Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.51 no.1 Córdoba mar. 2016
FICOLOGÍA
Valve morphology of Didymosphenia geminata (Bacillariophyceae) in Chubut province, Argentina
Noelia Mariel Uyua1*, Norma Herminia Santinelli1, Alicia Viviana Sastre1 and Silvia Estela Sala2
1 Laboratorio de Hidrobiología, Facultad de Ciencias Naturales sede Trelew, Universidad Nacional de la Patagonia San Juan Bosco, Av. 9 de Julio y Belgrano s/n, (9100). Trelew, Chubut, Argentina. Tel: 00542804421782.
2 División Ficología, Museo de La Plata, Universidad de La Plata, Paseo del Bosque s/n. (1900). La Plata, Buenos Aires, Argentina.
Corresponding author. E-mail: nuyua@conicet.gov.ar
Summary
Didymosphenia was recently found in Patagonia forming massive proliferations. In Argentina the species scattered in a short time to five provinces. Here, we analyse morphologic and morphometric data of specimens collected at Chubut Province, Argentina, and we compare them with other species of this genus reported from different regions of the world. Materials were collected in two basins during 2010- 2012. Samples analyzed with LM and SEM showed inter and intrapopulation variation in morphometric characteristics and in some valve structures such as spines. The studied material corresponds in valve outline and morphology to D. geminata ssp geminata Metzeltin & Lange-Bertalot. Our materials also resemble a group of species: D. clavaherculis, D. clavamagna, D. coronata and D. laticollis. The studied material can be easily confused to some specimens mentioned in the literature as D. clavaherculis but they clearly differ from the type material of the species. On the other hand, fine morphology of the studied materials coincides with materials from Chile. Although morphological revision of the genus recently published allowed separating many species, there are still doubts about specific limits of taxa closely related to D. geminata. Molecular analysis should be performed to clarify these issues.
Key words: Diatoms; Didymo; Patagonia; Morphometry; Nuisance species.
Resumen
Morfología valvar de Didymosphenia geminata (Bacillariophyceae) en la provincia de Chubut, Argentina.
Recientemente se verificó que Didymosphenia forma proliferaciones masivas en Patagonia. En Argentina se ha esparcido a cinco provincias en un corto tiempo. En este trabajo, analizamos datos morfológicos y morfométricos de especímenes recolectados en la provincia del Chubut, Argentina y los comparamos con otras especies de este género de diferentes regiones del mundo. El material de estudio fue recolectado en dos cuencas durante 2010-2012. Las muestras, analizadas con MO y MEB, mostraron variación inter e intra-poblacional en las características morfométricas y en algunas estructuras valvares, como espinas. La morfología del material estudiado corresponde a la de D. geminata ssp geminata Metzeltin & Lange-Bertalot. Nuestros materiales también se asemejan a las especies: D. clavaherculis, D. clavamagna, D. coronata y D. laticollis. Los materiales de Chubut pueden ser fácilmente confundidos con algunos ejemplares mencionados en la literatura como D. clavaherculis, pero difieren claramente del material tipo de esa especie. Por otro lado, la morfología de los materiales estudiados coincide con los hallados en Chile. Aunque recientemente se publicó una extensa revisión morfológica del género Didymosphenia, todavía hay dudas en torno al grupo de taxones estrechamente relacionados con D. geminata. Actualmente se están realizando análisis moleculares que ayudarían a establecer los límites específicos.
Palabras clave: Diatomeas; Didymo; Patagonia; Morfometría; Especie invasora.
INTRODUCTION
The history of the diatom Didymosphenia geminata (Lyngbye) Schmidt (commonly known as "didymo" or "rock snot") as a nuisance species forming blooms at the Northern Hemisphere has been documented for more than 20 years, with events in Vacouver Island in late 1980s, followed by blooms in Iceland, USA and Poland (Bothwell et al., 2014). Since then it has been reported as an aggressive invader in low nutrient streams worldwide. Following the outbreak in New Zealand in 2004 it was inferred that the problem was related to the anthropogenic introduction of the species, and in this context Patagonia was included as a vulnerable zone in a map published by McNyset & Julius in Spaulding & Elwell (2007). Alerted by this warning, three years later the species was found at Futaleufú River Basin in Chilean and Argentinean Patagonia (Sastre et al., 2013). Within one year its presence was confirmed in 20 rivers distributed over 800 km in Chile (Reid et al., 2012). This rapid spread and colonization of different substrates is ascribed to a high plasticity of the species (Merino et al., 2011). In Argentina although the first reports were restricted to Chubut Province (Sastre et al., 2010), the species spread in a short time to the north (Beamud et al., 2013) and to the south being found in Río Grande, Tierra del Fuego (Sala et al., 2013). Recently the causes of bloom formation were explained in terms of large-scale human intervention in climatic, atmospheric and edaphic processes (Bothwell et al., 2014) but it is not clear yet which were the agents that spread it all around the world. Nevertheless, is accepted the idea that anthropic activities as fishing and tourism have been responsible of the introduction and spread of the species in the Southern Hemisphere. Consequently management strategies in Argentina are focussed on control and disinfection of fishing equipment and boats.
