Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.52 no.2 Córdoba jun. 2017
BRIOLOGÍA
Identification of volatile compounds from three species of Cyathodium (Marchantiophyta: Cyathodiaceae) and Leiosporoceros dussii (Anthocerotophyta: Leiosporocerotaceae) from Panama, and C. foetidissimum from Costa Rica
Noris Salazar Allen1, Ana Isabel Santana2,3, Nélida Gómez1,4, Clementina Chung C.1,(†) and Mahabir Prashad Gupta3
1 Smithsonian Tropical Research Institute, P.O. Box 0843-03092, Balboa, Ancon, Panama, Republic of Panama. salazarn@si.edu
2 Department of Organic Chemistry, Faculty of Natural, Exact Sciences and Technology, University of Panama, Panama, Republic of Panama.
3 Center for Pharmacognostic Study of Panamanian Flora, College of Pharmacy, University of Panama, P.O. Box 0824- 00172, Panama, Republic of Panama. mahabirpgupta@gmail.com
4 Present Address: Asesora/Consultora.Innovación/Comercialización Saber Panama Consulting, P.O. Box 0819-02440.
(†) Deceased.
Summary
Cyathodium is a thalloid marchantialean liverwort with five species reported for the Neotropics. Three species that occur in Panama (C. bischlerianum, C. spruceanum, C. cavernarum) and one from Costa Rica (C. foetidissimum) were studied chemically. Female and male plants of the dioecious C. spruceanum were very similar in their chemical composition except for two compounds that were found only in female plants. All samples of C. spruceanum and C. bischlerianum contained, in less than three percent, the sesquiterpenes germacrene D and bicyclogermacrene. The presence of these compounds suggests a close affinity between these two species. Cyathodium bischlerianum contained mainly aromatic monoterpenes with nerolidol as the main compound. Cyathodium cavernarum also had a very distinct chemical composition with an octane derivative as its major compound. Indole compounds were found only in C. foetidissimum. The presence of these compounds in plants from Costa Rica and Tahiti suggests that they could be considered as potential chemosystematic markers for the species. Based on their chemical composition there is a clear distinction between the four species of Cyathodium studied. The chemistry of these species supports previous morphological and genetic studies. Only two compounds could be identified in Leiosporoceros dussii. There is a need for additional genetic and chemical studies on neotropical Cyathodium and Leiosporoceros.
Key words: Costa Rica; Diterpenes; Hornwort; Neotropical liverworts; Panama; Sesquiterpenes; Skatole.
Resumen
Identificación de compuestos volátiles de tres especies de Cyathodium (Marchantiophyta: Cyathodiaceae) y Leiosporoceros dussii (Anthocerotophyta: Leiosporocerotaceae) de Panama y C. foetidissimum de Costa Rica
Cyathodium es una hepática marchantial con cinco especies comunicadas para el Neotrópico. Se estudió la composición química de tres especies que crecen en Panamá (C. bischlerianum, C. spruceanum, C. cavernarum) y, una que crece en Costa Rica (C. foetidissimum). Plantas femeninas y masculinas del dioico C. spruceanum fueron muy similares en su composición química excepto por dos compuestos que se encontraron solo en plantas femeninas. Todas las muestras de Cyathodium spruceanum y C. bischlerianum contenían, en un porcentaje de menos del tres por ciento, los sesquiterpenos germacreno D y biciclogermacreno. La presencia de estos compuestos sugiere una afinidad muy cercana entre las dos especies. Cyathodium bischlerianum contiene principalmente monoterpenos aromáticos con nerolidol como el compuesto principal. Cyathodium cavernarum también tuvo una composición química muy distintiva con un derivado del octano como su compuesto principal. Compuestos de indol fueron encontrados solo en C. foetidissimum. La presencia de estos compuestos en plantas de Costa Rica y Tahiti sugiere que puedan ser marcadores quimosistemáticos para esta especie.
Las cuatro especies de Cyathodium estudiadas se pueden distinguir de acuerdo con su composición química. La química de estas especies apoya estudios genéticos y morfológicos previos. Solo dos compuestos pudieron ser identificados en Leiosporoceros dussii. Se necesitan estudios genéticos y químicos adicionales para los Cyathodium y Leiosporoceros neotropicales.
Palabras claves: Antocerote; Costa Rica; Diterpenos; Escatol; Hepáticas neotropicales; Panamá; Sesquiterpenos.
