Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.53 no.1 Córdoba mar. 2018
Sistemática de Plantas Vasculares - Systematics of Vascular Plants
First report of Duchesnea indica F. albocaput (Rosaceae) in Northwestern Argentina.
Primer reporte de Duchesnea indica F. albocaput (Rosaceae) en el Noroeste de Argentina.
MARIO A. DEBES1,2 *, INGRID G. ORCE3, ANA C. LUQUE1, JUAN C. DÍAZ-RICCI2, ATILIO P. CASTAGNARO3 and MARTA E. ARIAS1,4
1 Facultad de Ciencias Naturales e I.M.L, Universidad Nacional de Tucumán (UNT). Tucumán, Argentina. mariodebes@csnat.unt.edu.ar; anacatalinaluque@hotmail.com; arias@csnat.unt.edu.ar.
2 Instituto Superior de Investigaciones Biológicas (INSIBIO, CONICET-UNT) Tucumán, Argentina. mariodebes@gmail. com, juan@fbqf.unt.edu.ar
3 Instituto de Tecnología Agroindustrial del Noroeste Argentino (ITANOA, CONICET-EEAOC). Tucumán, Argentina. georginaorce@yahoo.com, atiliocastagnaro@gmail.com.
4 Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Catamarca (UNCa). Catamarca, Argentina. eumart2003@yahoo.com.ar.
*“Author for correspondence”: mariodebes@gmail.com, arias@csnat.unt.edu.ar.
“Address for correspondence”: Facultad de Ciencias Naturales - Universidad Nacional de Tucumán. Address: Miguel Lillo 205 (CP 4000), San Miguel de Tucumán, Tucumán, Argentina. Fax: +00 54 0381-4330633. Telephone: +00 54 0381-4239456.
Summary: The genus Duchesnea includes two species originally from India: D. indica and D. chrysantha. In Northwestern Argentina the monitoring of wild strawberry-like species was carried out; during 2002-2016, many populations of D. indica and none of D. chrysantha were discovered. Red- and white-fruited plants of D. indica were collected from disturbed areas and ex situ conserved in green-house and in nursery conditions. Were also consulted materials from different national and international herbaria, and we only found reports of D. indica with red fruit for Argentina. In the present work, we report for the frst time the presence of D. indica f. albocaput in Argentina and South America, cohabiting with populations of D. indica f. indica in the underwoods of Tucumán. This finding broadens the distribution range of D. indica f. albocaput, cited as endemic to Japan by Naruhashi 1992. The morphological and anatomical characters of the two botanical forms of D. indica (red fruit genotypes and white fruit genotypes) are also presented: fruit color, number of leafets, and crystal form.
Key words: Duchesnea, morpho-anatomical, wild-strawberry.
Resumen: Primer informe de Duchesnea indica f. albocaput (Rosaceae) en Noroeste de Argentina. El género Duchesnea incluye dos especies originarias de India: D. indica y D. chrysantha. Durante el período 2002-2016, se realizaron monitoreos de especies silvestres relacionadas con la frutilla cultivada en el Noroeste Argentino y se encontraron numerosas poblaciones de D. indica pero ninguna de D. chrysantha. Plantas de D. indica con frutos rojos y plantas con frutos blancos fueron colectadas y conservadas ex situ, en cámaras bajo condiciones controladas y en invernaderos a campo. Se consultaron también materiales de diferentes herbarios nacionales e internacionales, y solo se encontraron reportes de ejemplares de D. indica de frutos rojos para Argentina. En el presente trabajo se reporta por primera vez para Argentina y Sudamérica, la presencia de poblaciones de D. indica f. albocaput, co-habitando con poblaciones de D. indica f. indica en sotobosques de Tucumán. Con esta cita se amplía el rango de distribución de D. indica f. albocaput, citada como endémica para Japón. Se presentan además, las características morfo-anatómicas distintivas de ambas formas botánicas de D. indica (genotipos de frutos rojos y genotipos de frutos blancos): color del fruto, número de foliolos, y forma de cristales.
Palabras clave: Duchesnea, frutillas silvestres, Morfo-anatomía.
introDuction
The biodiversity of the Yungas eco-region is high; despite, demographic expansion, deforestation process and agricultural practices generated areas with endangered species or sensible to diversity-loss. Despite to the great process of transformation and degradation, the pre-montane forests in Northwestern Argentina have higher percentage of exclusive species and patches with good status of biodiversity conservation (Brown, 2009; Malizia et al., 2012).
