecific distribution) and to those shrubs forming micro-sites that act as potential generators of environmental diversity (interspecific distribution), in the central hyper-arid Monte desert.
Materials and Methods
Study area The study was conducted at the Matagusanos locality (31° 13′ 17′′ S-68° 39′ 7′′ W) in San Juan province, Argentina. Average annual precipitation is 84 mm, 72 % of which occurs during the summer period. Mean annual temperature is 20°C, with a max. mean of 40°C and an min. mean of 16°C (Poblete & Minetti, 1999). Shrubland is the dominant physiognomy, with an average plant cover of 20%. Dominant species are Larrea cuneifolia Cav., Bulnesia retama (Gillies ex Hook. & Arn.) Griseb., Zuccagnia punctata Cav., Bougainvillea spinosa (Cav.) Heimerl., among others. At working scale, the landscape is heterogeneous with sandy and rocky plains and hillsides with east and west exposure, both significantly affected by water erosion. At landscape scale, four physiographic units were detected in the study area (at geotope level), different in slope, exposure and shrub cover and diversity: alluvial plain (AP), east-exposed hillside (EH), west-exposed hillside (WH) and sandy plain (SP). Because no E. villicumensis individuals were recorded in the SP unit, this unit was not considered in the analysis.
The study area (Fig. 1) is one of the sites most densely populated with E. villicumensis. Close to this population are several industrial complexes related to mining and quarrying of limestone. Eriosyce villicumensis (Rausch) Katt. E. villicumensis has light grey-green sub globular stems, with whitish powdery surface, with black spines curved upward. The orange-yellow flowers are funnelform. Fruits are globular, dark brown, with hard wall and dry. The seeds are asymmetric with black-brown testa (Kattermann, 1994). The genus Eriosyce is distributed along the west of South America, from center of Chile to south-west of Perú. In the west of Argentina, from Mendoza to Salta. Particularly, E. villicumensis only was found on the Villicum hill from San Juan, Argentina (Katterman, 1994).

Fig. 1. Study Area (A). Industrial complexes for limestone mining (B y D). Waste deposits in the open (C). Protected Natural Area "Loma de las Tapias" (E).
Methodology
In each physiographic unit, eight plots of 6 x 10 m were randomly established. We measured height and the largest and smallest canopy diameters on all shrubs found within each plot, and determined average size for each shrub species. Because of the low plant density values, height and shrub cover were averaged for all physiographic units (Table 1). Nomenclature of plant species follows Zuloaga et al. (2008). To analyze the microenvironment under shrubs, photosynthetically active radiation (PAR) was measured with a digital radiometer (QMSS-S Apogee®), and temperature (Temp) 7 cm above ground using rapid response alcohol thermometers. All data were always taken from the same shrubs (all shrubs within each plot and considering the shrub species). Measurements were made at midday and at three positions on shrub canopies: centre, midpoint and edge, throughout one day in the middle of the spring, summer, autumn and winter periods. Simultaneously, PAR and Temp were recorded for bare soil at four random points on each plot; the average of these values was taken as reference value.
Table 1. ANOVA results showing the effect of shrubs in Δ PAR (μmol*m-2*s-1) and Δ Temp (°C). Different letters indicate significant differences for Tukey Test (p<0.05). Mean average and Standard Deviation of cover canopies (m2) and height (m) of shrubs in the physiographic units

To establish a benchmark for comparison and to eliminate variation in the readings due to the time elapsed between measurements in one plot and the next, the values of PAR and Temp recorded beneath shrubs were subtracted from the average reference value of PAR and Temp recorded for bare soil, in each plot and by season. Thus, one ΔPAR and one ΔTemp value was obtained for each combination of factors (shrub species and position beneath shrubs). Subsequently, the average annual value was calculated per shrub species and position beneath shrubs, averaging all three physiographic units, because the density of each shrub individuals in each physiographic unit was lower to statistical request. The effect of these factors on ΔPAR and ΔTemp and the interaction between them were analyzed using ANOVA factorial and mean separation with Tukey test (p <0.05). Normality and homoscedasticity of residuals were verified with Shapiro-Wilks (Mahibbur & Govindarajulu, 1997) and Levene (Montgomery, 1991) tests, respectively. In each physiographic unit, each plot was split into 1500 squares of 20 x 20 cm to obtain the Cartesian coordinates for each cactus as well as the shape, position and size of each shrub, following the methodology of Wiegand et al. (2006) for spatial point pattern analysis for objects of finite size and irregular shape.
