Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.53 no.3 Córdoba set. 2018
Fitoquimica - Phytochemistry
Chemical composition of essential oils of peltate glandular trichomes from leaves and flowers of Lepechinia floribunda (Lamiaceae)
JULIA L. CAMINA*1, JOSÉ S. DAMBOLENA12, JULIO A. ZYGADLO12 andLORENA ASHWORTH1
Summary: The knowledge of the chemical composition of different plant organs provides valuable Information for clarifying the functionality of secondary metabolites. Essential oils are secondary metabolites produced and stored in secretory structures on leaves, flowers, and steam. Many studies have shown that the chemical composition of the essential oil can differ depending on the plant organ and the secretory structures even if they are at the same organ. Such differences may be the consequence that organs also differ in type and density of glandular structure. Moreover, the same type of glandular structure can change its chemical composition depending on its position within the same organ. However, no study has been evaluating the chemical composition of the essential oil from the same glandular structure in leaves and fertile floral whorls. The aim of this work was to characterize the chemical composition of essential oils of peltate glandular trichomes from anthers and leaves of Lepechinia floribunda. We found a lower richness of chemical compounds and higher relative abundance of monoterpene hydrocarbons on anthers than leaves. Leaves showed an increased relative abundance of oxygenated sesquiterpenes. Such differences probably respond to a high tissue-specificity expression of genes in different plant organs.
Key words: Anther, glandular trichome, Lamiaceae, leaf, Lepechinia floribunda, terpenes.
Resumen: Composición química del aceite esencial de tricomas glandulares peltados en hojas y flores de Lepechinia floribunda (Lamiaceae). El conocimiento de la composición química del aceite esencial producido por distintos órganos en las plantas, brinda información para conocer las funciones de dichos compuestos. Los aceites esenciales son metabolitos secundarios producidos y almacenados en diferentes estructuras glandulares en hojas, flores y tallos. La composición química del aceite esencial difiere entre órgano de la planta y entre estructuras de secreción, incluso ubicadas en un mismo órgano. Tales diferencias podrían ser consecuencia del distinto tipo y densidad de estructuras glandulares en cada órgano. El aceite esencial del género Lepechinia ha sido escasamente estudiado, sin embargo su uso etnobotánico pone en evidencia propiedades medicinales y antisépticas. Hasta la fecha varios estudios han evaluado la composición química del aceite esencial en distintas partes de las plantas o de la misma estructura glandular en diferentes posiciones dentro de las mismas. Sin embargo, no se han encontrado estudios que evalúen la composición química del aceite esencial del mismo tipo de estructura glandular en hojas y verticilos florales fértiles. El objetivo de este trabajo fue caracterizar la composición química del aceite esencial proveniente de tricomas glandulares peltados de anteras y hojas de Lepechinia floribunda (Lamiaceae). Se encontró menor riqueza de compuestos químicos y un incremento en la abundancia relativa de monoterpenos hidrogenados en el aceite esencial de anteras que en hojas, mientras que en hojas hubo mayor abundancia de sesquiterpenos oxigenados. Estos cambios en la composición química probablemente respondan a la alta especificidad tisular en la expresión de genes que existe para cada órgano.
Palabras clave: Antera, hoja, Lamiaceae, Lepechinia floribunda, terpenos, tricoma glandular.
Lamiaceae is one of the greatest families of Angiosperms and most of species are aromatics (Li et al., 2016). The genus Lepechinia is exclusive from America and it is composed by 43 species with mainly an Andean distribution, ranging from California, USA to Buenos Aires, Argentina (Drew & Sytsma, 2012). The essential oils of Lepechinia have many medicinal and antiseptic properties (Stashenko et al., 1999; Malagón et al., 2003; Acevedo et al., 2005; Arias Toledo et al., 2010; Ruiz et al., 2015), but compared to other genus of Lamiaceae, its chemical composition has been scarcely studied (Bozin et al., 2006; De Martino et al., 2009).
