Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Boletín de la Sociedad Argentina de Botánica
On-line version ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.51 no.3 Córdoba Sept. 2016
ANATOMÍA Y MORFOLOGÍA
Vibrational and thermal characterization of seeds, pulp, leaves and seed oil of Rosa rubiginosa
Pablo Martín Ramos1*, Jesús Martín Gil2, M. Carmen Ramos Sánchez3, Luis Manuel Navas Gracia2, Salvador Hernández Navarro2 and Francisco Javier Martín Gil3
1 EPSH, Universidad de Zaragoza, Carretera de Cuarte s/n, 22071 Huesca, Spain. Phone: +34 974 292668; Fax: +34 974 239302; E-mail: pmr@unizar.es
2 ETSIIAA, Universidad de Valladolid. Avenida de Madrid, 44, 34004 Palencia, Spain.
3 Hospital Universitario Río Hortega. Calle Dulzaina, 2, 47012 Valladolid, Spain.
Summary
Rosa rubiginosa L. seed oil has been studied for its application in skin care products, but the chemical nature of seeds, pulp and even leaves, apart from that of oil, is also relevant with a view to the application of this weed for biodiesel production. All these vegetal materials were studied by infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC) for characterisation purposes. FTIR bands at 3005, 2924, 1740, 1654 and 1456 cm-1 were used to estimate the iodine index, suitable for biofuels, and the oxidation stability degree. From the viewpoint of the thermal stability, both the seed oil (for which pyrolysis occurs at 462ºC), the raw seeds and the rosehip pulp (with decomposition temperatures of 373ºC and 333ºC, respectively) showed potential as a biomass feedstock for conversion into biofuels.
Key words: A-linolenic acid; DSC; FTIR; Rose hip seed oil; R. rubiginosa.
Resumen
Caracterización térmica y vibracional de las semillas, pulpa, hojas y aceite de semillas de Rosa rubiginosa
El aceite de semillas de R. rubiginosa L. ha sido estudiado para su aplicación en productos para el cuidado de la piel, pero la naturaleza química de las semillas, pulpa y hojas, además de la del aceite, también es importante con miras a la aplicación de esta mala hierba para la producción de biodiesel. Los materiales vegetales mencionados se han estudiado mediante espectroscopía infrarroja (FTIR) y calorimetría diferencial de barrido (DSC) con fines de caracterización. Las bandas del espectro infrarrojo en 3005, 2924, 1740, 1654 y 1456 cm-1 han sido utilizadas para estimar el índice de yodo, adecuado para los biocombustibles, y el grado de estabilidad a la oxidación. Desde el punto de vista de la estabilidad térmica, tanto el aceite de semillas (para el que la pirólisis se produce a 462ºC) como las semillas crudas y la pulpa (con temperaturas de descomposición de 373°C y 333°C, respectivamente) mostraron potencial como materia prima de biomasa para su conversión en biocombustibles.
Palabras clave: Aceite de rosa de mosqueta; Ácido α-linolénico; DSC; FTIR; R. rubiginosa.
Introduction
Rosa rubiginosa L. (= R. eglanteria L.) is a shrub from Great Britain that is frequent in Southern Alps and grows massively in Southern America in the shadow of the Andes Mountains. More recently, it has also started invading some of the temperate regions of Australia (Hatton, 1989; Hunter, 2004).
This member of the rose family has flowers pink in colour, and when petals fall (the flowers are ethereal, only living about 24 hours), the shrub develops enlarged floral cups (receptacles) which surround numerous small, hard dry fruits (achenes) commonly called seeds (Fig. 1). Rose hips are bright orange and oval and become fleshy but are not true fruits (Joublan et al., 1996). The pulp is edible and has a high concentration of vitamin C and is often used to make jams and conserves. The leaves and the petals, with their astringent and refreshing properties, are used for baths and infusions.

Fig. 1. Rosa rubiginosa or "rosa mosqueta" hips and leaves.
The finest rosehip seed oil is derived from cold pressing hips. This oil has been used by local people for its soothing and moisturizing properties and is commonly known in South America as "Rosa mosqueta" and in Australia as "sweet briar".