For a homogeneous and isotropic point pattern in regular plots, the second-order characteristics depend only on distance r, but not on the direction or location of points. An appropriate geometry is therefore to adopt circular shapes, such as the circles of Ripley's K-function, as a basis for the spatial statistics (Ripley, 1976). This analysis is based on the bivariate Ripley function, were K12(r) is defined as the expected number of points of pattern 2 (in this case, individuals of E. villicumensis) within a given distance r of an arbitrary point of pattern 1 (in this case, each shrub species), divided by the intensity λ2 of points of pattern 2 (Wiegand & Moloney, 2004): λ2K12r = E [# (points of pattern 2 ≤ r from an arbitrary point of pattern 1)] where # means ‘‘number of'', and E[ ] is the expectation operator. Under independence of the two point patterns, K12(r) = pr2, without regard to the individual univariate point patterns. Using rings instead of circles has the advantage that one can isolate specific distance classes, whereas the cumulative K-function confounds effects at larger distances with effects at shorter distances. The mark-correlation function g12(r) is the analogue of Ripley's K12(r) when replacing the circles of radius r by rings with radius r, and the O-ring statistic O12(r) = λ2 g12(r) gives the expected number of points of pattern 2 at distance r from an arbitrary point of pattern 1:
O12(r) = λ2g12(r) = E [# (points of pattern 2 at distance r from an arbitrary point of pattern 1)]. The mark-correlation function g12(r) is related to Ripley's K-function:
![]()
We obtained O12(r) = λ2 for independent patterns, O12(r) < λ2 for repulsion, whereas O12(r) > λ2 for attraction. The O11(r) is based on the same analytical approach but for univariate point analysis (intraspecific distribution analysis) calculated from the probability of individuals in pattern 1 with respect to the distance r to other individuals in the same pattern (in this case, E. villicumensis with respect to other E. villicumensis individuals). Results of the eight plots (in each physiographic unit) were combined in one average graphic function, using the ‘combine replicates tool' included in the Programita software (Wiegand & Moloney, 2004). Theoretical issues are developed in Appendix A included in Raventós et al. (2010).
We analyzed the intraspecific distribution pattern [O11(r)] of E. villicumensis in each physiographic unit, comparing the values from Wiegand-Moloney's O11(r) empirical statistics with a null model of ‘complete spatial randomness' (CSR) distribution, where any point of the pattern (E. villicumensis individual) has an equal probability of occurring at any position in the plot area, and the position of a cactus is independent of the position of any other cactus (i.e. there is no interaction). This null model operates as a dividing hypothesis to detect further regularity or aggregation in univariate patterns (Wiegand & Moloney, 2004). To determine 95% confidence bands, 99 Monte Carlo simulations (Bailey & Gatrell, 1995; Haase, 1995) were performed, taking both the 5th highest and lowest values as confidence limits (Wiegand et al., 2000). Graphically, if the empirical distribution falls within the confidence limits, it is assumed that there are no significant differences with respect to the theoretical distribution model; values distributed above the upper confidence limit indicate spatial aggregation and values below the lower limit indicate a regular (uniform) spatial pattern (Diggle, 1983) at a determined distance.
The relationship between E. villicumensis and shrub species [inter-specific distribution pattern; O12(r)] was analyzed using a ‘Toroidal shift' null model (Wiegand-Moloney's O12(r) empirical statistics), in order to preserve the possible effect of the intraspecific distribution pattern of E. villicumensis, as well as the shape, size and position of shrubs. The null model testing for independence of two point patterns assumes that independent stochastic processes created the component patterns. Departure from independence indicates that the two patterns display attraction or repulsion, regardless of the univariate pattern of either group by itself. Preserving the separate second order structures of observed patterns, whilst breaking their interdependence, can be achieved by simulations that involve random shifts of the whole of one component pattern relative to the other (Dixon, 2002; Diggle, 1983; Goreaud & Pélissier, 2003). The same idea applies for plants of finite size and irregular shape: pattern 1 remains fixed whereas pattern 2 is randomly shifted as a whole across the study area, using a ‘torus' (toroidal shift) (Wiegand et al., 2006).