Introduction
Bryophytes are a major group of land plants that occur in most ecosystems and substrates from the Arctic to the Antarctic, except in the sea. Taxonomically, they are placed between the green algae and the vascular plants (ferns and flowering plants). There are, ca. 22,000-25,000 species of bryophytes in three lineages (Magill, 2010; Villarreal et al., 2010; von Konrat et al., 2010), the liverworts (Marchantiophyta), the hornworts (Anthocerotophyta) and the mosses (Bryophyta). They are considered the closest modern relatives of the ancestors of the first terrestrial plants (Renzaglia et al., 2007). The liverworts have been one of the most chemically studied of all bryophytes. This is due to the presence of oil bodies (membrane-bound organelles) in the cells of most liverworts that contain terpenoids suspended in a carbohydrate and/ or protein-rich matrix (Vanderpoorten & Goffinet, 2009). Spörle et al. (1991a, 1991b, 1991c) and Asakawa et al. (2013) reported, in Panamanian bryophytes, the presence of spiroterpenoids in Plagiochila moritziana Gottsche & Lindenb. ex Hampe, (-)-geosmin and other terpenoids in Sympyogyna brongniartii Mont. and lipophilic constituents from Monoclea gottschei Lindb. subsp. elongata Gradst. & Mues (published as M. gottschei Lindb. subsp. neotropica).
Cyathodium (Marchantiophyta: Cyathodiaceae) is a pantropical thalloid liverwort comprising 12 species distributed worldwide (Srivastava & Dixit, 1996; Salazar et al., 2004). Five of the species occur in the Neotropics, three of these are endemic to the New World: C. bischlerianum Salazar Allen, (until now endemic to Panama), C. spruceanum Prosk. and C. steerei Hässel, while two occur also in the Paleotropics, C. cavernarum Kunze and C. foetidissimum Schiffn. The center of diversity of the genus appears to be India, with eight species (Srivastava & Dixit, 1996). The plants are relatively simple in structure with the thallus composed of a central layer of air chambers covered by a dorsal and a ventral layer of cells. The air chambers are separated by uniseriate vertical rows of cells and are open to the upper surface by distinct pores flanked by narrow, elongated botuliform cells (Fig. 1). In some species, C. foetidissimum, the center part of the thallus has a multistratose area. In C. steerei the multistratose area is tuberculate and it is located at the base of the thallus (Hässel de Menéndez, 1961, 1962).

Fig. 1. A-E. Cyathodium spruceanum. A. Male plant with antheridial receptacles (arrows). B. Female plants, ES = Sporophytes, Arrows = apex of involucre. C. Transverse section of thallus showing air cavities. D. Upper surface of thallus with pores. E. Pore with 3(-4) rings of cells. F-G. Cyathodium bischlerianum. F. Growing on trunk of tree. G. Dorsal view of plant with sporophytes (dark areas). (A-B from Salazar Allen 16700; G from Salazar Allen 16765, C from Gudiño 398, D- E from Gudiño 337, F from Gudiño 340) (Photos A-B, G, Salazar Allen; C-E, F, J.A. Gudiño)
Cyathodium is poorly known in the Neotropics mainly by few herbarium collections (Salazar Allen et al., 2004). The paucity of collections may be related to the seasonal growth of these plants. They grow during the rainy season and start dying out at the onset of the dry season. Nevertheless, they can persist under very humid conditions on banks of creeks and rivers and in terraria for most of the year and in axenic agar cultures under controlled environmental conditions. In the field, only seasonal plants appear to produce sporophytes (Salazar Allen et al., 2004). Of the five Neotropical species three are monoecious (C. bischlerianum, C. cavernarum, C. foetidissimum) and two (C. spruceanum, C. steerei) are dioecious.
Each neotropical species is morphologically and genetically distinct (Salazar Allen et al., 2004; Salazar Allen, 2005; Salazar Allen & Korpelainen, 2006). Genetic variations in nucleotide sequences in the nuclear ribosomal DNA region, ITS1-5.8S rRNA-ITS2 were analyzed for three species (C. cavernarum, C. spruceanum and C. foetidissimum). Sequences for C. bischlerianum failed and were not included in the genetic analysis (Salazar Allen & Korpelainen, 2006). The largest genetic differences were found between C. foetidissimum and C. spruceanum. Samples from C. cavernarum and C. spruceanum from nearby geographical areas were shown to be genetically more closely related than those of geographical distant areas. In Cyathodium the oil bodies occur in specialized cells (idioblasts) of the thallus devoid of chloroplasts (C. cavernarum, C. bischlerianum, C. foetidissimum and, in C. spruceanum only in the border cells). In C. spruceanum the oil bodies are present in all cells with the chloroplasts (Fig. 2). Substances in the oil bodies confer a distinctive odor to some of the species, e.g., an unpleasant odor in C. foetidissimum (Ludwiczuk et al., 2009; Salazar Allen Pers. comm.), and a cedar oil smell in C. steerei (Hässel de Menéndez, 1962). Nevertheless, only one species, C. foetidissimum, has been chemically investigated. Skatole, which is responsible for the very intense unpleasant odor of the ether extract of C. foetidissimum (Ludwiczuk et al., 2009), is a well-known compound produced by biodegradation of tryptophan that is responsible for the fecal odor of this liverwort. This is the second record of skatole in the Marchantiophyta. Previously, this compound was detected in an Asterella-like liverwort collected in Malaysia (Askawa et al., 1995). Cyathodium foetidissimum also elaborates isolepidozene and lunularin. Isolepidozene is known as the main volatile component of Concephalum japonicum (L.) Dum. and Marchantia emarginata subsp. tosana (Stephani) Bischl. (as Marchantia tosana Stephani). Lunularin was previously isolated from or detected in Dumortiera hirsuta (Sw.) Nees, Marchantia polymorpha L., M. chenopoda L., M. berteroana Lehm. & Lindenb., M. paleacea var. diptera (Nees & Mont.) Inoue, and Ricciocarpos natans (L.) Corda. All these species are thallose liverworts in the order Marchantiales of the Marchantiophyta. Cyathodium foetidissimum is closely related chemically to the Marchantiopsida (Asakawa et al., 2013).