Even though it was initially considered that the species was not present in South America (Spaulding & Elwell, 2007), Asprey et al. (1964) and Rivera & Gebauer (1989) mentioned it in Chile. The latter author confirmed the presence of D. geminata at Mejillones (Antofagasta Region), Sarmiento Lake (Magallanes Region) and Cisnes River (Aysén Region) in a review of the Chilean diatom flora. In Argentina the species was not recorded until 2010 (Vouilloud, 2003 and further publications; Sastre et al., 2013). Nevertheless, considering that basic information in the country is scarce, it is not certain if the species was present in small populations before or, if it entered from Chile in the recent years. Native or exotic, the problem in Patagonia is that the species is rapidly increasing its colonized area. In Chile, Bus Leone et al. (2014) found that not only human but aquatic mammals function as vectors. As a first step to understand the problem in Patagonia, it was necessary to identify the taxon that forms blooms in the region as the genus Didymosphenia comprises around 22 species. Metzeltin & Lange-Bertalot (1995) conducted a detailed analysis of several species and described diagnostic features for species and morphotypes, delimiting five species, and three morphotypes for D. geminata. In 2014 Metzeltin & Lange-Bertalot published an actualized and detailed analysis of the genus, and studied for the first time, with Scanning Electron Microscopy (SEM), materials collected in 1819 from the Faroe Islands and selected a lectotype. Besides, they described 10 new species (6 from Lake Baikal): D. clavamagna, D. coronata, D. crassiporata, D. grunowii, D. laticeps, D. laticollis, D. mongolica, D. niponica, D. skvortzowii and D. strelnikovae and 2 new subspecies D. geminata ssp. geminata and ssp. crassa. They also gave a new status to D. dorogostaiskyi (Skvortzow) Metzeltin & Lange- Bertalot and D. subcapitata (Skvortzow & Meyer) Metzeltin & Lange- Bertalot. Through this detailed review the authors gave foundations to establish diagnostic features of the species of this genus and pointed out that micromorphology allows differentiating four clusters. The cluster around the type species (D. geminata) is characterized by its valve face ornamented with shallow pits, warts and papillae; ridge with apical spines on the valve face and mantle union and foraminae of volate areolae lying depressed in quasi circumvallated pits. They also point out that it is very difficult to make identifications based on the analyses of few specimens, or specimens representing only part of the cell cycle and that cryptic or semicryptic species are present behind the variability of valves shapes and dimensions. Guiry in Guiry & Guiry (2015) lists D. geminata with the varieties D. geminata var. baicalensis Skvortzov & Meyer, D. geminata var. dorogostaiskyi Skvortzov & Meyer and D. geminata var. neocaledonica Manguin, D. pumila Metzeltin & Lange-Bertalot, D. clava-herculis (Ehrenberg) Metzeltin & Lange-Bertalot, D. dentata (Dorogostaisky) Skvortzow & Meyer and D. tatrensis Mrozinska, Czerwik-Marcinkowska & Gradzinski as currently accepted taxa. Besides, they point out that D. curvata (Skvortzov & Meyer) Metzeltin & Lange-Bertalot, D. curvirostrum (Tempère & Brun) M. Schmidt and D. siberica (Grunow) M. Schmidt were included but have not been subject to full verification.
South American populations of this "invader" were analysed with electron microscopy for the first time by Rivera et al. (2013), and therefore their valve micromorphology could not be compared with populations from other regions of the world until that date. The aims of this paper were to describe the materials of Didymosphenia genus present in Chubut Province, Argentina, and to analyze morphometric parameters and fine morphology of several populations. In addition, we compare them with materials from Chile and from other regions of the world.