Results
Table 1 describes only those shrubs associated with E. villicumensis within the plots surveyed. ANOVA analysis shows significant differences between shrubs (ΔPAR: F= 41.51; p<0.0001; ΔTemp: F= 15.54; p<0.0001; table 1) and between positions under canopies (Δ Temp: F= 54.72; p<0.0001 and Δ PAR: F= 8.38; p<0.0001; table 2), but no significant interactions between the "shrub" and "position" factors (ΔPAR: F = 0.64; p= 0.6962 and ΔTemp: F= 1.42; p= 0.2046). When comparing differences in PAR and Temp between shrubs, we found that the soil under B. retama and Z. punctata was significantly colder and shadier (Table 1), and that both these species were taller and had higher canopy cover. A strong gradient of ΔPAR and ΔTemp was observed, decreasing from the centre of the shrub toward the edge of the canopy (Table 2).
Table 2. ANOVA of ΔTemp (°C) and ΔPAR (μmol*m-2*s-1) respect to the factor position beneath shrubs. Different letters indicate significant differences for Tukey Test (p<0.05)

Density of E. villicumensis was similar in the three analyzed environments: AP: 0.023 indiv/ m2; EH: 0.029 indiv/m2 and WH: 0.031 indiv/m2. The spatial distribution of this cactus was similar in all physiographic units, where a CSR pattern (null intraspecific interaction) was observed between individuals of E. villicumensis (Fig. 2).

Fig. 2. Analysis of the univariate spatial pattern of E. villicumensis in all physiographic units. Bold line show the empirical function Wiegand-Moloney's O11(r) and dotted lines shows the confidence interval generated by the 5th highest and 5th lowest values of 99 Monte Carlo simulations of random Poisson null model.
The interspecific spatial distribution was significantly different from the Poisson random distribution and highly associated to the dominant shrubs in each physiographic unit (Fig. 3). In AP, all cacti were associated to L. cuneifolia between 0-0.6 m from the centre of this shrub, and unassociated with B. spinosa (in this unit, Z. punctata was not found, and B. retama was not associated with E. villicumensis in any villicumensis was highly associated with L. cuneifolia between 0-0.4 m and with Z. punctata between 0-1.0 m from the centre of shrubs. These distances are the same as the radius of shrubs. In all these associations between E. villicumensis and shrubs, the empirical function observed decreased from the centre to the edge of the canopy. From this edge, the empirical function falls within the confidence limits, showing no significant differences from complete spatial randomness. As for the shrubs B. spinosa and B. retama, no significant differences were found with respect to the null model (CSR) on any of the three sites.
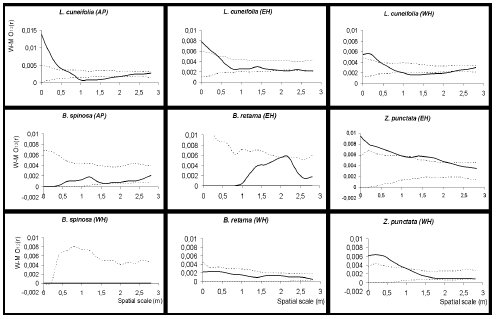
Fig. 3. Analysis of the spatial distribution of E. villicumensis in relation to shrubs found in all physiographic units: alluvial plain (AP), west hillside (WH) and east hillside (EH). Bold line shows the empirical function Wiegand-Moloney's O12(r) and dotted lines shows the confidence interval generated by the 5th highest and 5th lowest values of 99 Monte Carlo simulations of ‘Toroidal shift' null model (CSR).