Fig. 2. Cyathodium foetidissimum. A-B. Plants in their natural habitat. Dh = Dumortiera hirsuta, Dn = Dumortiera hirsuta subsp. nepalensis (Tayl.) Schust. C. Idioblasts (arrows) on cells of thallus. D. Pore on upper surface of thallus. (A-B from Salazar Allen et al. 17047; C-D from Salazar Allen 20627). (Photos by
Phytochemical analyses of polar and non-polar compounds were pursued by our group in 2004 to determine 1) if morphological and genetic differences that distinguish the species were reflected in their chemistry, and 2) if there were chemical variations related to the sexual state of the plants, particularly in the dioecious C. spruceanum (young or old males and females with sporophytes).
Leiosporoceros dussii Hässel (Anthocerotophyta, Leiosporocerotaceae) is a hornwort that, unlike other hornworts, has its associated cyanobacteria in longitudinal thallus channels (Villarreal & Renzaglia, 2006) (Fig. 3). It grows on rocks and in volcanic and sandy soils near creeks or in roadway ditches (Villarreal, 2009). It has been reported as occurring in Mexico, Costa Rica, Panama, the Caribbean region, Colombia and Ecuador (Villarreal, 2009). Morphological and phylogenetic studies have revealed that it is the most genetically and morphologically divergent hornwort (Duff et al., 2004; Villarreal et al., 2010). It is also the most basal of all hornworts (Villarreal et al., 2010). Nevertheless, there is a lack of information on its chemical composition with or without its symbiont cyanobacteria. The aim of this study was to determine the chemical differences in fresh samples of male and female plants and decomposing ones of Leiosporoceros dussii, collected from its natural environment.
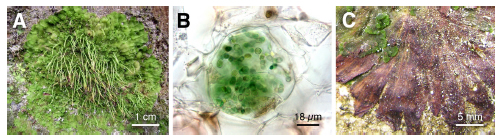
Fig. 3. Leiosporoceros dussii. A. Female plant in its natural environment. B. Transverse section of Nostoc canal. C. Decomposing thallus. (B from Gudiño 492; C from Salazar Allen 21368) (Photos A, M. Moya; B, J.A. Gudiño; C, Salazar Allen).
Materials and Methods
Forty milligrams of fresh plants of C. bischlerianum, C. cavernarum and C. spruceanum (female with young sporophytes, young male plants and senescence males) were selected from populations of Panama. Young male plants were those with their receptacles light green colored; senescence males had their male receptacles brown-colored with empty sperm cavities. Only plants collected in Panama were used for Leiosporoceros dussii and sterile young plants from axenic cultures (Salazar Allen & Korpelainen, 2006) for C. foetidissimum from Costa Rica. Using sterile tweezers, the samples were placed in sterile glass vials to which a 0.75 ml of n-hexane and n-octadecane (50 μg/ml of n-octadecane and n-hexadecanol as internal standards) was added to the samples to arrive at a final concentration of 20 μg/mg. The closed vials were placed in a rack and submerged in a 250 ml beaker containing 50 ml of water. The beaker was heated in a microwave at high power (700-800 W) for one minute. The vials were cooled at room temperature and the supernatant was transferred to clean sterile glass vials. The closed vials were stored at -18o C. Separation, quantification and determination of compounds were done using an Agilent Technologies, Model 6890N gas chromatograph, fitted with a Model 5973 mass selective detector (MSD) and managed by an Agilent Chem Station Data system. An HP- 5MS (5% phenyl methylpolysiloxane) fused silica capillary column (30 mm x 0,25 mm id., film thickness 0,25 μm) was used. Helium at a flow rate of 1.0 ml /min was used as carrier gas. An MS interface temperature of 270o C, electron impact (EI) ionization mode, an ionization voltage of 1,300 V; and a scanning range m/z 40-400 at 1 scan/s was used.