MATERIALS AND METHODS
This study was conducted within the framework of a monitoring program carried out by the Ministerio de Ambiente y Control de Desarrollo Sustentable and by the Universidad Nacional de la Patagonia San Juan Bosco due to the initial presence of the species at the lower section of Futaleufú River. The studied area comprises the northwest of Chubut Province (Fig. 1). Samples were collected at Futaleufú and Rivadavia rivers that belong to Futaleufú Basin and at Azul River that belongs to Lago Puelo Basin. Sampling was held at Futaleufú River during spring 2010, autumn and spring 2011 and at Azul and Rivadavia rivers during spring 2012, following international security recommendations (Duncan et al., 2007). The sites were located with GPS. Periphyton was collected by brushing a variable surface from different rocks and from submerged and emerging plants and macroalgae. All samples were preserved in 4% formaldehyde and were treated to eliminate organic matter following the method described in Hasle and Fryxell (1970). For light microscopy (LM) they were mounted in Naphrax® and, for SEM the samples were deposited on 1 cm2 pieces of glass mounted on metal stubs and then coated with gold-palladium. Analyses were held with an Olympus CKX41 microscope and photographed using an Olympus Evolt E-330 camera and, a Jeol JSM-6360 LV SEM at the Electron Microscopy Service of the Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Argentina.
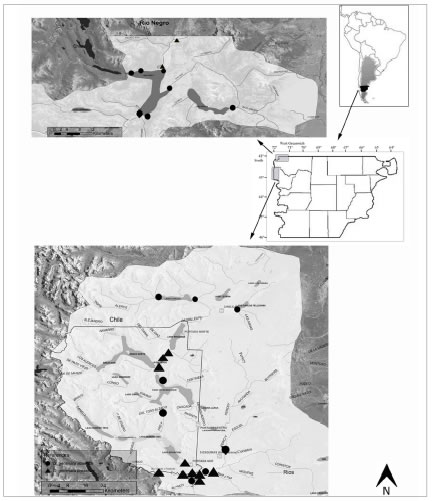
Fig. 1. Location of the study sites.
Uncleaned, cleaned subsamples and permanent slides were deposited at the Herbario of the División Científica Ficología, Museo de La Plata under the following numbers:
LPC 13400: Río Futaleufú, 43°10´23.4´´ S, 71°35´41.1´´ W; Chubut Province, Argentina; September 2010; Leg. Gabriel Bauer.
LPC 13401: Río Futaleufú, 43º10'24.2" S, 71º35'39.9" W; Chubut Province, Argentina; March 2011; Leg. Gabriel Bauer.
LPC 13402: Río Futaleufú, 43º10´20.1´´ S, 71º36'23.8" W; Chubut Province, Argentina; September 2011; Leg. Noelia Uyua and Gabriel Bauer.
LPC 13403: Río Futaleufú, 43º10´37.0´´ S, 71º37'41.1" W; Chubut Province, Argentina; April 2011; Leg. Noelia Uyua and Gabriel Bauer. LPC 13404: Río Azul, 42º05'12.4" S, 71º37'10.4" W; Chubut Province, Argentina; November 2012; Leg. Gabriel Bauer.
LPC 13405: Río Rivadavia, 42º40'27.2" S, 71º41'56.7" W; Chubut Province, Argentina; September 2012; Leg. Gabriel Bauer.
The terminology used to describe morphology is that proposed in Anonymous (1975), Ross et al. (1979) and Barber & Haworth (1981). For morphometric analyses 30 specimens from each sample were measured considering maximum length, maximum width, apical pole width, foot pole width, apical pole constriction, foot pole constriction, maximum width/head-pole width ratio, number of striae in 10µm, number of areolae in 10µm, and number of stigmata. SEM photographs were analyzed with Adobe Photoshop CS5 Extended software (Adobe Systems Incorporated 2010). Statistical analyses were carried out using the R statistical package version 2.15.1 (R development Core Team 2013). We performed one-way analysis of variance (ANOVA) to verify the significance of possible differences in length of D. geminata among the different rivers (populations).
RESULTS
We detected Didymosphenia for the first time in Argentina in August 2010 at Futaleufú River covering a few square meters in front of a fishing lodge. During 2011 the species spread in this basin forming colonies of several square meters. In February 2012 the species was also recorded in Rivadavia River and in August of the same year in small isolated patches. These rivers belong to the Futaleufú Basin upstream to Futaleufú River and are within the Alerces National Park. After a few months, the colonies enlarged the invaded area, covering approximately 10 km of the Rivadavia River and new rivers within the Futaleufú Basin. At the end of the year, the species was detected at the Azul River, northwest of the province bordering on Río Negro Province. In these cases the species formed blooms 2-4 cm thick covering all kind of substrates. Although it has expanded the occupied area in Azul River, at present Didymosphenia form blooms in summer and almost disappears in winter. In all cases, the initial colonies were attached to submerged rocks, and, when colonies grew, they were also attached to emerging rocks, plants and macroalgae (Figs 2, A-C).