Discusión
The random pattern observed in the intraspecific interaction among E. villicumensis individuals could indicate the absence of internal variables influencing their spatial distribution. Anyway, evaluation of this parameter is very important (intraspecific distribution), as it may be masked by the interspecific association (Wiegand & Moloney, 2004). The strongly positive associations with the dominant shrubs (L. cuneifolia and Z. punctata) decreased from the centre to the edge of their canopies, at similar distances from the radius of these shrub canopies, also coinciding with both the radiation and temperature gradients observed beneath these shrubs. A similar pattern was observed for E. leucantha ex Salm Dick Gilles, another columnar cactus (Almirón & Martinez Carretero, 2010) found at the same study site. Several other columnar or globose cacti were reported to be highly associated with surrounding shrubs in desert environments (Franco & Nobel, 1989; Valiente-Baunet et al., 1991; Valiente-Baunet & Ezcurra, 1991; Mandujano et al., 2002; Zuñiga et al., 2005). These studies did not evaluate the specific radiation and temperature gradients under each shrub species, considering the shade under shrubs as a homogeneous environment compared to bare soil. Perhaps the dispersal of these species is restricted to their reproductive mechanism, exclusively by seeds. Some shrubs could provide better sites for faunal dispersers, and for seed deposition (De Viana et al., 2000). In contrast, opuntioid cacti would be less dependent on shrubs, perhaps because of their type of growth and their ability to reproduce both sexually and in an agamic manner (Mandujano et al., 1998; Lopez & Valdivia, 2007; Méndez et al., 2004; Almirón & Martínez Carretero, 2013b).
The random distribution pattern of E. villicumensis in relation to B. retama and B. spinosa species indicates no interaction with these species, allowing us to think that E. villicumensis needs a narrow threshold of temperature and photosynthetically active radiation interacting simultaneously, a situation only achieved under both dominant shrubs: L. cuneifolia and Z. punctata. Perhaps, the microenvironment under B. retama was too cold (but good in light conditions), while under B. spinosa it was too luminous (but with good thermal conditions). Shrubs with higher and larger canopy areas (B. retama and Z. punctata) provided more different ΔTemp and ΔPAR values in relation to bare soil, generating cooler and shadier micro-sites (Table 1). In all three physiographic units, the dominant shrub was L. cuneifolia, and in the geotopes with steeper slope, this dominance was shared with Z. punctata, accompanied by other shrub species (B. retama, B. spinosa, Cercidum praecox, among others). These communities are commonly called "jarillal" and are characteristic of the Phytogeographic Province of Monte in Argentina, which dominates vast expanses of this country (Cabrera, 1976). Despite this, E. villicumensis appears to be restricted to certain sectors like low plains and hillocks, both with limestone and good drainage. In the sandy plain, there were no individuals of E. villicumensis or Echinopsis leucantha (Almirón & Martínez Carretero, 2010). Almirón & Martinez Carretero (2013a) observed on this site important soil differences that can determine the recruitment of shrubs and cacti. The community in the sandy plain is largely dominated by Larrea divaricata Cav. with relative frequency values of 70.8%, and has a much lower occurrence of L. cuneifolia (12.5%) than in the other units. We found no individuals of Z. punctata either in the sandy plain or in the alluvial plain.
The absence of E. villicumensis from the sandy plain may be determined by both soil conditions and lack of the shrub species (L. cuneifolia and Z. punctata) with which E. villicumensis has a strong spatial association in all three physiographic units analyzed. Like other columnar or globose cacti with only one reproductive mechanism (sexual), E. villicumensis is highly dependent on those shrubs that improve the environmental conditions under their canopies similarly to others cacti-shrub association complex (Franco & Nobel, 1989; De Viana et al., 2000; Mandujano et al., 2002; Mendez et al., 2004; Lopez & Valdivia, 2007).