The MS spectra obtained were compared to the spectral fragmentation patterns available from spectral libraries (AdamsWiley and NIST data base) (McLafferty, 1993; Adams, 1995; Vila et al. 2004; Vila et al. 2010).
The percentage represented by each of the identified compounds was obtained using the peak area normalization method. Not all compounds were identified. For example, from a sample analyzed by GC-MS, 100 peaks were produced but only 20 compounds could be identified representing 78% of the total peak area obtained.
Specimens studied. Cyathodium bischlerianum. PANAMA. Prov. Panama: Dtto. Panama, Parque Nacional Soberanía, Sendero El Charco, 1-XII- 2004, 70 m. Salazar Allen, Chung & De Gracia 20993 (PMA).
Cyathodium cavernarum. PANAMA. Prov. Coclé: Dtto. Antón, El Valle de Antón, cerca del Mirador, 21-VII-2004, +736 m, Salazar Allen, Chung, De Gracia & Ramírez 20926, 20927 (PMA). PANAMA. Prov. Coclé: Dtto. Antón, El Valle de Antón, sobre pared del puente sobre el río Guayabo, 21-VII-2004, 624 m, Salazar Allen, Chung, De Gracia & Ramírez 20939 (PMA).
Cyathodium spruceanum. PANAMA. Prov. Panama: Dtto. Panama, Parque Nacional Soberanía, Sendero Natural El Charco, 1-XII- 2004, 70 m, Salazar Allen, Chung, De Gracia 20992, 20994, 20995, 20997 (PMA). PANAMA. Prov. Panama: Dtto. Panama Parque Nacional Soberanía, La Cascada, 1-XII-2004, 76 m, Salazar Allen, Chung, De Gracia 20999 (PMA). PANAMA. Prov. Panama: Dtto. Panama Parque Nacional Chagres, Campo Chagres, 1-XII-2004, + 110 m. Salazar Allen, Chung, De Gracia 21002 (PMA). PANAMA. Prov. Panama: Dtto. Panama, en taludes a lo largo de la carretera después del mirador de la Represa Madden, 1-XII-2004, +118 m, Salazar Allen, Chung, De Gracia 21003, 21004 (PMA). PANAMA. Prov. Coclé: Dtto. Antón, El Valle de Antón, río Las Mozas, 21-VII-2004, 580 m, Salazar Allen, Chung, De Gracia & Ramírez 20943, 20947 (PMA). PANAMA. Prov. Coclé: Dtto. Antón, El Valle de Antón, río Las Mozas, 27- VIII-2004, 580 m, Salazar Allen, Chung, De Gracia & Ramírez 20946, 20950 (PMA).
Cyathodium foetidissimum.Tipo: COSTA RICA. Prov. Cartago, Cantón de Paraíso, Parque Nacional Tapantí, Valle del Río Grande de Orosi, II-2002, 1200 m, Salazar Allen, Lépiz, Villarreal, Carranza & Lizano 20618 (Paratipo (PMA!), 20619 (PMA, USJ).
Leiosporoceros dussii. PANAMA. Prov. Coclé: Dtto. Antón, El Valle de Antón, Monumento Natural Cerro Gaital, en laderas del río El Guayabo, 580 m, Salazar Allen, Chung, De Gracia & Ramírez 75, 77, 76, 80, 20940, 20942 (PMA). PANAMA. Prov. Coclé: Dtto. Antón, El Valle de Antón, en suelo, al borde de la carretera hacia La Mesa, 828 m, Salazar Allen, Chung, De Gracia & Ramírez 20932 (PMA).
Results
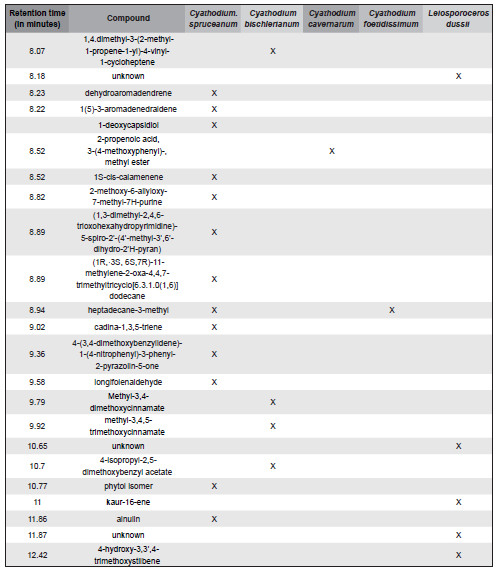
Compounds identified in the five species studied and their corresponding retention times (minutes) are listed in Table 1.
Table 1. Compounds identified in four species of Cyathodium and in Leiosporoceros dussii. Retention times are reported in minutes.