The genus Duchesnea includes two species native from India: Duchesnea indica and Duchesnea chrysantha. D. indica is reported as ubiquitous from different regions of the world and cited as adventitious from Argentina (Zardini 1973, 1999, Arias 2007, Zuloaga, 2008). D. chrysantha grows mainly in Japan, China, India, Korea, Taiwan, the Philippines, Indonesia, and it has not been reported in America (Kalkman, 1968; Sugimoto & Naruhashi, 1981; Kume et al., 1987; Sugimoto et al., 1991; Naruhashi, 1992; Naruhashi, 2001). Both species are very similar: plants in rosette, tri-foliated leaves, yellow fowers, runner producers and active ground colonizers (cespitose) (Zardini, 1973, 1999; Novara, 1993; Arias 2007); fruits of D. indica are scarlet-red and larger than the pink-reddish fruits of D. chrysantha (Naruhashi & Sugimoto, 1996). In both species, white-fruited genotypes have been reported: D. chrysantha f. leucocephala (Makino) Hara (Naruhashi & Iwatsubo, 1991), from Korea, and D. indica f. albocaput Naruh. endemic in Honshu (Fukui Pref.), Japan (Naruhashi, 1992, 2001).
Anatomical comparisons among D. indica, D. chrysantha and their hybrids were carried out by Naruhashi & Ishizu (1992). Arias (2007) reported foliar anatomical characters observed in two forms of D. indica. Cytological and genetic studies revealed the presence of fve ploidy forms in Duchesnea from Gifu Prefecture (Japan), 2n = 14, 21, 49, 56, 84 with a basic haploid set x = 7 (Iwatsubo & Naruhashi, 1991; Naruhashi & Iwatsubo, 1991). Plants with 2n = 49 (heptaploid) and 2n = 56 (octoploid) were considered as natural hybrids between D. chrysantha (2n = 14) and D. indica (2n = 84) (Naruhashi & Takano 1987). One example is D. x harakurosawae N. Naruhashi & M. Sugimoto, a heptaploid (2n = 49) hybrid that despites being sterile, propagates actively by vegetative reproduction (Naruhashi & Sugimoto, 1996).
In this paper we report the presence of D. indica f. albocaput growing in low highland forests of Tucumán (Argentina). We also present the chromosome number and a comparative analysis of the morpho-anatomical characters between D. indica f. albocaput and D. indica f. indica.
Materials anD MethoDs
Study area and collecting sites
The presence of wild strawberries-like species in different sites of Northwestern Argentina was monitored during 2002-2016 at summer and winter seasons (Fig. 1A, B). Areas with different environmental conditions (mountain forests, highland pastures, and riparian habitat) were monitoring. D. indica genotypes from different sites were selected and collected for ex situ conserving in green-house of Banco de Germoplasma de Frutilla de la Universidad Nacional de Tucumán (BGF-UNT).
Comparative analyses between red- and white-fruited plants
From ex situ conserved genotypes clones of both forms of D. indica were obtained by agamic propagation in BGF-UNT (by stolon rooting) for morphological analyses. Vegetative and reproductive traits were analyzed in different plants of successive propagations. Achenes germination in green-house was also evaluated.
Leaves of 15 red- and 15 white-fruited plants were collected and fixed in FAA (formalin-acetic acid-alcohol 80° (3:1:5)) (D' Ambrogio de Argüeso, 1986), and treated according conventional techniques (Dizeo de Strittmater, 1980; D' Ambrogio de Argüeso, 1986). Anatomical analysis with light microscope was made; in addition, polarized light microscope for crystals analysis was used.
Meiotic studies from young immature fowers of white- and red-fruited plants were performed; the samples were fxed in absolute alcohol and glacial acetic acid solution (3:1) during at least 12 hours at 4 ºC. Immature anthers were squashed with a drop of propionic acid (45%), hematoxylin (2%); ferric citrate (1%) as a mordant were used (Nuñez, 1968). Cytological analyses using a minimum of 20 pollen mother cells in different meiotic stages were carried out; the chromosomal counting on diakinesis stage was only made.
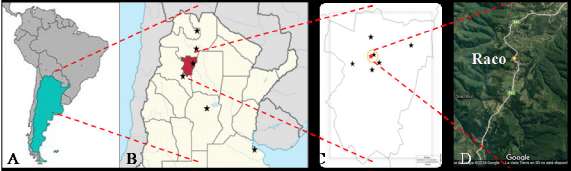
Fig. 1. Geographical distribution of Duchesnea indica in South America. A, regional distribution in Argentine. B, reported sites in central and northwestern region. C, distribution in different Tucumán province localities. D, satelital image of Raco locality. Black star signaling occurrence of D. indica f. indica, and red star indicate presence of D. indica f. albocaput.