For this type of cacti, the seed and seedling status is perhaps the most critical one, in relation to both abiotic (high temperatures and solar radiation, low humidity) and biotic conditions (granivory and herbivory, competitors for resources, among others) as mentioned by others authors (Rojas-Arechiaga & Vazquez-Yanez, 2000; Mendez, et al., 2004; Godínez-Alvarez et al., 2003). According to IUCN (2013), E. villicumensis is categorized in LC (Least Concern). Anyway, in this same categorization, it is stated that this specie is distributed in a small area (500 km2, approximately) around the Villicum hill. Although in the justification of conservation category selected, the authors said "it is abundant and there are no threats affecting it, in addition the area where it grows is very dry and unsuitable for human settlements and activities", in this work were observed in the south face of Villicum hill two limestone mines with strong impact on natural landscape (Fig. 1). Added to this, nearby to this zone were observed waste deposits where waste elimination is with fire, implicating a real threat for this endemic cacti. The increasing impact of human activities on natural ecosystems, and particularly on cactus populations that are highly sensitive (Martínez Carretero, 1984, 1987), evidences a lack of information regarding the different responses that these populations of E. villicumensis may have in the future. Further studies about germination and demography of E. villicumensis added to this paper and considering their small distribution area (with strong anthropic changes in land use), the exclusive reproduction mode by seed germination and the high proportion of fruits with early lepidopterans deposition affecting to actual population, could help to dilucidate the real conservation state of this endemic cacti.
Close to the study area, there is a Protected Natural Area called Loma de las Tapias (5.200 Ha) (Dirección de Conservación y Áreas Protegidas de San Juan, 2009) de that contains several specimens of E. villicumensis. In this protected area, it would be vital to highlight and strengthen the conservation objectives, including E. villicumensis as a priority.
Acknowledgements
Lic. Juan Aguilera and Juan Cáceres for field assistance and Nelly Horak for the English version, and to anonymous reviewers for their suggestions. This work is dedicated to Mariano G. Ariza, a friend and colleague who worked hard in the field. This work was supported by postdoctoral grant of the National Council of Scientific and Technical Research (CONICET, Resol. D 2327).
Bibliography
1. ALMIRÓN, M. & E. MARTÍNEZ CARRETERO. 2010. Echinopsis leucantha (Gilles ex Salm-Dick) Walp. (Cactoideae). Interacciones con plantas nodrizas en el Desierto Central Argentino. Multequina 19: 77-87. [ Links ]
2. ALMIRÓN, M. & E. MARTÍNEZ CARRETERO. 2013a. Spatial distribution of Tephrocactus aoracanthus (Lem.) Lem. in relation to shrubs in the hyperarid regions of west-central Argentina. Austral Ecol. 38: 131-138. [ Links ]
3. ALMIRÓN, M. & E. MARTÍNEZ CARRETERO. 2013b. Tephrocactus aoracanthus (Lem.) Lem. Reproducción sexual y clonal en un cactus dominante del desierto hiperárido argentino. J. Prof. Assoc. Cactus 15: 20-31. [ Links ]
4. ARMAS, C. & F. PUGNAIRE. 2005. Plant interactions govern population dynamics in a semiarid plant community. J. Ecol. 93: 978-989. [ Links ]
5. BAILEY, T & A. GATRELL. 1995. Interactive spatial data analysis. Longman Scientific and Technical, Harlow. [ Links ]
6. BERTNESS, M. & R. CALLAWAY. 1994. Positive interactions in communities. Trends Ecol. Evol. 9: 191-193. [ Links ]