The chemical composition of essential oils of the genus Lepechinia has been studied in less than a half of the 43 Lepechinia species (Drew & Sytsma, 2012). Overall the essential oils of this genus are composed mainly by terpenes, although the identity and kind of them are highly variable. Three groups of species were identified based on the mayor compound of the essential oils. Species like L. meyenii (Walp.) Epling, L. schiedeana (Schltdl.) Vatke, L. mutica (Benth.) Epling, L salviaefolia (Kunth) Epling, L. betonicifolia (Lam.) Epling, L. Salviifolia (Kunth) Epling, L. rufocampii Epling & Mathias, and L. Calycina (Benth.) Epling, produce essential oils mostly conformed by monoterpenes (Lawrence & Morton, 1979; Ciccio et al., 1999; Eggers et al., 2001; Malagón et al., 2003; Caballero-Gallardo et al., 2011; Brand et al., 2016; Ruiz et al., 2015). While species like L. graveolens (Regel) Epling, L. paniculata (Kunth) Epling, L. bullata (Kunth) Epling and L. floribunda (Benth.) Epling, show dominance of sesquiterpene compounds (Velazco-Negueruela et al., 1994; Eggers, 2000; Arze et al., 2009; Valarezo et al., 2012). The third group formed by L. urbanii (Briq.) Epling, L. caulesens (Ortega) Epling, L. chamaedryoides (Balb.) Epling, L. vulcanicola J. R. I. Wood, L. radula (Benth.) Epling and L. conferta (Benth.) Epling have essential oils composed by equivalent proportions of mono and sesquiterpene compounds (Valenzuela et al., 1992; Zanoni & Adams, 1991; Acevedo et al., 2005; Borges et al., 2006; Brand et al., 2016; Morocho et al., 2017).
At intraspecific level the Chemical composition of the essential oil can vary by seasonal changes, by effect of different extrinsic factors, throughout the life stages of individuals and, or given a particular life stage, among vegetative and reproductive structures (Turner et al., 2000; Glas et al., 2012). Essential oils are produced and stored in specialized structures that allow the plant to make use of their biological functions in the right place at the right time (Fahn, 2000). Structures such as glandular trichomes can be found on approximately 30% of all vascular plants, and these trichomes are the main sources of production and secretion of essential oils in Lamiaceae (Glas et al., 2012; Rehman, 2016). Plants have many kinds of glandular trichomes, and it is currently known that different kind of trichomes have distinct biological functions as they produce different secondary metabolites (reviewed by Glas et al., 2012). The Lamiaceae species are well known for their often densely haired aromatic leaves (Metcalfe & Chalk, 1950). The presence of both glandular (peltate and capitate) and non-glandular trichomes is a characteristic feature of this family (Maffei & Codignola, 1990). Glandular trichomes are widely distributed over the aerial reproductive and vegetative organs and they are the source of aromatic, volatile oils and terpenes (Bhatt et al., 2010). Peltate glandular trichomes comprise a basal and stalk cell and a head composed by several secretory cells, which are surmounted by a large sub-cuticular storage cavity (Turner et al., 2000). Peltate trichomes have been found on anthers of flowers and on leaves of some species of Lamiaceae like Leonotis leonurus L. R. Br., Leonurus sibiricus L., Orthosiphon stamineus Benth. and Prostanthera gilesii B. J. Conn & T. C. Wilson (Ascensao et al., 1995; Moyano et al., 2003; Keng & Siong, 2006; Conn & Wilson, 2015). Despite the presence of peltate glandular trichomes in different plant organs, little is known about the chemical composition of their essential oils and therefore about their functional variability (Ascensao et al., 1995; Moyano et al., 2003; Keng & Siong, 2006; Conn & Wilson, 2015). Many studies have shown that the chemical composition of the essential oil differs among plant organs (e.g. Angioni et al., 2006; Borges et al., 2006; Marzoug et al., 2011), and such differences may be consequences that organs also differ in the type and density of glandular structures (e.g. Maffei & Sacco, 1987). Moreover, the same type of glandular structure can change its chemical composition depending on the position of the organ within the plant and the position of the glandular structure within the same organ (Maffei et al., 1989; Rohloff, 1999; Stesevic et al, 2016). Thus, as example, the chemical composition of the same glandular structure can ever vary among different position within a flower. Up to date, no study has evaluated the chemical composition of the essential oils from the same type of glandular trichome in leaves and flower fertile whorls.