Taxonomic treatment
Same plants collected were ex situ conserved (in green-house of BGF-UNT) and others, were herborized and deposited in the Herbarium of the Fundation Miguel Lillo (LIL). Different herbaria collections: La Plata (LP), Darwinion (SI), Museo de Ciencias Naturales de Salta (MCNS), Museo Botánico de Córdoba (CORD) and Herbario Miguel Lillo (LIL) were consulted. Additionally, digitals copies of white-fruited specimens from Makino Herbarium, Metropolitan Tokyo University (MAK) were also analyzed.
Results
Duchesnea indica (Andrews) Focke, Nat. Pfanzenfam. 3 (3): 33. 1888.
Potentilla grandifora, auct. non L., Thunb., Fl. Jap.: 219. 1784. Fragaria indica Andrews, Bot. Repos. 7 Tab. 479.1807. Tipo «in alpibus indiae orientales» (typus not showed). Duchesnea fragiformis Sm., Trans. Linn. Soc. London 10: 373.1811, nom. illeg. Pontetilla denticulosa Ser., DC. Prodr. 2: 573. 1825. Type: «in Napauliâ»
(Typus not showed). Potentilla wallichiana Ser., DC. Prodr. 2: 574. 1825. Type: «in Napauliâ» (Typus not showed). Duchesnea indica (Andrews) Teschem., Hort. Reg. & Gard. Mag. 1(12): 460. 1835. Fragaria malaya Roxb., Fl. Ind. Ed. 2: 520. 1832. Fragaria nilagirica Zenker, Pl. Ind. 1:7, t. 9. 1835. Potentilla durandii Torr. et A. Gray, Fl. N. Amer. 1: 444. 1840. Potentilla trífda Lehm., Add. ad Ind. Sem. Hort. Bot. Hamburg. Anno. 1851: 10. 1853. Duchesnea indica (Andrews) Focke var. wallichiana (Ser.) Franch. et Sav., Enum. Pl. Jap. 1: 129. 1873. Potentilla indica (Andrews) Th. Wolf, Syn. Mitteleur. Fl. 6: 661. 1904. nom. inv. Potentilla indica (Andrews) Th. Wolf var. serrulata Th. Wolf, Biblioth. Bot. 16(71): 666. 1908. Duchesnea indica (Andrews) Teschem. var. major Makino, Bot. Mag. Tokyo 28: 184. 1914. Duchesnea major (Makino) Makino, J. Jap. Bot. 2:19. 1921. Duchesnea indica Andrews, Fieldiana. Bot. 24 (4): 432-484. 1946.
Hirsute, perennial and stoloniferous herbs, with short rhizome (crown) ofiten thickened; green runner with rooting nodes and caulinar tripartite stipules. Trifoliolated green opaque leaves, elliptic leafets with margin serrate and long petioles with loosely pubescence (adpresous) and bipartite foliar stipules. Flowers perfect, solitary, axillary (on long peduncles arising from nodes), 5-merous; calyx and epicalyx persistent and green and slightly pubescent; petals 5, yellow, obovate early deciduous; numerous pistils sessile and free. The styles are persistent in lateral position at the achenes. Stamens in several cicles. Fruiting receptacle scarlet-red, feshy, insipid and edible. Achenes red, numerous, reniform and prominent (Fig. 2C and E).