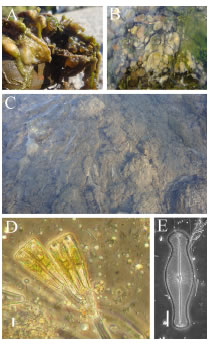
Fig. 2. Didymosphenia geminata colonies at the Futaleufú River. A-B: Late winter-early spring. C: Summer. D: LM. D. geminata stalk. E: LM. D. geminata. Scale bar = 20 µm (Figs D-E).
At the beginning of the bloom (late winterearly spring), the colonies were cushion shape, dark brown and gelatinous (Figs 2, A-B), but then they became long hair masses of different lengths (Fig. 2, C). The increasing amount of extracellular slime and the development of increasing numbers of branched stalks (Fig. 2, D) occur as the masses enlarge. At the time of the samplings, blooms persisted throughout the whole year and covered areas of several kilometers at Futaleufú River. At Azul and Rivadavia rivers the occupied area was smaller than at Futaleufú River since the former rivers were recently colonized. At Azul River, in October 2013 the species formed a small bloom covering 1 m2 at the same places where D. geminata formed large blooms during January and February 2014.
Description
Didymosphenia geminata produces large masses of stalks, brown dense soggy carpet-like layers rough to the touch in the study area. The cells have heavily silicified frustules (Fig. 2, E), in gelatinous colonies. Frustules are slightly cuneate with few copulae. Valves are strongly heteropolar, symmetric or slightly asymmetric respect the apical plane with capitate head and foot poles (Figs 3, A-J). The valve face is plane; at the union with the mantle there are marginal ridges that end in spines at the head pole (Figs 4, A-K). Uniseriate striae are strongly radial all alongside. The striae appear to progress around the head pole in a fanlike manner (Figs 4, A-E). SEM analyses shows that the apical striae have a slightly irregular pattern when contrasted with the valve face striae. The striae about the central area are irregularly and alternately shortened. Poroid areolae are occluded by volae with dendritic slits between the inside and outside (Fig. 4, G; 5, G). On the external side there are small papilae around the areolae (Fig. 4, G). Internally, the valve face presents transapical ribs strongly branched at valve center and poles (Figs 5, A-G). In transversal section the ribs are spongy and with a variable thickness, constricted at the middle (Figs 5, F-G). The foot pole has a large apical pore field, with small poroids aligned in longitudinal rows more or less ordered, 44 in 10 µm (Figs. 4, I-J). The raphe sternum is narrow widening abruptly to an elliptic central area that shows externally "ghost areolae". Central area mostly apically elliptical, extended c. 1/4 - 1/5 of the valve breadth, pierced by 2 to 5 stigmata with external oval openings surrounded by a thin flange and internally cushion shaped with a spongy appearance (Figs 5, D-E). The raphe is lateral slightly curved with terminal fissures long and abruptly bent towards the opposite side of the stigmata (Figs 3, A-E). Although both terminal fissures are curved, basal pole terminal fissures are deflected in an almost right angle whereas terminal fissures at head pole are deflected in an obtuse angle (Figs 3, A-E). Internal distal ends slightly bent towards the same side, ending in small helictoglossae (Figs 5, A-C). External proximal raphe endings are expanded in a simple teardrop shaped opening (Figs 4, A and F-G). Internal proximal endings are obscured by a nodular outgrowth of silica on the primary or secondary side of the valve, in some cases there is a small slit in the apical direction (Figs 5, D-E). Girdle is composed of few (4) bands opened at the foot pole with a row of poroids (Fig 4, H).

Fig. 3. SEM. Didymosphenia geminata. A-E: Frustules in external view: A: General valve view from Rivadavia River. B-C: General valve views from Futaleufú River. D-E: General valve views from Azul River. F: Frustule in girdle view from Futaleufú River. G-J: Valves in internal view: G: Azul River. H-I: Futaleufú River. J: Rivadavia River. Scale bars= 20 µm (Figs A-J).
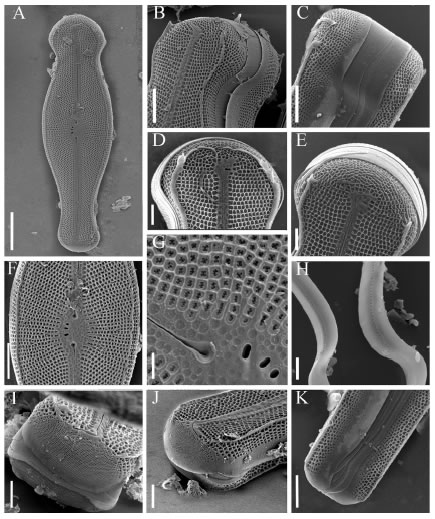
Fig. 4. SEM. Didymosphenia geminata frustules in external view. A: Frustule in valve view. B: Detail of the head-pole. Futaleufú River. C: Detail of the head pole in girdle view. Azul River. D-E: Detail of the head pole in valve view. Azul River. F: Detail of valve center. G: Detail of the central area. H: Band with a row of poroids. I-J: Detail of the apical pore field. K: Detail of the foot pole in girdle view. Scale bars = 20 µm (Fig. A); 10 µm (Figs B-C, F, K); 5 µm (Figs D-E, H-J); 2 µm (Fig. G).