In Table 2, the results of secondary metabolites identified in samples of Cyathodium spruceanum at different growth stages and collected from different sites are presented. Germacrene D and bicyclogermacrene were present in all samples of C. spruceanum studied. In the female sample with sporophytes (FS) from Campo Chagres, nine compounds were identified representing a total of 73,3%. The principal components were 12-norcyercene B (70,2%), germacrene D (1,6%) and longifolenaldehyde (1,4%). In the senescent male sample (SM) collected from rocks, 68,4% of the total compounds could be detected. The major components were cyercene (63,2%) and germacrene D (1,6%), while 11 compounds representing a total of 69,5% were identified in the female sample (FS) collected from Madden, a site close to Campo Chagres. This sample also contained cyercene (65,8%) and germacrene D (1,6%) (Table 2). At the Cascada (Parque Nacional Soberanía), the female sample (FS) was found to contain 14 compounds (67,8%), of which the major compounds were: 4-(3,4-dimethoxybenzylidene)- 1-(4-nitrophenyl)-3-phenyl-2-pyrazolin-5-one (61,0%) and germacrene D (1,6%). In the senescent male sample (SM) collected from the nature trail El Charco we identified 9 compounds (totaling 76,6%). The major components were cyercene (74,6%) and germacrene D (1,2%). From the female sample (FS) from the same site, 9 compounds (81,1%) were also detected, of which the major components were cyercene (51,0%), 12-norcyercene B (21,7%), phytol isomer (3,5%) and germacrene D (3,2%). In the young male sample (YM) collected at Las Mozas, we identified 14 compounds (79,0%), of which the principal components were: 4-(3,4-dimethoxybenzylidene-1-(4-nitrophenyl)- 3-phenyl-2-pyrazolin-5-one (43,8%), cyercene (20,3%), (1R,3S,6S,7R)-11-methylene-2-oxa-4,4,7- trimethyltricyclo[6.3.1.0(1,6)]dodecane (6,7%), 1S-cis-calamenene (2,5%), germacrene D (1,7%) and (+)-clavukerin A (1,2%). From the female sample (FS) collected also at Las Mozas nine compounds (77,6%) were identified, of which the principal components were 4-(3,4-dimethoxybenzylidene)- 1-(4-nitrophenyl)-3-phenyl-2-pyrazolin-5-one (66,9%) and (1R,3S,6S,7R)-11-methylene-2-oxa- 4,4,7-trimethyltricyclo[6.3.1.0 (1,6)]dodecane (6,8%).
Table 2. Percentages of major volatile compounds in Cyathodium spruceanum from plants of different sites (A: El Charco, B: La Cascada, C: Campo Chagres, D: Madden, E: Las Mozas) and various sexual condition (FS: female plants with sporophytes, YM: Young males, SM: senescence males).


In Cyathodium bischlerianum, 12 compounds were identified representing a total of 70,3%, of which the main compounds were nerolidol (26,7%), γ-terpinene (15,7%) and limonene (7,2%) (Table 3). (+)-Nerolidol is present in Gymnocolea inflata (Huds.) Dumort. and Lophocolea heterophylla (Shrad.) Dumort. The same alcohol has been isolated from the essential oils of Plagiochila ovalifolia Mitt. and Wiesnerella denudata (Mitt.) Stephani (Asakawa, 1995).
Table 3. Percentages of major volatile compounds in Cyathodium bischlerianum collected in Sendero El Charco (Soberanía National Park), Province of Panama.

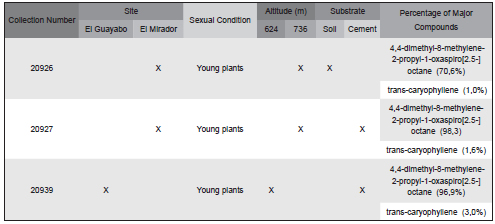
In C. cavernarum, collected from El Valle de Antón, two compounds were identified (representing 99.94%), and the major component was 4,4-dimethyl-8-methylene-2-propyl-1- oxaspiro [2.5-]octane (70,6%) (Table 4).
Table 4. Percentages of major volatile compounds from various samples of Cyathodium cavernarum collected in two sites in El Valle de Antón, Province of Coclé.
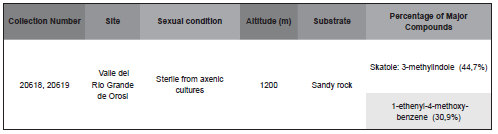
In axenically grown samples of Cyathodium foetidissimum of Costa Rica, six compounds were identified representing a total of 87,9 %. The main compounds found were: 3-methylindole (44,7%) and 1-ethenyl-4-methoxy-benzene (30,9%) (Table 5). This is the only species in this genus that has been previously studied chemically (Ludwiczuk et al., 2009). Skatole or 3-methylindole is a mildly toxic white crystalline organic compound belonging to the indole family. It occurs naturally in feces (it is produced from tryptophan in the mammalian digestive tract) and coal tar and has a strong fecal odor. However, at low concentrations, it has a floral odour and is present in several flowers and essential oils, including those of orange blossoms, jasmine, and Ziziphus mauritiana Lam. It is used as a fragrance and fixative in many perfumes and as an aroma compound. Its name is derived from the Greek root skato-meaning "dung". Skatole was discovered in 1877 by the German physician Ludwig Brieger (1849 – 1919). It is used by the U.S. military in its non-lethal weaponry arsenal; specifically as malodorants (Patent 6,386,11).