Rosa rubiginosa oil has a high content of essential fatty acids: over 77% in PUFA (oleic, linoleic or LA, and linolenic or αLNA). The percentage of SFA (palmitic and stearic acids) is below 5%. The LA and αLNA acids are considered important for the maintenance of a healthy skin. Besides non-saturated fatty acids and significant quantities of α-tocopherol (300 ppm), the presence of carotenoids, flavonoids and trans-retinoic acid has also been detected and these could be responsible for some of the pharmacological and therapeutic properties of rosehip seed oil (Zielinski, 1984; Dourado et al., 2000; Moure et al., 2001, 2005; Franco et al., 2007a, b; Romero et al., 2007; Merrill et al., 2008).
The characterization of R. rubiginosa seed oil has been of particular interest in refining processes; however, the chemical nature of plant parts such as seeds, pulp and even leaves, apart from that of crude oil, is now also relevant with a view to the more recent application of R. rubiginosa for biodiesel production. In this line, the aim of this work is to investigate their physicochemical properties by FTIR spectra and their thermal behaviour by differential scanning calorimetry (DSC).
Material and Methods
Fruits of R. rubiginosa come from Calafate, Santa Cruz, Argentina. Seeds were separated from pulp, air-dried for two weeks in the dark, grinded and sieved. High-purity commercial seed oil was supplied by Bariloche SilvestreTM (Provincia de Rio Negro, Argentina). This commercial oil contains 1.5±0.5 g oleic acid/100 g in FFA; 10-20 % in oleic (C18:1), 41-50% in LN (C18:2 n-6); 26-37% in LN (C18:3 n-3), 3-5% in palmitic (C16:O), and 1-3% in stearic (C18:0), in agreement with the characterization carried out for rosehip oils by Concha et al. (2006) and Pareja & Kehl (1990). R. rubiginosa oil FFA content was determined by AOAC method (protocol 940.28) using a chromatograph (Hewlett-Packard 5890 series II) equipped with an FID and fitted with an SP-2330 column. After methylation, a hexane extract containing FAME was injected into the GC column. FAME were separated using a stated temperature program and its weights calculated on the basis of their relative area vs. tridecanoic acid.
The infrared spectra were recorded with a Thermo Nicolet 380 FT-IR apparatus equipped with Smart Orbit Diamond ATR system.
DSC curves were obtained using a TA Instruments Q100 under N2 flow (20 mL·min-1), at a heating rate of 20ºC·min-1, in 40-μL sealed aluminium capsules. After cooling of the samples down to -100ºC, the scans were registered on the heating cycle from -100°C to 500 °C. For the study of the glass transition at low temperatures, samples were cooled to -100ºC and then heated from -60ºC to +20ºC at a rate of 10ºC·min-1. The data obtained were analysed using TA Instruments Universal Analysis V4.1D software.
Simultaneous analysis of TG and DSC (mW) for two samples was conducted with a Perkin- Elmer STA6000, DTA/DTG equipment, by heating the samples in a slow stream of N2 (20 mL/min) from room temperature up to 800ºC, with a heating rate of 20ºC/min. Pyris v.11 software was used for data analysis.
Results and Discussion
Vibrational characterization
Table 1 shows the frequencies and assignments of the bands observed in the vibrational spectra (Fig. 2) for the various samples of R. rubiginosa. To facilitate such assignments, the spectra of other plant samples with different percentages of immediate principles (lipids, proteins and carbohydrates) have been included.
Table 1. Main bands in the ATR- FTIR spectra of different parts of R. rubiginosa plant and of other vegetal species and natural products (for comparison purposes). All wavenumber values are in cm-1.
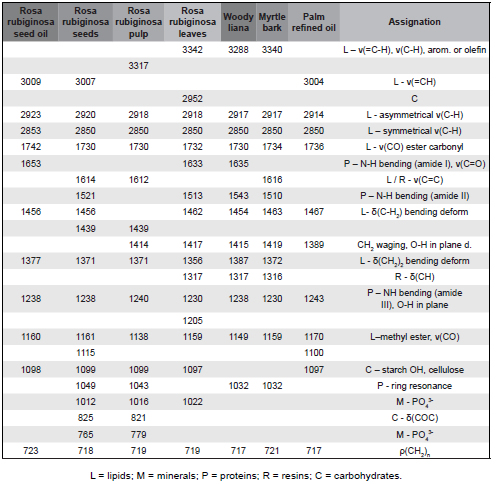

Fig. 2. ATR-FTIR spectra of different parts of R. rubiginosa plant.