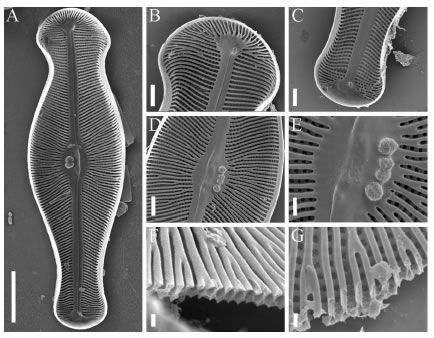
Fig. 5. SEM. Didymosphenia geminata. Valves in internal view. A: General valve view. B: Detail of the head pole. C: Detail of the foot pole. D: Valve center. E: Detail of the central area. F: Cross section of the valve at the central area. G: Detail of the valve structure. Scale bars = 20 µm (Fig A); 5 µm (Figs B-D); 2 µm (Fig. E); 1 µm (Figs F-G).
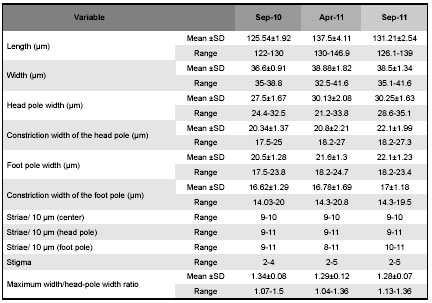
The comparison of the valve morphology of several specimens of the same population shows some morphological variations e.g., some specimens collected at the Azul River have bilobed or trilobed spines (Fig 4, D) while in others the spines are poorly developed (Figs 4, B-C), and the marginal ridge is almost continuous at the head pole (Fig 4, D). On the other hand the comparison of specimens from the 3 studied rivers shows that valves of some specimens appear slightly asymmetric about the apical plane, principally those from the Azul River while others are symmetric such as those from Futaleufú and Rivadavia rivers (Figs 3, A-J). Besides, the terminal raphe fissures vary beings more or less bent and sometimes are irregular instead of straight. The analyses of morphometric parameters show that specimens present in Futaleufú River are larger than those at Rivadavia (p<0.001, ANOVA test) and Azul rivers (p<0.001, ANOVA test) (Table 1). Besides, the analyses of morphometric parameters of specimens collected in Futaleufú River at different dates showed variation principally in the length of the cell: the cells registered in the first bloom are smaller than those collected a year later (p<0.001, ANOVA test) (Table 2). The ratio maximum width/head-pole width is highly variable within all populations ranging between 1.04 and 1.56.
Table 1: Morphometric data of D. geminata specimens collected at three rivers from Chubut. SD: standard deviation.
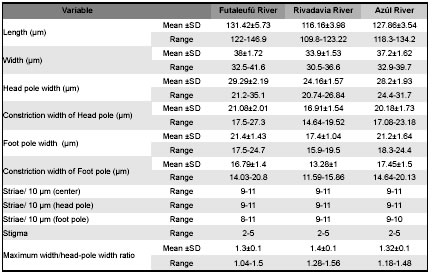
Table 2: Morphometric data of specimens of D. geminata collected at different moments at Futaleufú River. SD: standard deviation.
DISCUSSION
Didymosphenia geminata presents a large variation in some of its morphological and morphometric features and sometimes these characters seem to be insufficient to establish with certainty their limits with allied species (Metzeltin & Lange-Bertalot, 2014). The specimens collected in Chubut Province have some morphological features in which they differ from the previously described D. geminata and share others with other species of the genus. The studied specimens show variations within and among populations, principally in the morphometric parameters and in some structures such as spines and terminal raphe fissures. The variability of the studied materials in valve morphology could be attributed to differences in environmental conditions, but there are no available data to prove it. In comparison with specimens described from different regions of the world, specimens from Chubut have a narrow range of size variation that represents just a restricted sector of the entire cell cycle, excluding smaller forms. Nevertheless, they coincide in size with the type material of the species from Faroe Islands (Table 3). Specimens named as D. geminata morphotype geminata from different regions of the world are different from each other in valve outline (Whitton et al., 2009; Stoermer et al., 1986). Our specimens are less variable in the marked constrictions at the head and foot poles but have a slightly wide range of striae density and maximum width/width headpole ratio. Stoermer et al. (1986) pointed out that the most important characteristic that differentiate population from Lake Baikal from those occurring in other habitats was the frustule asymmetry (feature considered diagnostic), in relation to this character in Chubut we found specimens with and without cymbelloid symmetry.