Specimens examined. ARGENTINA. Buenos Aires: Isla Martín García, 12-XI-1994, Hurrell et al. 2097 (LP); Isla Martín García, camino de los Álamos hacia el oeste, 12-XII-1992, Hurrell et al. 1404 (LP); Isla Martín García, 16-XII-1997, Hurrell, Belgrano, Jankwski et Mehltreter 3748 (LP). Pdo. Zárate, Las Palmas, 30-XI-1951, Boelcke 5095 (SI); Delta, Isla Sudamérica, 21-X-1973, Zardini 233 (LP). Pdo. Tigre, Tigre, 3-IX-1936, Burkart 7821 (SI). Catamarca: Dpto. El Rodeo, inmediaciones del Río Ambato, 15-XII-1971, Ariza Espinar 2588 (CORD). Córdoba: Dpto. Colón, Sierra Chica, Los Quebrachitos, 6-XI-1955, Hunziker 11276 (SI); Sierra Chica, Río Ceballos, en la Quebrada, 2-XI-1963, Subils & Articó 650 (SI). Dpto. Capital, La Carolina, 15-X-1962, Hunziker 15948 (SI). Jujuy: Dpto. Capital. Laguna Yala, 4-XI-1971, Abbiatti, Holgado & Figueroa 3290 (LIL); Yala, 8-IV-1945, O' Donell 3002 (LIL); Laguna Yala, 13-I-1947, Garolera & Romero s.n. (LIL 208259); Camino a Laguna de Yala, 25-VI-1948, Pereyra s.n. (LIL 232563); Laguna Yala, 10-I-1947, Garolera & Romero s.n. (LIL 195972); Camino a Laguna de Yala, 25-VI-1948, Rojas s.n. (LIL 230027); Laguna de Yala, 20-VII-1948, Pereyra s.n. (LIL 329290); Laguna Yala, 13-I-1947, Garolera & Romero s.n. (LIL 204711); Laguna Yala, 28-II-1945, Abbiatti & Claps 839 (LIL); Yala, 7-I-1947, Garolera & Romero 112 (LIL); Laguna de Yala, 23-V-1950, Krapovickas 7254 (LIL); Lagunas de Yala, 13-II-1951, Meyer 16983 (LIL); Yala, 11-X-1981, Zardini 1438 (LP); Quebrada de Yala, 28-X-1964, Cabrera 16360 (LP); Camino a Laguna Yala, 24-IV-1943, Lourtey 418 (LIL); Yala, 2-XI-1974, Schinini, Quarín, Arbo & Pire 10041 (LP). Dpto. Ledesma, 14-X-1961, Ahumada, Vaca, Legname 2135 (LIL); Ledesma, 7-XI-1973, Cabrera, Kiesling & Zardini 23974 (LP); Ledesma, 17-X-1963, Fabris 4485 (LP); Ledesma, 18-X-1963, Fabris 4499 (LP); Dpto. Valle Grande, Valle Grande, 21-XI-1958, Villa Carenzo & Legname 683 (LIL). Dpto. Dr. Manuel Belgrano, Termas de Reyes 12-I-1947, Garolera & Romero s.n. (LIL 202459); Reyes, Alt. 1364, 5-II- 1947, Garolera & Romero 96 (LIL). Dpto. San Antonio, El Morado, 15-IX-1981, Rotman 539 (SI). Salta: Dpto. Capital, 16-IX-1988, Novara 8089 (MCNS); Río Vaqueros, 15-X-1981, Novara & Varela 2102 (MCNS); Río Vaqueros, 4-X-1981, Novara 2066 (MCNS); Vaqueros, Arroyo Chaile, 21-VIII-1986, Núñez, Martín & Novara 37 (MCNS); Quebrada de San Lorenzo, 18-IX-1992, Novara 10575 (MCNS); San Lorenzo, 19-II-1949, Montenegro 438 (LIL); Quebrada de San Lorenzo, 5-IX-1992, Tolaba 1400 (MCNS); Quebrada de San Lorenzo, 30-III-1991, Tolaba 114 (MCNS); Quebrada de San Lorenzo, 10-IX-1953, Meyer 18078 (LIL); Quebrada de San Lorenzo, 16-II-1970, Abbiatti, Holgado & Figueroa 3194 (LIL). Dpto. Anta, 7-VIII-1959, Türpe & West s.n. (LIL 485706); Anta, 19-VI-1985, Palaci 54 (MCNS), Parque Nacional El Rey, 16-VIII-1981, Brown & Malmierca 1460 (MCNS); Parque Nacional El Rey, Brown & Malmierca 1660 (MCNS). Dpto. La Caldera, Quinta La Angostura, 1-III-1994, Tolaba 443 (MCNS); Camino a la Cornisa, límite con Jujuy, 5-XII-1984, del Castillo & Varela 520 (MCNS); La Caldera, 21-VIII-1941, Salvador Rosa 214 (MCNS). Dpto. Orán, 12-III-1997, Hilgertim & Lamas 1679 (MCNS). Dpto. San Carlos, Los Sauces-La Angostura, 10-X-1989, del Castillo 1116 (MCNS). Dpto. Chicoana, 9-II-1987, Novara 5998 (MCNS); Quebrada de Escoipe, 4-X-1981, Zardini 1402 (LP). Dpto. Rosario de Lerma, 3-XII-1992, Pensiero & Marino 4239 (MCNS), Dique Las Lomitas, 10-X-1998, Juárez de Varela 1643 (MCNS); Finca El Manantial a 10 Km. de La Silleta, 31-VIII-1983, del Castillo 82 (MCNS). Dpto. Santa Victoria, 5-X-1973, Legname & Cuezzo 