The lipid characteristic band at 3009 cm−1 (which only appears for oil and seeds samples) is due to C-H stretching vibration of the carbon- carbon double bond (Guillén & Cabo, 1997). The strong absorption band at 2924 cm-1, shown by all the samples of R. rubiginosa, also has a lipid origin. A third lipid characteristic band at 1742 cm-1 for oils and seeds (shifted at 1730 cm-1 for other samples) is due to the stretching vibration of the triglyceride ester carbonyl (-C-C=O-) group. FTIR absorbance ratios A(3009 cm-1)/A(2924 cm-1), A(3009 cm-1)/A(2854 cm-1), and A(3009 cm-1)/A(1740 cm-1) were considered to measure the iodine values (El-Bahy, 2005). These ratios (0.21, 0.32 and 0.24, respectively) revealed that the iodine value of rosehip oil is similar to that of corn and higher than those of, for example, olive oil or cotton oil.
The band between 1653 and 1637 cm-1, common to Rosa moschata oil and leaves and woody liana, should be ascribed to C=O (amide I). The peak that appears between 1543-1521 cm-1 should be assigned to N-H bending vibrations (amide II). The intermediate band at 1614 cm-1 is due to lipids and resins (Yoshida & Yoshida, 2003; Abdelmalik et al., 2011). Bands between 1456 and 1317 cm-1 are due to CH2 and CH bending vibrations and are usual for vegetal fats and resins. From the above bands, one that appears at 1317 cm-1 is common to R. rubiginosa leaves, woody liana and myrtle bark.
According to the equation deduced by Sadeghi-Jorabchi et al. (1990), ν(C=C)/δ(CH) = A(1653 cm-1)/A(1456 cm-1) = (6.845⋅10-3)IV - (2.489⋅10-2), it was found that the iodine value of rosehip oil is ca. 115 gI2/100g (again very close to that of corn, which ranges from 103 to 123).
Since the oxidative stability is dependent on the iodine value (the higher the iodine value, the less the stable the oil), a presumably moderate stability could be expected. However, in agreement with Steel et al. (2005), we suggest that such oxidative stability would be enhanced by the high quantity of tocopherols present (300 ppm), which act as stabilizers.
The absorption band at around 1240 cm-1 is attributed to N-H bending (amide III) and the following bands (down to 1100 cm−1) are a fingerprint of methyl ester of long-chain fatty acids (Silverstein et al., 2005). The FTIR band at 1097 cm-1 indicates the presence of starch. The bands at wavenumbers around 1020 cm-1 and 810-765 cm-1 are due to the phosphate component. Finally, the band at 720 cm-1 can be assigned to (CH2)n rocking.
Thermal behaviour
Raw seeds
DSC curves of the heating cycle (after cooling down to -100ºC) of R. rubiginosa raw seeds are depicted in Fig. 3a. The endotherm at around -37ºC is assigned to the crystallization of α-LNA and LA polyunsaturated fatty acids as well as remaining fractions of di- and triglycerides, in good agreement with the values reported by Ueno et al. (2000) and Franco et al. (2013).

Fig. 3. DSC thermal effects of R. rubiginosa raw seeds: (a) detail of the low temperature endotherm after 5 freezing-thawing cycles; (b) details on the four main thermal effects.
The main thermal effect at around 111ºC is related to gelatinization of starch (an order-disorder transition for the starch/moisture system). The endotherm at 242ºC indicates seed protein crystallization to β-crystals accompanied by the random-coil β-form conformational transition. The exothermic events above 270ºC are related to the decomposition of hemicellulose and other fibre components (302ºC) and to the decomposition of either unsaturated fatty acid triglycerides or flavonoids (373ºC). Such two effects are in excellent agreement, for example, with those observed in the DSC scan of the salmon coloured bark of the myrtaceae Luma apiculata, rich in flavonoids, which has been included for comparison purposes (see Fig. 4).
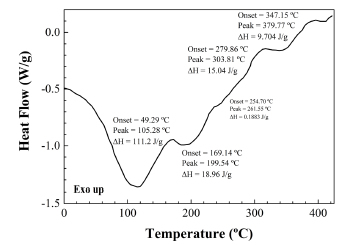
Fig. 4. DSC scan of myrtle bark.
The assignation of the last exothermic effect at 373ºC to decomposition of triglycerides is in agreement with values previously reported for pure fatty acids: according to Santos et al. (2002), the decomposition for polyunsaturated and monounsaturated fatty acids occurs in the 300- 380ºC range and from 380 to 480ºC, respectively. Alternatively, this same effect could be also assigned to degradation of flavonoids, according to da Costa et al. (2002).