7. CABRERA, A. 1976. Regiones fitogeográficas argentinas. En: Enciclopedia Argentina de agricultura y jardinería. Tomo 2, fasc. 1. Acme, Buenos Aires. [ Links ]
8. CALLAWAY, R. & L. WALKER. 1997. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78: 1958-1965. [ Links ]
9. CARRILLO-GARCÍA, A., Y. BASHAN & G. BETHLENFALVAY. 2000. Resource-island soils and the survival of the giant cactus, cardón of Baja California Sur. Plant Soil 218: 207-214. [ Links ]
10. CONNELL, J. (1983). On the prevalence and relative importance of interespecific competition: evidence from field experiment. Amer. Nat. 122: 661-696. [ Links ]
11. CAZÓN A., M. DE VIANA & J. GIANELLO. 2002. Comparación del efecto fitotóxico de aleloquímicos de Baccharis boliviensis (Asteraceae) en la germinación de Trichocereus pasacana (Cactaceae). Ecol. Austral 12: 73-78. [ Links ]
12. DE VIANA, M., S. SUHRING & B. MANLY. 2000. Application of randomization methods to study the association of Trichocereus pasacana (Cactaceae) with potential nurse plants. Plant Ecol. 156: 193- 197. [ Links ]
13. DIGGLE, P. 1983. Statistical analysis of spatial point patterns. Academic Press, London. [ Links ]
14. DIRECCIÓN DE CONSERVACIÓN Y ÁREAS PROTEGIDAS, SECRETARIA DE AMBIENTE, GOBIERNO DE SAN JUAN. 2009. Áreas Naturales Protegidas, Provincia de San Juan. [ Links ]
15. DIXON, P. 2002. Ripley's K function. Encyc. Environmetrics 3: 1796-1803. [ Links ]
16. FLORES-MARTÍNEZ, A., E. EZCURRA & S. SÁNCHEZ-COLÓN. 1994. Effect of Neobuxbaumia tetetzo on growth and fecundity of its nurse plant Mimosa luisiana. J. Ecol. 82: 325-330. [ Links ]
17. FRANCO, A. & P. NOBEL. 1989. Effect of nurse plants on the microhabitat and growth of cacti. J. Ecol. 77: 870-886. [ Links ]
18. GODÍNEZ-ALVAREZ, H., VALVERDE T. & P. ORTEGA-BAES. 2003. Demographic trends in the Cactaceae. Bot. Rev. 69: 173-203. [ Links ]
19. GOREAUD, F. & R. PÉLISSIER. 2003. Avoiding misinterpretation of biotic interactions with the intertype K-12-function: population independence vs. random labelling hypotheses. J. Veget. Sci. 14: 681-692. [ Links ]
20. HAASE, P. 1995. Spatial pattern analysis in ecology based on Ripley's K-function: introduction and methods of edge correction. J. Veget. Sci. 6: 575- 582. [ Links ]
21. HACKER, S. & S. GAINES. 1997. Some implications of direct positive interactions for community species diversity. Ecology 78: 1966-1975. [ Links ]
22. HOLMGREN, M., M. SCHEFFER & M. HUSTON. 1997. The interplay of facilitation and competition in plant communities. Ecology 78: 1966-1975. [ Links ]
23. IUCN. 2013. IUCN Red List of Threatened Species (ver. 2013.1). Available at: http://www.iucnredlist.org. [ Links ]
24. KATTERMANN, F. 1994. Eriosyce (Cactaceae). The genus revised and amplified. Succulent Plant Res. 1: 1-176. [ Links ]
25. LEIRANA-ALCOCER, J. & V. PARRA-TABLA. 1999. Factors affecting the distribution, abundance and seedling survival of Mammillaria gaumeri, an endemic cactus of coastal Yucatán, Mexico. J. Arid Environ. 41: 421-428. [ Links ]
26. LÓPEZ, R. & S. VALDIVIA. 2007. The importance of shrub cover for four cactus species differing in growth form in an Andean semi-desert. J. Veget. Sci. 18: 263-70. [ Links ]
27. MAHIBBUR, R. & Z. GOVINDARAJULU. 1997. A modification of the test of Shapiro and Wilks for normality. J. Appl. Stat. 24: 219-235. [ Links ]
28. MANDUJANO, M., C. MONTAÑA, I. MÉNDEZ & J. GOLUBOV. 1998. The relative contribution of sexual reproduction and clonal propagation in Opuntia rastrera from two habitats in the Chiuahuan Desert. J. Ecol. 86: 911-921. [ Links ]
29. MANDUJANO, M., A. FLORES-MARTINEZ, J. GOLUBOV & E. EZCURRA. 2002. Spatial distribution of three globose cacti in relation to different nurse-plant canopies and bare areas. Southw. Nat. 47: 162-168. [ Links ]