9628 C. (LIL); Santa Victoria, Pueblo Baritú, 14-VII-1999, Novara, de la Sota, Martínez &. Gamem 11303 (MCNS). Los Valcones, 2-XI-1989, Juárez 1942 (MCNS). Tucumán: Dpto. Tafí del Valle, Quebrada de los Sosa, 12-IX-1962, Cuezzo, Legname & Vaca 3015 (LIL); Quebrada de los Sosa, 8-X-1963, Meyer, Legname & Cuezzo 4198 (LIL); Quebrada de los Sosa, Monumento al Indio, 11-XI-1963, Meyer 23102 (LIL); Monumento al Indio, 29-X-1996, Lucena 335 (LIL); Tafí del Valle, 9-XII-1997, Figueroa, Slanis & Muruaga 1248 (LIL); Tafí del Valle, 9-X-1997, Lucena, Cuezzo & Vega 420 L. (LIL); Tafí del Valle, 8-VIII-1999, Albornoz 1 (LIL); Dpto. Tafí Viejo, Taficillo, 17-VIII-1998, Arias, Albornoz & Monteros 522 (LIL); Taficillo, 3-VII-1998, Albornoz, Arias & Monteros 528 (LIL); Taficillo, 17-VIII-1998, Monteros, Albornoz & Arias 527 (LIL), El Nogalar, Ruta 307, 18-VIII-1997, Sidán & Rossi s.n. (LIL 603220 a). Dpto. Yerba Buena, El Rulo, 25-X-1995, Sosa, Albornoz & Arias 526 (LIL); El Rulo, 25-X-1995, Sosa s.n. (LIL 603313). Dpto. Chicligasta, Río Cochuna, 20-XI-1965, Lefebure s.n. (LIL 557162). Dpto. Burruyacu, Villa Padre Monti, 12-VIII-1999, Albornoz 2 (LIL); Burruyacu, Nogalito, 29-XI-1988, Slanis 119 (LIL). Dpto. Trancas, 8-V-1981, Roig & Roig Juñent 10342 (LP); El Potrero, 31-VIII-1999, Albornoz 2 (LIL); El Potrero, Alt. 1600m, 12-IX-1996, Arias, Kirschbaum & Castagnaro 524 (LIL). Dpto. Monteros, Pueblo Viejo, 12-XII-1962, Neuman 28 (LIL); Río Lozano, 5-XI-1971, Abbiatti, Holgado & Figueroa 3302 (LIL).

Fig. 2. Habitat and morphological analysis of both botanical form of Duchesnea indica. A, Aspect to monitoring site. B-F, ex situ conserved genotypes in green-house of Banco de Germoplasma de Frutilla de la Universidad Nacional de Tucumán (BGF-UNT). B, General aspect of D. indica f. albocaput. C, General aspect of D. indica f. indica. D, white fruit. E, red fruit. F, 5-foliolated leave. G, seasonal light difference in color-fruit. Scale bar = 3 cm (B, C and F) and Scale bar = 1 cm (D, E and G).
Typus. JAPAN. Honshu island, Chubu: Fukui prefecture, Fukui city, Asuwa-sanzan, 21-VI-1991, Wakasugi 34420 (FR); ibidem, 24-X-1991, Wakasugi & Naruhashi 91102401 (FR); and, 10-VI-1991, Wakasugi & Naruhashi 92061001 (FR; HOLO-: KANA; ISO-: KYO, MAK, TI & TNS) not seen.
Perennial, stoloniferous herbs with crown ofiten thickened; green runner with rooting nodes and caulinar tripartite stipules. Dark green leaves, trifoliolated and sometimes tetrafoliolated or pentafoliolated, leafets obovate-eliptic with margin serrate; venation semicraspedodromous (scarcely branched secondary venation). Petiole long, with variable length (5-14cm), green and pubescence. Trichomes: glandular (with pluricellular, uniseriate foot and pluricellular head) and single (unicellular) in epidermis of petiole and leaf blade. Crystals: prismatic and cubic in the mesophyll of the leaf. Flowers axillary perfect (15-25mm diameter), solitary, 5-merous; calyx and epicalyx persistent and green; petals 5, yellow obovate-obcordate early deciduous; numerous pistils sessile and free. The styles are persistent in lateral position at the achenes. Stamens in several cicles. Fruiting receptacles fesh, convex, accrescent, insipid and edible; the fruiting receptacles white, with 60-120 cream achenes, prominent and reniform smoothly; in addition, slight changes in color-achenes of this genotypes were registered, to brown-cream and to cream-pinkish in spring. However, the new fruits of self-plants in others season return to typical cream color (Fig. 2 and 3).
Specimens examined. Tucumán: Dpto. Tafí Viejo, Raco, 1.IX.2004, Arias & Gómez 601 (LIL).