Table 5. Percentages of major volatile compounds in Cyathodium foetidissimum of Costa Rica grown in axenic cultures.
Four compounds were identified in the hornwort Leiosporoceros dussii. The major compounds were 4-hydroxy-3,3',4-trimethoxystilbene (56,5%) and kaur-16-ene (31,4%). The sample in decomposition had kaur-16-ene (64.9%) and an unidentified compound. The sterile samples contained 4-hydroxy-3,3',4-trimethoxystilbene (57,0%), kaur- 16-one (28,6%) and an unidentified compound. All of the male samples also produced 4-hydroxy- 3,3',4-trimethoxystilbene (57.6%) and kaur-16-ene (36,3%) as major compounds (Table 6).
Table 6. Percentages of major volatile compounds in the hornwort Leiosporoceros dussii according to its sexual condition and of a decomposing thallus from samples collected in El Valle de Antón, Province of Coclé.

Discussion
There is a clear distinction between the four neotropical Cyathodium species studied according to their chemical composition. The chemistry of these species supports previous morphological and genetic studies (Salazar Allen, 2005; Salazar Allen & Korpelainen, 2006). According to these studies each species is clearly distinct based on gametophytic characters [frequency of pores, oil bodies in idioblasts (C. bischlerianum, C. cavernarum and C. foetidissimum) or with chloroplasts in the same cell (C. spruceanum)], type of rhizoids; sexual condition (monoecious or dioecious) position and size of male receptacles and rhizoid morphology among some and, also according to sporophytic characters (presence or absence of an operculum, ornamentation of the upper cells of the sporophyte and spore ornamentation). According to genetic studies (Salazar Allen & Korpelainen, 2006), each species is also genetically distinct but no differences between male and female plants of C. spruceanum were genetically studied. Nevertheless, although male and female plants of this species were found to be similar regarding their chemistry, three compounds, 12-norcyercene-B, longifolenaldehyde and 1(5)-3-aromadenedraidene were found only in female plants. These differences point to the need to pursue genetic studies in this dioecious species to determine if the chemical differences observed can be genetically determined.
Although C. bischlerianum was not included in the genetic studies of Salazar Allen & Korpelainen (2006), this species shares with C. spruceanum the sesquiterpenes germacrene D and bicyclogermacrene, although they were present in low percentages. The presence of these sesquiterpenes suggests a genetic affinity between C. bischlerianum and C. spruceanum, the latter considered the most plesiomorphic of all neotropical Cyathodium species. Additional work is needed, which should include samples from other sites in the Neotropics, to determine if this chemical similarity is characteristic for both species. Both sesquiterpenes have been reported in other foliose and thalloid liverworts (Asakawa et al., 2013).
Cyathodium bischlerianum unlike the other species contains mainly aromatic monoterpenes with nerolidol as the main compound (Table 1). (E)–nerolidol has a floral and woody fragance (Padalia et al., 2015). It is used in fine fragrances, cosmetics, shampoo, soaps, detergents and cleaning products. The world consumption of the compound is ca. 10-100 tons/year (Queiroga et al., 2014). It has also been approved by the Food and Drug Administration of the United States as a flavoring agent for food. γ-Terpinene, identified in this species, is one of the major components of the oil of the African and Iranian "ajowan" or Bishop's weed seed oil (Carum copticum Benth, Apiaceae) (Boskabadi et al., 2017).
It is noteworthy that this species is the smallest in size of the neotropical species and occurs as an epiphyte on the bark of trees near creeks, or in soil and humus accumulated in the forks or concavities of trees, and on rocks in humid places (Fig. 1).
The chemistry of C. spruceanum and C. cavernarum coincides with morphogenetic results previously reported (Salazar Allen and Korpelainen, 2006) where samples from close geographical areas were genetically more closely related than those from geographically distant samples (e.g., plants from El Valle de Antón all share the same compounds). Chemical differences in the same species growing in different sites have been reported for other liverworts, e.g., Bryopteris filicina (Sw.) Nees, a foliose liverwort collected in Costa Rica that produced two germacrenes that were not found in the same species collected in Panama (Asakawa et al., 2013). It would be interesting to compare the chemical composition of Cyathodium species from Panama with those from Costa Rica.