Lepechinia floribunda is a perennial subshrub distributed from Bolivia to central-east Argentina (Epling, 1938). The plant surface is coated with capitate and peltate glandular trichomes, has white, hermaphroditic and tubular flowers. Each flower has four purple anthers with peltate glandular trichomes (Camina, Pers. Obs.). Previous studies on the chemical composition of the essential oils of L. floribunda have shown four groups of terpenes: monoterpene hydrocarbons, where camphene was the most representative compound; oxygenated monoterpenes with borneol and 1,8-cineole as the most abundant compounds; sesquiterpene hydrocarbons with P-caryophyllene and y-cadinene as mayor compounds and oxygenated sesquiterpenes where ledyl acetate, guaiol and P-eudesmol were the most abundant compounds (Velasco-Negueruela et al., 1994; Viturro et al., 2002; Fuselli et al., 2008; Arze et al., 2009). The aim of this work was to determine for the first time the chemical composition of the essential oils from glandular peltate trichomes on leaves and anthers of Lepechinia floribunda.
In October 2014 we selected three healthy (without herbivory or florivory) individuals of L. floribunda from a natural population located in the "Reserva Hídrica Natural Municipal Los Manantiales" (31° 10 21,3S, 64° 20 47,5O; Río Ceballos, Córdoba, Argentina). Selected plants were of similar size separated by at least 10 m. Two or three flowering branches per plant (Fig. 1A) were cut early in the morning when the corolla of the flower was just opening in order to avoid pollinator visitation. Branches were maintained in a styrofoam bucket with water for 2 hours until the essential oils extraction. Images of peltate glandular trichome in anthers and leaves were taken on the fresh material by a magnifying glass (Leica EZ5, range zoom 1 to 5 with 1x objective and 10x eyepieces) and reflection confocal microscopy (OLYMPUS LEXT OLS4000, lens:MPLAPONLEXT20, zoom X1, scanning mode: XYZ step scan + Color). These images were edited by Adobe Photoshop CS6 (Fig. 1). The essential oil extraction was performed in the laboratory under a magnifying glass (Leica EZ5, zoom: 50x). We used a filter paper of 0.5 mm cut as an isosceles triangle, simulating a needle (Schleicher and Schuell, 0859 type, 90 mm) to break the trichome cuticle and absorb by capillarity the essential oil. Special care was taken to avoid contact the filter paper with any other structures. Anthers of L. floribunda have only peltate trichomes (Fig. 1 B-D) and leaves have also non-glandular trichome and capitate trichomes (Fig.1E, F). However, as peltate trichomes are bigger (approximately 90 pm) than other type of trichome, and they are easily to view under magnifying glass, we were sure that any other structure was touched during the extraction of the essential oil. For each plant, we collected the essential oil of all peltate trichomes of all anthers of three to five flowers (total 70-200 trichomes per plant) and ten peltate trichomes from the adaxial surface of three to four apical leaves (total 30-40 trichomes per plant). Following this protocol, each plant is a replicate. Each fragment of filter paper was placed in an Eppendorf at -18°C until processing. The extraction of the essential oil from the filter paper was made with 10 pl of hexane per Eppendorf. Extractions were performed inside Eppendorf tubes.

Fig. 1. A: Branch with flower showing the anthers. B: Peltate glandular trichomes on anthers. C: Peltate glandular trichome (arrow) on anther. D: Detail of a peltate glandular trichome on anthers. E: Peltate glandular trichomes (arrow) on the abaxial surface of a leaf. F: Detail of peltate glandular trichome (arrow) on the abaxial surface of a leave.