Taxonomic considerations
According to species description, the Holotypus is housed in KANA and ISOTYPUS in KYO, MAK, TI & TNS (Naruhashi, 1992). Afiter of an exhaustive search in those herbaria, we not found theses materials. Answering to this confict, we considered that one NEOTYPUS is necessary for this taxon (International code of botanical nomenclature article 9.6).
Distribution of Duchesnea sp.
In highlands of Tucumán province, in Northwestern Argentina, plants of D. indica f. albocaput were found in 2004 (Fig. 1C, D). This particular white-fruited genotype, grow under pinewood co-habiting with red-fruited D. indica (Fig. 2A).

Fig. 3. Morphological and anatomical characters of Duchesnea indica f. albocaput. A, General aspect to plant with trifoliolate leaves, short crown with stolons leaves and axiliary fower; details: fower and fruit. B, Aspect to venation pattern (secondary and tertiary veins). C, Marginal arrangement (semicraspedodromous). D, Simple trichoma. E, Glandular trichoma, arrow: cubic crystals. F, Epidermical-cells disposition in simple trichoma base of adaxial surface Simple trichoma. G, Foliar cross section with different crystals in parenchyma; the arrow indicates a cubic crystal. H, Meiotic chromosomes.
During more than 10 years of monitoring, many populations of D. indica f. indica (red-fruited) and only one population of D. indica f. albocaput (white-fruited) were found. However, none population of D. chrysantha in different sites of this region was found.
Comparative analyses between D. indica f. indica and D. indica f. albocaput
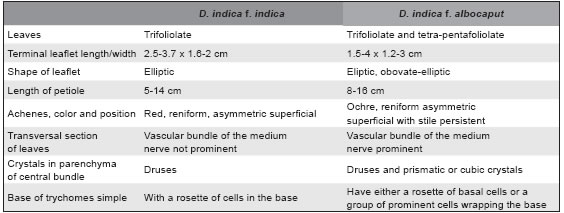
The evaluation of vegetative and reproductive characters of both forms ex situ conserved, showing similar stolons number (average =10 ± 2) with 3-5 daughter plants by stolons; however, the number of clones obtained was minor in D. indica f. albocaput than the other form (Fig. 2B, C); foliar and others vegetative characteristics were summarized in Table 1. The number of fowers for plant in both form of D. indica were variable according to season (3-7) showing a peak in October (spring season); the fruiting receptacle production was also variable (3-5) depending on the season. The number of achenes by fruiting receptacle ranged from 60 to 120 (± 10) with major production in December (Fig. 2D, E).
Table 1. Distinctive characters observed between red-fruited D. indica and white-fruited D. indica.
On more than 10 years of evaluation in BGF-UNT, the color-fruit was a stable trait in all conserved genotypes: red fruit in D. indica f. indica and white fruit in D. indica f. albocaput (Fig. 2B, C). Nonetheless, slight changes in achene-color of D. indica f. albocaput at some seasons were observed. In summer instead of cream color to brown-cream and cream color to pinkish-cream- in spring (Fig. 2F); however, red color in this botanical form was never observed.
The germination of achenes was successfully for all of genotypes of D. indica. However, the major percentage of germination was observed in red achenes of D. indica f. indica (95%) regard to cream achenes of D. indica f. albocaput (62%). The plantlets with good development and similar aspect to the mother plants were obtained in both forms.
Similar epidermical characters such as: isodiametric cells in adaxial epidermis and lobulated cells in abaxial epidermis, anomocitic stomata, glandular trichomes (with pluricellular head), single trichome (unicellular) in both botanical forms of D. indica were found. Spheroidal aggregate of prismatic crystals of calcium oxalate (druses) were observed in parenchyma cells of the mesophyll and in the middle bundle. However, distinctive characters such as: the epidermical cells disposition (in rosette or a group of prominent cells wrapping) on base of simple trichomes and type of calcium oxalate crystals inclusions (prismatic and cubic) in the mesophyll and the parenchymatic phloem cells were only found in white-fruited D. indica (Fig. 3B-E).
Cytological studies revealed that the white-fruited D. indica is dodecaploid (2n = 12x = 84) as happens in the red-fruited genotype (Fig. 3F).
Discussion
The several D. indica populations found in different areas confrmed the high plasticity of this species to colonize many places. The capacity of fowers and fruits production of white-fruited D. indica is similar to the red-fruited plants. However, slight differences in stolons rootings and percentage of achenes germination could explain the minor occurrence of white-fruited populations or a low capacity to colonize new lands regard to the red fruited plants.