Chemically, C. foetidissimum is very distinct from the three Cyathodium species analyzed in this study. It does not share any of the major compounds found in the other species. The presence of indol compounds in C. foetidissimum from Costa Rica and Tahiti suggests that these compounds may be potential chemosystematic markers for the species. Skatol responsible for the fecal odor of the plants, was found in plants from Tahiti as well as from axenically grown plants from Costa Rica. It will be useful to have samples from other areas where the species have been reported, e.g., Java, Sumatra, Nukahiva (Srivastava & Dixit, 1996) and Cameroon (Wigginton & Grolle, 1996; Wigginton, 2002) to compare their chemical composition.
The hornwort Leiosporoceros dussii contains a great number of compounds that could not be identified. Nevertheless, the diterpene found (in low quantities) is known for Anthoceros caucasicus Steph. (Sonwa & Köning, 2003). It is important to grow Leiosporoceros in axenic cultures to determine if the secondary metabolites it produces are, at least in part, the result of its association with its Nostoc endosymbiont.
There is a need to continue the study of these plants by collecting samples during different seasons and from different substrates throughout the year, to determine if there are variations in the type of compounds produced or in their concentrations according to the substrate on which they occur, their growth and sexual states. Also, extracts from axenically grown plants will be helpful in clarifying the role of endosymbionts (e.g., in Leiosporoceros) in the production of these chemicals. Another interesting line of research is the study of the bioactivity of these compounds.
Acknowledgements
This work was supported by research funds from Smithsonian Tropical Research Institute, Panama, to N. Salazar Allen and N. Gómez. Our sincere thanks to Nelly Rivas and Johant Leaky, for help with processing samples; in Costa Rica to M.I. Morales, J. Carranza, M. Bermúdez, E. Lépiz, geologist A.L. Velerio and to J.C. Villarreal, J.E. De Gracia & I. Ramírez for assistance with field work. To the Ministerio de Ambiente y Energía, Sistema Nacional de Áreas de Conservación of Costa Rica and the Ministerio de Ambiente (former Autoridad Nacional del Ambiente – A.N.A.M.) of Panama for issuing collecting permits. The deepest gratitude of NSA to the late H. Bischler for encouraging her to undertake research on neotropical Cyathodium. MPG thanks SENACYT for SNI distinguished scientist award. Special acknowledgements to two anonymous reviewers.
Bibliography
1. ADAMS, R. P. 1995. Identification of essential oils by gas chromatography/mass spectroscopy. Carol Stream, IL: Allured. [ Links ]
2. ASAKAWA, Y., M. TOYOTA, H. TANKA, T. HASHIMOTO & D. JOULAIN. 1995. Chemical constituents of an unidentified Malaysian liverwort Asterella (?) species. J. Hattori Bot. Lab. 78: 183. [ Links ]
3. ASAKAWA, Y., A. LUDWICZUK & F. NAGASHIMA. 2013. Chemical constituents of bryophytes. Bio-and chemical diversity, biological activity, and chemosystematics. Prog. Chem. Org. Nat. Prod. 95. Springer-Verlag, Wien. [ Links ]
4. BOSKABADY, M. H., S. ALITANEH & A. ALAVINEZHAD. 2014. Carum copticum L.: A herbal medicine with various pharmacological effects. Review article. BioMed. Res. Int. 2014: 1-11. [ Links ]
5. DUFF, R. J., D. C CARGILL, J. C. VILLARREAL & K. S. RENZAGLIA. S. 2004. Progress and challenges towards developing a phylogeny and classification of hornworts. Bryologist 110: 214-243. [ Links ]
6. HÄSSEL DE MENÉNDEZ, G. G. 1961. Una nueva especie de un género nuevo para Argentina: Cyathodium steerei. Rev. Bryol. Lichénol. 30: 223- 231. [ Links ]
7. HÄSSEL DE MENÉNDEZ, G. G. 1962. Estudio de las Anthocerotales y Marchantiales de la Argentina. Opera Lilloana VIII. Tucumán, Argentina. [ Links ]370
8. LUDWICZUK, A., I. KOMALA, A. PHAM, J-P. BIANCHINI, P. RAHARIVELOMANANA & Y. ASAKAWA. 2009. Volatile components from selected Tahitian liverworts. Nat. Prod. Commun. 4: 1387 – 1392.
9. MAGILL, R. 2010. Moss diversity: New look at old numbers. Phytotaxa 9: 167 – 174.
10. McLAFFERTY, F. W. 1993. Wiley registry of mass spectral data, 4th edition. New York: John Wiley & Sons. [ Links ]
11. QUEIROGA, C. L., M. Q. CAVALCANTE, P. C. FERRAZ, R. N. COSER, A. SARTORATTO & P. M. DE MAGALHÃES. 2014. High-speed countercurrent chromatography as a tool to isolate nerolidol from the Baccharis dracunculifolia volatile oil. J. Essent. Oil Res. 26: 334-337. [ Links ]
12. PADALIA, R., R. S. VERMA & A. CHAUHAN. 2015. The essential oil composition of Melaleuca leucadendra L. grow in India: A novel source of (E)- nerolidol. Ind. Crops Prod. 69: 224 – 227.