The identifications of Chemical compounds was made through their mass pattern fragmentations obtained by gas chromatography-mass spectrometry (GC-MS Perkin Elmer 600), equipped with a capillary column DB-5 (60 m x 0.25mm i.d. and 0.25 pm coating thickness). Chromatography conditions were as follows: oven temperature profile of 60 °C to 240 °C by 2 °C/min; helium was the carrier gas with a constant flow of 0.9 ml/min and 70 eV ion source. The injector was operated in splitless mode at 250 °C, same as the detector temperature. The volatile compounds were identified by comparing their retention indices (RI) determined on the basis of an homologous series of n-alkane hydrocarbons (C8-C25), mass spectra and pure reagents (SIGMA, USA) with those of mass spectral databases from the Wiley library and NIST 98 MS Library and with bibliography (Adams, 2007).
To compare the chemical composition of essential oils of glandular trichomes between leaves and anthers, four groups of terpenes were considered: monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpenes hydrocarbons and oxygenated sesquiterpenes. The mean relative abundance of each group, and the mean relative abundance of the main compounds of the essential oil (those that had more than 5% of relative abundance) were compared between leaves and anthers by a t-test with Welch correction to correct the degree of freedom when the homogeneity of variance assumption was not met (Zar, 1999). All statistical analyses were performed by R software, version 3.3.1 (R Development Core Team).
Lepechinia floribunda has between three to eight peltate glandular trichomes per theca, and on average 9.82 ± 2.21 per anther. Because each flower has four anthers, there are in total between 30-48 peltate trichomes per flower. Peltate glandular trichomes were located on the ventral face of the anthers (Fig. 1B-D), and they remain intact until a mechanical disruption by some external agent, e.g. a floral visitor makes contact with anthers breaking the cuticle of the trichomes and thus releasing their content.
The essential oil from peltate glandular trichomes of leaves had 19 compounds (Table 1), among them, 1,8-cineole, P-caryophyllene, ledol and an undefined oxygenated sesquiterpene were the most abundant compounds (Fig. 2). In contrast, the essential oil of peltate glandular trichomes of anthers had only 12 compounds (Table 1), all found also in the leaves. Moreover, in contrast to leaves the relative abundances of these compounds in anthers were rather equitable since there were no dominant compounds (Fig. 2). The seven compounds absent in the essential oil of anthers were found in low relative abundance in leaves, excepting guaiol and aromadendrene which were found in non-negligible amounts (Table 1).
Significant differences were found in the composition of the essential oils from peltate glandular trichomes from anthers and leaves. As a general pattern, the essential oil from anthers had a higher relative proportion of monoterpenes, while leaves had a higher relative proportion of sesquiterpenes (Table 1). The essential oils from anthers had a higher relative proportion of monoterpenes hydrocarbon (t = 6.04, P = 0.021, Fig. 2) and a lower relative proportion of oxygenated sesquiterpenes compared to leaves (t = -5.5, P = 0.0061, Table 1). There were no significant differences in the relative proportion of oxygenated monoterpenes and sesquiterpene hydrocarbons between anthers and leaves (t = -0.953, P = 0.4 and t = -1.19, P = 0.325, respectively, Table 1). Among monoterpene hydrocarbons only the relative proportion of a-pinene, camphene, P-terpinene and limonene were significantly higher in anthers than in leaves (t=2.039, P=0.0338; t=3.99, P=0.048; t=2.039, P=0.0338; t=2.07, P=0.0071, respectively, Fig. 2). Additionally, the relative proportion of ledol and the unidentified oxygenated sesquiterpene did not differ statistically between anthers and leaves; however their abundance tends to be higher in leaves. Finally, it is notable that from the seven compounds absent in the essential oil from anthers (but present in leaves), four of them were oxygenated sesquiterpenes (Table 1).