Table 3: Comparison of morphometric data of the studied materials and the closest taxa of the genus. * : measured from illustration.
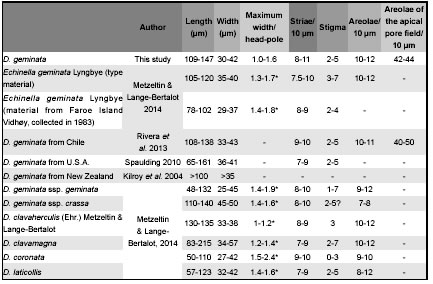
Metzeltin & Lange-Bertalot (2014) rejected the morphotypes geminata sensu stricto, capitata and subcapitata created by them in 1995 and proposed two subspecies: Didymosphenia geminata ssp geminata and Didymosphenia geminata ssp crassa. The former is apparently a cosmopolitan taxon while the latter is restricted to Siberia and The Netherlands. Differences between these two subspecies are related to valve breadth and areolae density. Materials from Chubut coincide in morphometric data with Didymosphenia geminata ssp. geminata, although larger specimens exceed the maximum length of 132 µm given by Metzeltin & Lange-Bertalot (2014) and we did not found specimens smaller than 109 µm (Table 3). In 1995 Metzeltin and Lange-Bertalot described D. siberica (Grunow) M. Schmidt, D. curvata (Skvortzow & Meyer) Metzeltin & Lange- Bertalot, D. clavaherculis (Ehrernberg) Metzeltin & Lange-Bertalot and D. pumila Metzeltin & Lange-Bertalot. In 2014 they redefined D. siberica and D. clavaherculis excluding the materials that they described under these names in 1995. Besides, in this new revision they erected 10 new species: D. clavamagna, D. coronata, D. crassiporata, D. grunowii, D. laticeps, D. laticollis, D. mongolica, D. nipponica, D. skvortzowii, D. strelnikovae and also described in detail D. curvata (Skvortzow & Meyer) Metzeltin & Lange Bertalot, D. curvirostrum Tempere & Brun, D. dentata (Dorogostaisky) Skortzow, D. dorogostaiskyi (Skovort & Meyer) Metzeltin, Lange-Bertalot & Kulikovsskiy, D. fossilis Horikawa & Okuno, D. lineata Skabichevsky, D. pumila, D. tatrensis Mrozinska et al. and D. subcapitata (Skvorzow & Meyer) Metzeltin & Lange-Bertalot. Comparing the materials from Chubut with all the taxa described in Metzeltin & Lange-Bertalot (2014), they are undoubtedly different from D. siberica, D. mongolica and D. pumila with less constricted valve outline, apices slightly differentiated and different areolae structure. From D. tatrensis and D. grunowii they differ in the absence of the marginal rib; from D. curvata in their strongly asymmetry respect the apical axis and from D. fossilis and D. nipponica are easily distinguished by the valve outline, symmetry and areolae. Dydimosphenia dentata and D. subcapitata are similar in valve outline but have strong spines around valve margin and D. dorogostaiskyi differs not only in the presence of spines but also in valve outline.