The color fruit is an important characteristic to attract disperser agents. The red or withe color of fruiting receptacle and achenes was the more conspicuous character that differences the two forms of D. indica analyzed in this work. Furthermore, few changes of achenes-color of white-fruited D. indica were observed seasonally but never in the red-fruited populations. Successful crosses between D. indica f. indica and D. indica f. albocaput in both directions and self-pollinations were carried out by Debes et al. (2011); those authors report compatibility between them and variations in color of fruiting-receptacle and achenes in offspring.
In vascular plants, anthocyanin and betacyanin pigments are wide distributed and their accumulations are involved in the red color of flowers and fruits (Shimada et al., 2004). According to Espley et al. (2009), the pigment biosynthesis in apple may be induced by light, particularly U V, and various stress treatments including cold. In addition, mutations in genes encoding for the biosynthetic or transcriptional regulators of the anthocyanin pathway have been linked to changes in color phenotypes (Espley et al., 2009). Molecular studies in red- and white-fruited D. indica reported that impaired in ANS gene expression is responsible of white color of fruits (Debes et al., 2011).
On other hand, the presence of small cubic and prismatic calcium oxalate crystals in leaves of D. indica f. albocaput, different of druses in leaves of D. indica f. indica, constitutes a new diagnostic trait not reported for Duchesnea genus. Factors involved in the crystals morphology regulation were studied by Nakata-Paul (2002); according to this research, the control of crystal morphology is a tightly regulated genetic process and a single point mutation can drastically alter the crystals size and shape. From to this, and based on our anatomical observations, we can thought that the new local white-fruited D. indica here described, could be result from a single or a few loci point mutations that may have occurred in the normal red-fruited D. indica f. indica.
Conversely, we could speculate that the local white-fruit genotype is, in fact, a new botanical form originated from an independent spontaneous mutation event that took place in the ubiquitous local red-fruit D. indica f. indica affecting the fruit color locus. Is also probably, that the occurrence of this white-fruited genotype has been produced by a "Wright effect" from a small population of red-fruit D. indica (Naruhashi personal communication, 2011).
Taken together these results, we concluded that white-fruited D. indica found in Tucumán (Argentina) is D. indica f. albocaput. This discovery increases the knowledge about the diversity of the Duchesnea genus.
Regard to taxonomic considerations, we consider that in order to elucidate the confict in question, a more profound taxonomy study should be carried out to establish, according to the international code of botanical nomenclature, a new reference material for this taxon; however, these aspect of this research exceeds the objectives of the present paper.
Acknowledgements
This work was partially supported by the Consejo de Investigaciones de la Universidad Nacional de Tucumán (PIUNT D544/1); Agencia Nacional de Promoción Científica y Tecnológica, FonCyT (INTA-PICT 2011 N° 1170). IGO and MAD are fellows of CONICET. and ACL, is fellow of CIUNT. Authors wish to thank: Dr. N. Naruhashi at the Department of Biology - University of Toyama (Japan) for kindly providing achenes of Duchesnea chrysantha f. leucocephala; Professor N. Murakami at the Makino Herbarium - Tokyo Metropolitan University (Japan) for digital images of Duchesnea indica var leucocephala Ing. Lemme, MC for technical assistance and Banco de Germoplasma de Frutilla de la Universidad Nacional de Tucumán (BGF-UNT) for providing plants and installations used.
Bibliography
ARIAS, M. E. 2007. Frutillas silvestres y especies relacionadas con la cultivada. Universidad Nacional de Tucumán. Tucumán, Argentina. [ Links ]
ARIAS, M. E., A. C. LUQUE, L. F. FERNÁNDEZ-DATTOLI & M. A. DEBES. 2014. Wild and cultivated strawberries: diversity, pigments and metabolic changes. In: NATHAN MALONE (ED.), Strawberries: Cultivation, Antioxidant Properties and Health Benefits, pp 215-238. Nova Science Publisher Inc., Hauppauge, New York-USA [ Links ]
BROWN, A. D. 2009. Las selvas pedemontanas de las Yungas: manejo sustentable y conservación de la biodiversidad de un ecosistema prioritario del noroeste argentino. In: BROWN, A. D., BLENDINGER, P. G., LOMÁSCOLO, T., GARCÍA BES, P. (EDS), Selva pedemontana de las Yungas Historia natural, ecología y manejo de un ecosistema en peligro, pp 14-15. Ediciones del Subtrópico. [ Links ].