Table 1. Mean relative abundance (% ± SD) and richness of Chemical compounds of the essential oil of peltate glandular trichomes from anthers and leaves of Lepechinia floribunda. Compounds are listed in order of elution from DB-5 column. RI: Retention Index. 1-Monoterpenes hydrocarbons, 2-Oxygenated monoterpenes, 3-Sesquiterpenes hydrocarbons and 4-Oxygenated sesquiterpenes. Different letters above means value show significant differences in the relative abundance of a compound between anthers and leaves.
| RI DB-5 | Compounds | Anther | Leaf | Methods of identification |
| 936 | a-Pinene1 | 8.6±5.07a | 1.28±0.76b | GCMS.Co |
| 953 | Camphene1 | 5.61±1.34a | 2.56±1.30b | GCMS |
| 982 | P-Terpinene1 | 15.53±4.79a | 1.12±0.47b | GCMS |
| 1034 | Limonene1 | 17.51±2.66a | 0.36±0.36b | GCMS.Co |
| 1038 | 1,8-Cineole2 | 12.27±3.62a | 13.03±6.66a | GCMS |
| 1185 | Borneol2 | 14.43±2.56a | 17.91±4.7 a | GCMS |
| 1204 | Terpineol2 | - | 0.77±0.39 | GCMS |
| 1294 | Bornyl Acetate2 | - | 1.22±0.34 | GCMS |
| 1438 | P-Caryophyllene3 | 8.72±2.23a | 9.61±2.96a | GCMS |
| 1458 | Aromadendrene3 | - | 3.18±0.31 | GCMS |
| 1475 | a-Humulene3 | 2.65±0.44a | 1.56±0.49a | GCMS |
| 1510 | Ledene3 | 0.34±0.61a | 0.61±0.32a | GCMS |
| 1573 | Nerolidol4 | - | 0.75±0.37 | GCMS |
| 1602 | Spathulenol4 | 0.52±0.91a | 1.25±0.24a | GCMS |
| 1608 | Caryphyillene Oxide4 | - | 0.96±0.62 | GCMS |
| 1618 | Guaiol4 | - | 1.54±2.67 | GCMS.Co |
| 1622 | Unidentified Oxygenated Sesquiterpene4 | 9.38±7.53a | 33.07±15.26a | GCMS |
| 1633 | Ledol4 | 3.82±2.45a | 8.88±14.72a | GCMS |
| 1684 | a-Eudesmol4 | - | 0.25±0.43 | GCMS |
| Identified compounds | 90.62 | 66.93 | ||
| Monoterpene Hydrocarbons | 47a | 5b | ||
| Oxygenated Monoterpenes | 27a | 33a | ||
| Sesquiterpenes Hydrocarbons | 12a | 15a | ||
| Oxygenated Sesquiterpenes | 14a | 47b |
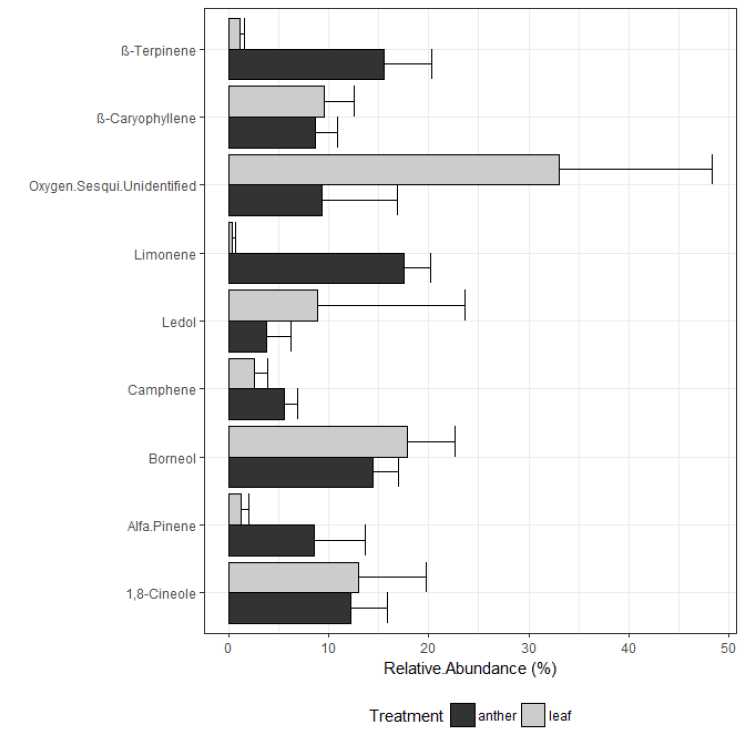
Fig. 2. Mean relative abundance (% ± SD) of the main Chemical compounds of the essential oil of peltate glandular trichomes from anthers and leaves of Lepechinia floribunda.