Rose hip pulp
The DSC scan of pulp from R. rubiginosa rose hips (Fig. 5) differs significantly from the DSC of raw seeds. The thermal effects that appear during the pulp heating cycle occur 30-70ºC below than those that appear for the raw seeds and have a different origin. Thus, the fine endotherm detected at around 175ºC (attributed to sucrose) can be attributed to a conversion of pentahydrate into an anhydride upon temperature increase. Nevertheless, an alternative adscription to lycopene is also plausible: the melting of this red carotenoid pigment takes place at 173ºC. Even a third assignation to melting of trans retinoic acid would be possible: when this nutrient is in nanosuspension, its melting point decreases by 11ºC to 173ºC (Zhang et al., 2006).
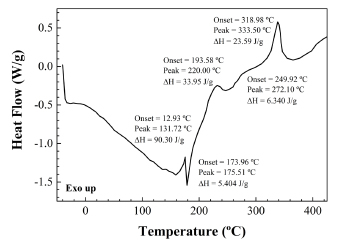
Fig. 5. DSC thermal effects of R. rubiginosa rose hip pulp.
With regard to the exotherms at 220ºC and 272ºC, they may correspond to decomposition of hemicellulose and starch, respectively. Nonetheless, since an exotherm at 218ºC has been reported for auto-oxidation of liposomes and other lipid systems containing water (Ayala-Zavala et al., 2007), this alternative assignation to the exotherm at 220ºC cannot be excluded.
Regarding the exotherm at 333ºC, it can be attributed either to decomposition of a mixture of α-LNA and LA acid starch esters produced from starch (Kapusniak et al., 2003) or to the decomposition of high molecular weight lignin components. This latter hypothesis arose when comparing the exotherm temperature for pulp with the one that appears in a DSC scan of a woody liana (see Fig. 6).

Fig. 6. DSC thermal effects of a woody liana.
Leaves
The low temperature effect at -1ºC, attributed to ice melting, appears lower than the one expected for pure water (0ºC) and is possibly conditioned by the temperature at which thylakoid membrane lipids undergo a phase transition (Fig. 7). The endotherm at 109ºC, attributed to usual evolved water (liberation or decomposition of water molecules) in cellulose membranes, is also in agreement with Raoult's Laws.

Fig. 7. DSC thermal effects of R. rubiginosa leaves.
Seed oil
The thermal decomposition of R. rubiginosa seed oil occurs in two stages. The first takes place between 200ºC and 400ºC and is sensitized by endotherms at 216ºC and 323ºC (see Fig. 8), at temperatures similar to those found for other oils such as refined palm oil, which features endotherms at 215ºC and 322ºC (Fig. 9). These thermal effects are assumed to be due to partial volatilization of triacylglycerides. The other decomposition stage, due to pyrolysis, starts at around 350ºC and is characteristic from each oil: whereas for refined palm oil occurs in a single step sensitized by a peak at 440ºC, for R. rubiginosa the decomposition takes place in two steps (peaks at 416ºC and 462ºC), in a similar fashion to -for example- the decomposition of olive pomace oil (with peaks at 284ºC and 429ºC) (Franco et al., 2013), albeit shifted to higher temperatures (by 33ºC). This second stage is the most significant because it is accompanied by an almost complete weight loss.

Fig. 8. TG/DSC thermal analysis of R. rubiginosa seed oil.
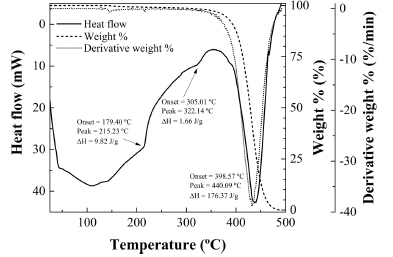
Fig. 9. TG/DSC thermal analysis of refined palm oil.
As a final remark to the overall discussion on the curves discussed above, the changes that result in endothermic and exothermic peaks must be considered as a summation of various thermal processes, out of which the main process still needs to be clarified in some cases. For example, the ambiguity in the assignation of the final decomposition thermal effects either to triglycerides or to flavonoids (in relation to the DSC of raw seeds) can be solved in favour of triglycerides because their concentration is higher than that of flavonoids, whereas in the case of rose hip pulp the elucidation of the contribution degree of polyunsaturated acid starch esters and lignin to the effect at 333ºC remains unsolved and further research is still required.