D' AMBROGIO DE ARGÜESO, A. 1986. Manual de técnicas en histología vegetal. Hemisferio sur. Buenos Aires, Argentina. [ Links ]
DEBES, M. A., M. E. ARIAS, C. F. GRELLET-BOURNONVILLE, A. F. WULFF, M. G. MARTÍNEZ-ZAMORA, A. P. CASTAGNARO & J. C. DÍAZ-RICCI. 2011. White-fruited Duchesnea indica (Rosaceae) is impaired in ANS gene expression. Am. J. Bot. 98: 2077-2083. [ Links ]
DIZEO DE STRITTMATER, C. 1980. Coloración con violeta de Cresyl. Bol. Soc. Argent. Bot. 19: 273-276. [ Links ]
ESPLEY, R. V., C. BRENDOLISE, D. CHAGNÉ, S. KUTTY-AMMA, S. GREEN, R. VOLZ, H. J. SCHOUTEN, S. E. GARDINER, R. P. HELLENS & A. C. ALLAN. 2009. Multiple repeats of promoter segment causes transcription factors autoregulation in red apples. Plant Cell 21: 168-183. [ Links ]
IWATSUBO, Y. & N. NARUHASHI. 1991. Cytological study of triploid Duchesnea chrysantha (Zoll. et Mor.) Miquel (Rosaceae). CIS 51: 18-20. [ Links ]
KALKMAN, C. 1968. Potentilla, Duchesnea and Fragaria in Malasia (Rosaceae). Blumea 16: 325-354. [ Links ]
KUME, O., T. WAKE & N. NARUHASHI. 1987. Distribution and habitat of Duchesnea in Kagama prefecture. J. Phytogeogr. Taxon. 32: 95-98.
MALIZIA, L., S. PACHECO, C. BLUNDO & A. D. BRAWN. 2012. Altitudinal characterization, use and conservation of subtropical Yungas of Argentina. Ecosistemas 21: 53-73.
NAKATA-PAUL, A. 2002. Calcium oxalate crystal morphology. Trends Plant Sci. 7: 324.
NARUHASHI, N. 1992. Duchesnea indica f. albocaput. J. Phytogeogr. Taxon. 40: 2-131.
NARUHASHI, N. 2001. Flora of Japan II. Kodansha ltd. Tokio, Japan.
Naruhashi, N. & N. Ishizu. 1992. Comparative anatomy of Duchesnea chrysantha, D. indica and their hybrids (Rosaceae). J. Phytogeogr. Taxon. 40: 5-12.
NARUHASHI, N. & Y., IWATSUBO. 1991. Comparative morphology and chromosome numbers in Duchesnea indica (Rasaceae) from Nepal and Japan. In: OHBA H., MALLA S.B. (EDS), The Himalayan plants II, pp 11-15. University of Tokyo press. Tokyo, Japan.
NARUHASHI, N. & M. SUGIMOTO. 1996. The foral biology of Duchesnea (Rosaceae). Pl. Spec. Biol. 11: 173-184.
NARUHASHI, N. & H. TAKANO. 1987. Chromosome numbers and distribution of Duchesnea (Rosaceae) in Gifu prefecture. Acta Phytotax. Geobot. 155-160.
NOVARA, L. J. 1993. Flora del valle de Lerma. Rosaceae juss. Aportes Botánicos de Salta. Serie Flora 2: 34-35.
SHIMADA, S., K. TAKAHASHI, Y. SATO & K. SAKUTA. 2004. Dihydroflavonol 4-reductase cDNA from non-anthocyanin-producing species in the Caryophyllales. Plant Cell Physiol. 45: 1290-1298.
SUGIMOTO, M., H. ISHIZU, & N. NARUHASHI. 1991. Morphologycal study of Duchesnea (Rosaceae). J. Phytogeogr. Taxon. 39: 87-95.
SUGIMOTO, M. & N. NARUHASHI. 1981 Seasonal growth cycles and dry matter allocation of two Duchesnea species. J. Phytogeogr. Taxon. 29: 85-90.
ZARDINI, E. M. 1973. Los géneros de Rosaceas espontáneos en la República Argentina. Bol. Soc. Argent. Bot. 15: 209-228.
ZARDINI E. M. 1999. Rosaceae. In: ZULOAGA, F.O. & M. MORRONE (ED) Catálogo de las plantas vasculares en la República Argentina II. pp 987-990 Monography. Systemmatics. Missouri Botanical Garden press. St. Louis, USA.
ZULOAGA, F. O, O. MORRONE & M. J. BELGRANO (EDS.). 2008. Catálogo de las plantas vasculares del Cono Sur (Argentina, sur de Brasil, Chile, Paraguay y Uruguay) II, pp. 107. Monography. Systemmatics. Missouri Botanical Garden press. St. Louis, USA.
Recibido el 3 de agosto de 2017, aceptado el 23 de octubre de 2017. Editor: Franco E. Chiarini.