30. MARTÍNEZ CARRETERO, E. 1984. El incendio de la vegetación en la Precordillera mendocina III. Los pastizales disclimáxicos de la Quebrada de Villavicencio. Parodiana 3: 175-183. [ Links ]
31. MARTÍNEZ CARRETERO, E. 1987. El incendio de la vegetación en la Precordillera mendocina V. Pérdida de la calidad nutritiva del sistema natural. Parodiana 5: 121-123. [ Links ]
32. MCAULIFFE, J. 1984. Sahuaro-nurse tree associations in the Sonoran Desert: competitive effects of Sahuaros. Oecologia 64: 319-321. [ Links ]
33. MÉNDEZ, E., J. GUEVARA & O. ESTEVEZ. 2004. Distribution of cacti in Larrea spp. shrublands in Mendoza, Argentina. J. Arid Environ. 58: 451-4462. [ Links ]
34. MONTGOMERY, D. 1991. Diseño y análisis de experimentos. Grupo Editorial Iberoamérica, México. [ Links ]
35. POBLETE, A. & J. MINETTI. 1999. Configuración espacial del clima de San Juan. Síntesis del Cuaternario de la Provincia de San Juan. 11º Reunión de Campo del Cuaternario. INGEO, Universidad Nacional de San Juan, San Juan. [ Links ]
36. RAVENTÓS, J., T. WIEGAND & M. DE LUIS. 2010. Evidence for the spatial segregation hypothesis: a test with nine-year survivorship data in a Mediterranean fire-prone shrubland. Ecology 91: 2110-2120. [ Links ]
37. RIPLEY, B. 1976. The second-order analysis of stationary point processes. J. Appl. Prob. 13: 255-266. [ Links ]
38. ROJAS-ARÉCHIGA, M. & C. VÁZQUEZ-YANES. 2000. Cactus seed germination, a review. J. Arid Environ. 44: 85-104. [ Links ]
39. SCHOENER, T. 1983. Field experiments on interspecific competition. Amer. nat. 122: 240-285. [ Links ]
40. TEWKSBURY, J. & J. LLOYD. 2001. Positive interactions under nurse-plants: spatial scale, stress gradients and benefactor size. Oecologia 127: 425-434. [ Links ]
41. TIELBÖRGER, K. & R. KADMON. 2000. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology 81: 1544-1553. [ Links ]
42. VALIENTE-BAUNET, A., A. BOLOGNAROCRAVENNA, O. BRIONES, E. EZCURRA, M. ROSAS, M. NÚÑEZ, G. BARNARD & E. VAZQUEZ. 1991. Spatial relation between cacti and nurse shrubs in a semi-arid Environment in central Mexico. J. Veget. Sci. 2: 15-20. [ Links ]
43. VALIENTE-BAUNET, A. & E. EZCURRA. 1991. Shade as a cause of the association between the cactus Neobouxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacán Valley, México. J. Ecol. 79: 961-971. [ Links ]
44. WIEGAND K., J. FLORIAN & D. WARD. 2000. Do spatial effects play a role in the spatial distribution of desert-dwelling Acacia raddiana? J. Veget. Sci. 11: 473-478. [ Links ]
45. WIEGAND, T. & K. MOLONEY. 2004. Rings, circles and null-models for point pattern analysis in ecology. Oikos 104: 209-229. [ Links ]
46. WIEGAND, T., W. KISSLING, P. CIPRIOTTI. & M. AGUIAR. 2006. Extending point pattern analysis to objects of finite size and irregular shape. J. Ecol. 94: 825-837. [ Links ]
47. YEATON, R. & M. CODY. 1976. Competition and spacing in plant communities: The Northern Mohave Desert. J. Ecol. 64: 689-696. [ Links ]
48. YEATON, R. 1978. A cyclical relationship between Larrea Tridentata and Opuntia Leptocaulis in the Northern Chihuahuan Desert. J. Ecol. 66: 651-656. [ Links ]
49. ZULOAGA, F., MORRONE, O. & M. BELGRANO (eds.). 2008. Catálogo de las plantas vasculares del Cono Sur (Argentina, sur de Brasil, Chile, Paraguay y Uruguay). Monogr. Syst. Bot. Missouri Bot. Gard. 107: 1-983. [ Links ]
50. ZUÑIGA, B., G. MALDA & M. SUZÁN. 2005. Interacciones planta-nodriza en Lophophora diffusa (Cactaceae) en un desierto subtropical de México. Biotropica 37: 351-356. [ Links ]
Recibido el 30 de Junio de 2014,
aceptado el 27 de octubre de 2014.