Much progress has been performed in order to disentangling the ecological function of chemical compounds produced in different organs of plants (Dobson et al., 1999; Anderson et al., 2002; Marín-Loaiza & Céspedes, 2007; Borghi et al., 2017). Many studies have characterized the chemical composition of the essential oil of flowers and leaves in different species of the aromatic Lamiaceae (Werker et al, 1985; Ascensao et al., 1995; Borges et al., 2006;
Touati et al., 2011), nonetheless essential oils from glandular trichomes located on reproductive organs has rarely been analyzed so far (Spring & Bienert, 1987; Duke & Paul, 1993; Gopfert et al, 2005). We found that the composition of the essential oils from peltate glandular trichomes located on anthers differ from those located on leaves.
The essential oil of peltate glandular trichomes from leaves had a significantly higher relative proportion of oxygenated sesquiterpenes than those obtained from anthers. The chemical composition of the essential oil from peltate glandular trichomes of leaves of L. floribunda resemble to those obtained from the leaves of L. conferta and L. shiedeana, where ledol had a high relative proportion (24.2% and 36.9% respectively) (Stashenko et al., 1999; Borges et al., 2006). Likewise, previous studies on another wild populations of L. floribunda from Córdoba province (Fester et al., 1961; Viturro et al., 2002; Duschatzky et al., 2007), found a common pattern in chemical composition of leaves which was similar to our finding. Compounds like 1,8-cineole, limonene, borneol, camphene and P-caryophyllene has been reported as effective for the control of different kind of microorganism and against insect growth (Kessler & Baldwin, 2002; Bakkali et al., 2008; Muhlemann et al., 2014; Amby et al., 2016). Consequently, the combination of chemical compounds found in the essential oil of peltate glandular trichomes from the leaves of L. floribunda would have a defensive function against herbivores and pathogens.
The essential oil of peltate glandular trichomes from anthers of L. floribunda has less richness of chemical compounds than peltate glandular trichomes from leaves. Moreover, the relative abundance of monoterpene hydrocarbons, specially a-pinene, camphene, P-terpinene and limonene, were ten times higher than in leaves. The lower richness of compounds observed in the essential oil of anthers trichomes was mainly due to the absence of many oxygenated sesquiterpens. A similar pattern has been previously observed in relation to the richness of compounds between the essential oil from flowers and leaves of other Lamiaceae such as Hyssopus oficinalis L. and Ocimum basilicum L. (Schulz & Stahl-Biskup, 1991; Chalchat & Ozcan, 2008). In contrast, the opposite pattern has been observed in Lavandula dentata L., Lepechinia conferta and Salvia sclarea L. (Farka et al., 2005; Borges et al., 2006; Touati et al., 2011). However, these studies did not allow to disentangling if the differential composition was due to a different kind of dominant trichomes at each organ or, if it was the same kind of trichome which changes its secretion products according to the its location in the plant. Here we demonstrated that chemical composition of peltate glandular trichomes varies with the location in the plant.