The studied specimens resemble a group of similar species: D. clavaherculis, D. clavamagna, D. coronata and D. laticollis. D. coronata has subcapitate poles, smaller length range and stigma foramina circular and smaller than Chubut´s material. Besides, it is described as a rare species exclusively from Asia. Our specimens are similar in valve outline to some specimens of D. laticollis but this species has less marked head pole constriction. Nevertheless, the validity of this taxon is questionable as the authors themselves point out that only molecular investigations can give more accurate results about its identity. The materials from Chubut are morphologically similar to D. clavaherculis (Ehrenberg) sensu Metzeltin & Lange-Bertalot 1995 but, according to Metzeltin & Lange-Bertalot, 2014: Plate 57, D. geminata ssp geminata and thus the specimens from Chubut clearly differ from the type material of this species with clavated valves and subcapitated ends. Although the authors point out that D. clavaherculis is a "weakly substantiated species" they still consider other morphodemes as D. clavaherculis s. str. that can be easily confused with D. geminata. On the other hand these authors mentioned that the specimens that they considered D. clavaherculis in Metzeltin & Lange-Bertalot (1995) (Figs 4:5-8, 7:6 and 8:4) belong to D. clavamagna. Although bigger specimens of D. clavamagna clearly differs from D. geminata, the smaller specimens can be easily confused but they can be distinguished in the narrow central area and the striae becoming considerably convergent below both valve ends vs. subparallel/parallel above foot pole respectively. Besides, D. clavamagna has several spines at the end of the marginal costae and terminal raphe fissures are in right angle. In relation to morphometric characters in our materials the length range falls within the length range of D. geminata ssp. geminata (although as we mentioned above in Chubut we did not found specimens shorter than 109 µm long), D. geminata ssp. crassa, D. clavaherculis, D. clavamagna and D laticollis. Considering maximum width/ width head-pole ratio, our specimens have a wider range of variation than all the mentioned species. Nevertheless, we consider that materials from Chubut belong to D. geminata ssp. geminata but it is not possible to establish this with certainty because there is a great variability within the species and subspecies. In relation to South American materials documented up to now, the studied specimens are similar to those described by Rivera et al. (2013) from Chile although in Argentina we found larger cells. The later authors described their specimens as D. geminata morphotype capitata, nevertheless they recognized that some features keep these specimens separated from those described by Metzeltin & Lange-Bertalot (1995). The materials described by Rivera & Gebauer (1989, fig. 54) as Gomphonema geminatum from Chile, present a similar outline to D. clavaherculis, with a less marked head and foot poles constrictions and a more developed head pole. It is highly probable that materials from Argentina and Chile belong to the same species and presumably they were transported from one to other country since Futaleufú Basin is a shared watershed. Also, molecular data are necessary to confirm or reject this hypothesis.
Although Metzeltin & Lange-Bertalot (2014) performed a detailed morphological and morphometrical revision, there are still many doubts around the group of Didymosphenia species that are closely related to D. geminata. Variations on valve outline along the cell cycle difficult the differentiation of species based on these data, thus only molecular analyses will help to clarify specific limits. Consequently, it is still uncertain whether it is only one species that can produce nuisance blooms and if it was transported by men.
ACKNOWLEDGEMENTS
We thank Chubut Province Government for sampling support in the framework of the Didymosphenia geminata Prevention and Monitoring Program. We thank to Dirección de Pesca Continental (Gobierno de la Provincia del Chubut) and Gabriel Bauer for his assistance and field sampling support.
BIBLIOGRAPHY
1. ADOBE SYSTEMS INCORPORATED. 2010. Adobe©Photoshop© CS5 Extended. San Jose, California. [ Links ]
2. ANONYMOUS. 1975. Proposal for standardization of diatom terminology and diagnosis. Nova Hedwigia Beih. 53: 323-354. [ Links ]
3. ASPREY, J.F., K. BENSON-EVANS & J.E. FURET. 1964. A contribution to the study of South American freshwater phytoplankton. Gayana Bot. 10: 1-18. [ Links ]
4. BARBER, H.G. & E.Y. HAWORTH. 1981. A guide to the morphology of the diatom frustule. Freshwater Biological Association, Scientific Publication No 44. [ Links ]