13. RENZAGLIA, K. S., S. SCHUETTE, R. J. DUFF, R. LIGRONE, A. J. SHAW, B. D. MISHLER & J. G. DUCKETT. 2007. Bryophyte phylogeny: Advancing the molecular and morphological Frontiers. Bryologist 110: 179 – 213.
14. SALAZAR ALLEN, N. 2005. Cyathodiaceae. In: BISCHLER-CAUSSE, H., S. R. GRADSTEIN, S. JOVET-AST., D. LONG & N. SALAZAR ALLEN (eds.), Marchantiidae. Flora Neotropica 97: 131- 146. [ Links ]
15. SALAZAR ALLEN, N., E. LÉPIZ & J. E. DE GRACIA. 2004. Cyathodium foetidissimum (Marchantiales), an Asiatic species new to Tropical America. Bryologist 107: 41-46. [ Links ]
16. SALAZAR ALLEN, N. & H. KORPELAINEN. 2006. Notes on neotropical Cyathodium. Cryptogam. Bryol. 27: 85-96. [ Links ]
17. SONWA, M. M. & W. A. KÖNING. 2003. Chemical constituent of the essential oil of the hornwort Anthoceros caucasicus. Flavour Frag. J. 18: 286- 289. [ Links ]
18. SPÖRLE, J., H. BECKER, N. SALAZAR ALLEN & M. P. GUPTA. 1991a. Spiroterpenoids from Plagiochila moritziana. Phytochemistry 91: 52-80. [ Links ]
19. SPÖRLE, J., H. BECKER, N. SALAZAR ALLEN & M. P. GUPTA. 1991b. Occurrence of (--) geosmin and other terpenoids in an axenic culture of the liverwort Symphyogyna brongniartii. Z. Naturforsch. 46c: 183-188 [ Links ]
20. SPÖRLE, J., H. BECKER, N. SALAZAR ALLEN & M. P. GUPTA. 1991c. Lipophillic constituents from the Panamanian liverwort Monoclea gottschei subsp. neotropica. J. Hattori Bot. Lab. 70: 151-155.
21. SRIVASTAVA, S. C. & R. DIXIT. 1996. The genus Cyathodium Kunze. J. Hattori Bot. Lab. 80: 149 – 215.
22. VILA, R., J. IGLESIAS, S. CAÑIGUERAL & M. P. GUPTA. 2004. Constituents and biological activity of the essential oil of Eugenia acapulcensis Steud. J. Essent. Oil Res. 16: 384 – 386.
23. VILA R., A. I. SANTANA, R. PÈREZ-ROSÈS, A. VALDERRAMA, M. V. CASTELLI, S. MENDONCA, S. ZACCHINO, M. P. GUPTA & S. CAÑIGUERAL. 2010. Compositionn and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of α-bisabolol. Bioresour. Technol. 101: 2510 – 2514.
24. VILLARREAL, J. C. 2009. La briofita del mes. Leiosporoceros dussii Hässel. Available in: Briología blogspot.com 2009/06/la briofita-del-mes-Leiosporoceros dussii Hässel.html [Accessed: February 2016]. [ Links ]
25. VILLARREAL, J. C. & K. S. RENZAGLIA. 2006. Structure and development of Nostoc strands in Leiosporoceros dussii (Anthocerotophyta). A novel symbiosis in land plants. Amer. J. Bot. 93: 693-705. [ Links ]
26. VILLARREAL, J. C., D. C. CARGILL, A. HAGBORG, L. SODERSTRÔM & K. S. RENZAGLIA. 2010. A Synthesis of hornwort diversity: Patterns, cause and future work. Phytotaxa l9: 150–166.
27. VANDERPOORTEN, A. & B. GOFFINET (eds.). 2009. Introduction to bryophytes. Cambridge University Press, Cambridge. [ Links ]
28. VON KONRAT, M., L. SODERSTRȎM, M. A. M. RENNER, A. HAGBORG & L. BRISCOE. 2010. Early Land Plants Today (ELPT): How many liverwort species are there? Phytotaxa 9: 22-40. [ Links ]
29. WIGGINTON, M. J. 2002. Checklist and distribution on the liverworts and hornworts of sub-Saharan Africa, including the East African Islands. Trop. Bryol. Res. Rep. 3: 22. [ Links ]
30. WIGGINTON, M. J. & R. GROLLE. 1996. Catalog of the hepaticae and anthocerotae of sub-Saharan Africa. Bryophyt. Biblioth. 50: 1-267. [ Links ]
Recibido el 1 de abril de 2017,
aceptado el 9 de junio de 2017.