Interestingly, monoterpenes as camphene and limonene, found in higher relative abundance in anthers of L. floribunda, have been reported as compounds used by Bombus spp. to transmit information about rewarding floral resources to nestmates and thus promote floral constancy (Molet et al., 2009). It suggests that these compounds can have a key function in the process of pollination in L. floribunda, as Bombus sp. are the most frequent floral visitors (Camina, 2018). Moreover, compounds such as camphene, P-Terpinene, 1,8-Cineole, limonene and P-caryophyllene, were founds in high proportions in anthers and they have demonstrated cytotoxic activities against floral pathogenic bacteria (Bakkali et al., 2008; Junker et al., 2011; Valiollahi et al., 2014). Thus, the higher proportion of monoterpenes in the essential oil of anthers might act as both, pollinator attraction and microorganism defense. In Lamiaceae several developmental gland stages can occur simultaneously in a single leave, producing a short secretory phase of volatile compounds (Turner et al., 2000). Changes in the composition of secreted products during leaf maturation, in the same organ at different positions within the plant or in different parts of the same organ were reported (Maffei et al, 1989; Rohloff, 1999; Court et al, 1993; Hallahan, 2000). Thus, questions arise regarding to the potential changes in the composition of the essential oil from peltate glandular trichomes of the anthers and leaves of L. floribunda, Does its composition change throughout the maturation of the organ? Does it composition change among trichomes located at different positions at the same organ?.
In synthesis our study shows for the first time differential chemical composition (relative abundance and richness) in essential oils produced by the same type of glandular trichomes placed on different organs. These results suggest a high tissue specificity expression of genes in different organs (Stesevic et al., 2016). This gene specificity expression has been demonstrated in several species where genes are expressed or silenced depending on the organ (Glas et al., 2012). Finally, such variation in chemical composition suggests different ecological functions for the same type of glandular trichome according to the organ.
This work was supported by grants from FONCyT (PICT 2011-1606, and PICT 20122146), CONICET (PIP 11220150100371CO and PIP 11220120100661CO), and the Universidad Nacional de Córdoba (SECyT). J.L.C. is fellowship holder from CONICET, J.S.D., J.A.Z and L.A. are researchers of the same institution. We thank Dra. Elena Galindez for the assistance on the assembling of figures, Lucas Carbone, Melanie
Hughes and two anonymous reviewer for valuable comments that helped to improve the manuscript, and Marcela Palacios for assistance on chemical sample processing.
ACEVEDO, J. G. A., J. L. Muñoz López, A. Martínez Cortés, A. M. García Bores, G. Martínez Cortés & I. Peñalosa Castro. 2005. In vitro anti-Vibrio cholerae activity of essential oil from Lepechinia caulescens. Fitoterapia 76:104-107.
ADAMS, R. P. 2007. Identification of essential oil components by gas chromatography/mass spectrometry, 4th ed., Allured publishing corporation, U.S.A. [ Links ]
AMBY, D. B., T. MANCZAK, M. A. PETERSEN, T SUNDELIN, C. WEITZEL, M. GRAJEWSKI & B. JENSEN. 2016. Role of the Colletotrichum acutatum sesquiterpene synthase CaTPS in the biosynthesis of sesquiterpenoids. Microbiology 162:1773-1783. ANDERSSON, S., L. A. NILSSON, I. GROTH & G. BERGSTROM. 2002. Floral scents in butterflyD pollinated plants: possible convergence in chemical composition. Bot J Linn Soc. 140:129-153. [ Links ]
ANGIONI, A., A. BARRA, V. CORONEO, S. DESSI & P. CABRAS. 2006. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. agricult. food chem. 54:4364-4370. ARIAS TOLEDO, B., C.TRILLO & M. GRILLI. 2010. Uso de plantas medicinales en relación al estado de conservación del bosque en Córdoba, Argentina. Ecol. Austral 20:235-246. [ Links ]
ARZE, J. B. L., G. COLLIN, F. X. GARNEAU, F. I. JEAN & H. GAGNON. 2009. Essential oils from Bolivia. VI. Lamiaceae: Lepechinia graveolens (Reg.) Epling L. floribunda (Benth.) Epling, and L. meyeni (Walp.) Epling. J. Essent Oil Res. 21:36-40. [ Links ]
ASCENSAO, L., N. MARQUES & M. S. PAIS. 1995. Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceae). Ann Bot. 75: 619-626. [ Links ]
BAKKALI, F., S. AVERBECK, D. AVERBECK & M. IDAOMAR. 2008. Biological effects of essential oils -A review. Food Chem. Toxicol. 46: 46-475. [ Links ]
BHATT, A., Y NAIDOO & A. NICHOLAS. 2010. An investigation of the glandular and non-glandular foliar trichomes of Orthosiphon labiatus NE Br.[Lamiaceae]. N.Z. J. Bot. 48:153-161. [ Links ]
BORGES, R., L. B. ROJAS, J. A. CEGARRA & A. USUBILLAGA. 2006. Study of the essential oils from the leaves and flowers of Lepechinia conferta (Benth) Epl. FlavourFragr. J. 21:155-157.