5. BEAMUD, G., G. BAFFICO, F. PEDROZO & M. DÍAZ. 2013. First record of the invasive algae Didymosphenia geminata in the Lake Nahuel Huapi: Argentina, Patagonia. Rev. Chil. Hist. Nat. 86: 493-496. [ Links ]
6. BOTHWELL, M.L., B.W. TAYLOR & C. KILROY. 2014. The Didymo story: the role of low dissolved phosphorus in the formation of Didymosphenia geminata blooms. Diatom Res. 29: 229-236. [ Links ]
7. BUS LEONE, P., J. CERDA, S.E. SALA & B. REID. 2014. Mink (Neovison vison) as a natural vector in the dispersal of the diatom Didymosphenia geminata. Diatom Res. 29: 259-266. [ Links ]
8. DUNCAN, M., C. KILROY, C. VIEGLAIS & F. VELVIN. 2007. Protocol for the collection of samples for delimiting surveys for Didymosphenia geminata for microscopic analysis. NIWA Client Report: CHC2007-110. [ Links ]
9. HASLE, G. & G. FRYXELL. 1970. Diatoms: cleaning and mounting for light and electron microscopy. T. Am. Microsc. Soc. 89: 469-474. [ Links ]
10. GUIRY, M.D. & G.M. GUIRY. 2015. AlgaeBase. Worldwide electronic publication, National University of Ireland, Galway. http://www.algaebase.org [searched on 27 May 2015]. [ Links ]
11. METZELTIN, D. & H. LANGE-BERTALOT. 1995. KritischeWertung der Taxa in Didymosphenia (Bacillariophyceae). Nova Hedwigia 60: 381-405. [ Links ]
12. METZELTIN, D. & H. LANGE-BERTALOT. 2014. The genus Didymosphenia M. Shmidt. A critical evaluation of established and description of 11 new taxa. Iconogr. Diatomol. 25: 1-293. [ Links ]
13. MERINO, R., G. BAUER, V. SASTRE, N. SANTINELLI, M. HAMAME, P. MONTERO & B. REID. 2011. Initial invasion and colonization patterns of Didymosphenia geminata in Patagonia. 2nd World Conference on Biological invasions and Ecosystem Functioning. November 21-24, Mar del Plata. [ Links ]
14. R DEVELOPMENT CORE TEAM. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, http://www.R-project.org. [ Links ]
15. REID, B.L., K.L. HERNÁNDEZ, M. FRANGÓPULOS, G. BAUER, M. LORCA, C. KILROY & S. SPAULDING. 2012. The invasion of the freshwater diatom Didymosphenia geminata in Patagonia: prospects, strategies, and implications for biosecurity of invasive microorganisms in continental waters. Conservation Letters 5: 432-440. [ Links ]
16. RIVERA, P. & M. GEBAUER. 1989. Chilean diatoms included in the Boyer´s, Cleve & Moeller´s, Schulze´s and Smith´s collections, deposited at the Academy of Natural Sciences of Philadelphia. Gayana Bot. 46: 89-116. [ Links ]
17. RIVERA, P., S. BASUALTO & F. CRUCES. 2013. Acerca de la diatomea Didymosphenia geminata (Lyngbye) M. Schmidt: su morfología y distribución en Chile. Gayana Bot. 70: 154-158. [ Links ]
18. ROSS, R., E.J. COX, D.G. KARAYEVA, D.G. MANN, T.B. PADOCK, R. SIMONSEN & P.A. SIMS. 1979. An emended terminology for the siliceous components of the diatom cell. Nova Hedwigia 64: 513-533. [ Links ]
19. SALA, S.E., S. SPAULDING, M. FERRARIO & A. LAMARO. 2013. Presencia de Didymosphenia geminata en Tierra del Fuego. Bol. Soc. Argent. Bot. 48 (Suppl.): 174-175. [ Links ]
20. SASTRE, A.V., G. BAUER, G. AYESTARAN & N. SANTINELLI. 2010. Monitoreo de Didymosphenia geminata. Informe Nº 5: Resultados muestreo 31 de Agosto, 2 y 7 de Septiembre de 2010. Laboratorio de Hidrobiología, Facultad de Ciencias Naturales, Universidad Nacional de la Patagonia San Juan Bosco Sedes Trelew-Esquel. [ Links ]
21. SASTRE, A.V., N.H. SANTINELLI, G.A. BAUER, M.G. AYESTARÁN & N.M. UYUA. 2013. First record of the invasive diatom Didymosphenia geminata (Lyngbye) Schmidt in a Patagonian Andean river of Argentina. BioInvasions Records 2: 11-17. [ Links ]
22. SKABICHEVSKIJ, A.P. 1983. A new species of Didymosphenia lineate (Bacillariophyta) and its variability. Bot. Zhurn. (Moscow & Leningrad) 68: 1254-1260. [ Links ]
23. SPAULDING, S.A. & L. ELWELL. 2007. Increase in nuisance blooms and geographic expansion of the freshwater diatom Didymosphenia geminata. Open- file report 2007-1425. U.S. Geological Survey. [ Links ]
24. SPAULDING, S. 2010. Didymosphenia geminata. In: Diatoms of the United States. Available at: http://westerndiatoms.colorado.edu/taxa/species/didymosphenia_geminata [Searched on 18 December 2014]. [ Links ]
25. STOERMER, E.F., Q. YU-ZAO & T.B. LADEWSKI. 1986. A quantitative investigation of shape variation in Didymosphenia (Lyngbye) M. Schmidt (Bacillariophyta). Phycologia 25: 494-502. [ Links ]
26. VOUILLOUD, A.A. 2003. Catálogo de diatomeas continentales y marinas de Argentina. Asociación Argentina de Ficología. [ Links ]
27. WHITTON, B.A., N.T. ELLWOOD & B. KAWECKA. 2009. Biology of the freshwater diatom Didymosphenia: a review. Hydrobiologia 630: 1-37. [ Links ]
Recibido el 24 de agosto de 2015,
aceptado el 16 de noviembre de 2015.