Conclusion
Different parts of R. rubiginosa plant ("Rosa mosqueta") and the most well known product derived from it, rosehip seed oil, were characterized by vibrational spectroscopy and differential scanning calorimetry.
ATR-FTIR allowed to observe similarities in the absorption bands for the various components of the plant (particularly evident for seeds and oil) and their correspondence with those of other plant species. By considering the absorbance ratios of the bands at 3005, 2923 and 1740 cm-1, assigned to lipids, the iodine index value for R. rubiginosa seed oil has been estimated to be close to 115 gI2/100 g, an intermediate value for oils. Since this iodine value is within the limits fixed by EN 14111:2003 regulation, R. rubiginosa seeds can be deemed as suitable for biodiesel production.
From the point of view of thermal stability, both the seed oil (with a pyrolysis temperature of 462ºC, higher than that of olive pomace oil or refined palm oil), the raw seeds and the rosehip pulp (which exhibit decomposition temperatures as high as 373ºC and 333ºC, respectively) also show a remarkable potential as biomass feedstock for biofuels. Further research on the reported raw materials (together with mill solid wastes) of R. rubiginosa is needed in order to explore their potential as a fuel crop, apart from their current use in skin care products.
Acknowledgements
Access to TAIL-UC facility funded under QREN-Mais Centro project ICT-2009-02-012-1980 is gratefully acknowledged.
Bibliography
1. ABDELMALIK, A. A., A. P. ABBOTT, J. C. FOTHERGILL, S. DODD & R. C. HARRIS. 2011. Synthesis of a base-stock for electrical insulating fluid based on palm kernel oil. Ind. Crop. Prod. 33: 532-536. [ Links ]
2. AYALA-ZAVALA, J. F., G. OMS-OLIU, I. ODRIOZOLA-SERRANO, G. A. GONZÁLEZ-AGUILAR, E. ÁLVAREZ-PARRILLA & O. MARTÍN-BELLOSO. 2007. Bio-preservation of fresh-cut tomatoes using natural antimicrobials. Eur. Food Res. Technol. 226: 1047-1055. [ Links ]
3. CONCHA, J., C. SOTO, R. CHAMY & M. E. ZÚÑIGA. 2006. Effect of rosehip extraction process on oil and defatted meal physicochemical properties. J. Amer. Oil Chem. Soc. 83: 771-775. [ Links ]
4. DA COSTA, E. M., J. M. B. FILHO, T. G. DO NASCIMENTO & R. O. MACÊDO. 2002. Thermal characterization of the quercetin and rutin flavonoids. Thermochim. Acta 392-393: 79-84. [ Links ]
5. DOURADO, F., P. VASCO, F. M. GAMA, M. A. COIMBRA & M. MOTA. 2000. Characterisation of Rosa Mosqueta seeds: cell wall polysaccharide composition and light microscopy observations. J. Sci. Food Agric. 80: 1859-1865. [ Links ]
6. EL-BAHY, G. M. S. 2005. FTIR and Raman spectroscopic study of Fenugreek (Trigonella foenum graecum L.) seeds. J. Appl. Spectrosc. 72: 111-116. [ Links ]
7. FRANCO, D., M. PINELO, J. SINEIRO & M. NUNEZ. 2007a. Processing of Rosa rubiginosa: Extraction of oil and antioxidant substances. Bioresour. Technol. 98: 3506-3512. [ Links ]
8. FRANCO, D., J. SINEIRO, M. PINELO & M. J. NÚÑEZ. 2007b. Ethanolic extraction of Rosa rubiginosa soluble substances: Oil solubility equilibria and kinetic studies. J. Food Eng. 79: 150-157. [ Links ]
9. FRANCO, J. M., L. A. GARCÍA-ZAPATEIRO, C. VALENCIA, M. A. DELGADO, C. GALLEGOS & M. V. RUIZ-MÉNDEZ. 2013. Chemical, thermal and viscous characterization of high-oleic sunflower and olive pomace acid oils and derived estolides. Grasas Aceites 64: 497-508. [ Links ]
10. GUILLÉN, M. D. & N. CABO. 1997. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 75: 1-11. [ Links ]
11. HATTON, T. J. 1989. Spatial patterning of sweet briar (Rosa rubiginosa) by two vertebrate species. Aust. J. Ecol. 14: 199-205. [ Links ]
12. JOUBLAN, J., M. BERTI, H. SERRI, R. WILCKENS, F. HEVIA & I. FIGUEROA. 1995. Wild rose germplasm evaluation in Chile. In: Proceedings of the Third National Symposium New Crops: New Opportunities, New Technologies, pp. 584-588. Indianapolis, Indiana. [ Links ]
13. KAPUŚNIAK, J., P. SIEMION & P. TOMASIK. 2003. Thermal reactions of starch with proteogenic amino acids. Thermochim. Acta 397: 209-218. [ Links ]
14. MERRILL, L. I., O. A. PIKE, L. V. OGDEN & M. L. DUNN. 2008. Oxidative stability of conventional and high-oleic vegetable oils with added antioxidants. J. Amer. Oil Chem. Soc. 85: 771-776. [ Links ]
15. MOURE, A., D. FRANCO, R. SANTAMARÍA, C. SOTO, J. SINEIRO, H. DOMÍNGUEZ, M. E. ZÚNIGA, M. J. NUÑEZ, R. CHAMY, A. LÓPEZ-MUNGUÍA & J. M. LEMA. 2001. Enzyme-aided alternative processes for the extraction of oil from Rosa rubiginosa. J. Amer. Oil Chem. Soc. 78: 437- 439. [ Links ]
16. MOURE, A., M. RÚA, J. SINEIRO & H. DOMÍGUEZ. 2005. Fractionation and characterization of proteins from Gevuina avellana and Rosa rubiginosa seeds. J. Amer. Oil Chem. Soc. 82: 169-173. [ Links ]
17. PAREJA, B. & H. KEHL. 1990. Contribution to the identification of Rosaaff. rubiginosa L. oil rose active principles. Anal. Real. Acad. Farm. 56: 283- 294. [ Links ]
18. ROMERO, N., P. ROBERT, L. MASSON, J. ORTIZ, K. GONZÁLEZ, K. TAPIA & C. DOBAGANES. 2007. Effect of α-tocopherol, α-tocotrienol and Rosa mosqueta shell extract on the performance of antioxidant-stripped canola oil (Brassica sp.) at high temperature. Food Chem. 104: 383-389.
19. SADEGHI-JORABCHI, H., P. J. HENDRA, R. H. WILSON & P. S. BELTON. 1990. Determination of the total unsaturation in oils and margarines by fourier transform raman spectroscopy. J. Amer. Oil Chem. Soc. 67: 483-486. [ Links ]
20. SANTOS, J. C. O., I. M. G. SANTOS, A. G. SOUZA, S. PRASAD & A. V. SANTOS. 2002. Thermal stability and kinetic study on thermal decomposition of commercial edible oils by thermogravimetry. J. Food Sci. 67: 1393-1398. [ Links ]
21. SILVERSTEIN, R. M., F. X. WEBSTER & D. J. KIEMLE. 2005. Spectrometric identification of organic compounds. 7th. John Wiley & Sons, Hoboken. [ Links ]
22. STEEL, C. J., M. C. DOBARGANES & D. BARRERA-ARELLANO. 2005. The influence of natural tocopherols during thermal oxidation of refined and partially hydrogenated soybean oils. Grasas Aceites 56: 46-52. [ Links ]
23. UENO, S., A. MIYAZAKI, J. YANO, Y. FURUKAWA, M. SUZUKI & K. SATO. 2000. Polymorphism of linoleic acid (cis-9, cis-12-Octadecadienoic acid) and α-linolenic acid (cis-9, cis-12, cis-15- Octadecatrienoic acid). Chem. Phys. Lipids 107: 169-178.
24. YOSHIDA, S. & H. YOSHIDA. 2003. Nondestructive analyses of unsaturated fatty acid species in dietary oils by attenuated total reflectance with Fourier transform IR spectroscopy. Biopolymers 70: 604- 613. [ Links ]
25. ZHANG, X., Q. XIA & N. GU. 2006. Preparation of all-trans retinoic acid nanosuspensions using a modified precipitation method. Drug Dev. Ind. Pharm. 32: 857-863. [ Links ]
26. ZIELINSKI, J. 1984. Rosa rubiginosa L. and Rosa heckeliana Tratt. in Bulgaria. Fragmenta Floristica Geobot. 30: 207-212. [ Links ]
Recibido el 20 de febrero de 2016,
aceptado el 3 de marzo de 2016.