BORGHI, M., A. R. FERNIE, F. P. SCHIESTL & H. J. BOUWMEESTER. 2017. The Sexual Advantage of Looking, Smelling, and Tasting Good: The Metabolic Network that Produces Signals for Pollinators. TrendsPlant Sci. 22:338-350.
BOZIN, B., N. MIMICA-DUKIC, N. SIMIN & G. ANACKOV. 2006. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. agric. food chem. 54:1822-1828.
BRAND, Y. M., V. C. ROA-LINARES, L. A. BETANCUR-GALVIS, D. C. DURÁN-GARCÍA & E. STASHENKO. 2016. Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on Human Herpes viruses. J. Essent. Oil Res. 28:130-137.
CABALLERO-GALLARDO, K., J. OLIVERO-VERBEL & E. E. STASHENKO. 2011. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. J. Agric. Food. Chem. 59:1690-1696.
Camina, J. L. 2018. Importancia de interacciones ecológicas en la provisión de servicios ecosistémicos: La producción de aceites esenciales en Lepechinia floribunda (Benth.) Epling (LAMIACEAE). Tesis doctoral para optar por el título de PhD. Universidad Nacional de Córdoba, Córdoba, Argentina.
CHALCHAT, J. C. & M. M. OZCAN. 2008. Comparative essential oil composition of flowers, leavesand stems of basil (Ocimum basilicum L.) used as herb. Food Chem. 110:501-503.
CICCIÓ, J. F., V. H. SOTO & L. J. POVEDA. 1999. Essential oil of Lepechinia schiedeana (Lamiaceae) from Costa Rica. Rev. Biol. Trop. 47:373-375.
CONN, B. J. & T. C. WILSON. 2015. Two new species of Prostanthera (Lamiaceae) in New South Wales. Telopea 18:463-474.
COURT, W. A., R. POCS & R. C. ROY. 1993. Effect of harvest date on the yield and quality of the essential oil of peppermint. Can. J. Plant Sci. 73:815-824.
DE MARTINO, L., V. DE FEO & F. NAZZARO. 2009. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 14:4213-4230.
DOBSON, H. E. M., E. M. DANIELSON & I. D. VAN WESEP. 1999. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Sci. Biol. 14:153-166.
DREW, B. T. & K. J. SYTSMA. 2012. The South American radiation of Lepechinia (Lamiaceae): phylogenetics, divergence times and evolution of dioecy. Bot. J. Linn. Soc. 171:171-190.
DUKE, S. O. & R. N. PAUL. 1993. Development and fine structure of the glandular trichomes of Artemisia annua L. Int. J. Plant Sci. 154:107-118.
DUSCHATZKY, C. B., M. L. POSSETTO, M. C. FERNANDEZ BELMONTE, M. PEROTTI, C. SCHUFF, C. NUÑEZ, C. GARCÍA, E. ACOSTA y D. DAMONTE. 2007. Composición química y actividad antiviral de aceites esenciales de especies autóctonas del centro oeste de Argentina. Primer Simposio Iberoamericano de Química Orgánica (SIBEAQO I); Mar del Plata.
EGGERS, M. D. 2000. Zusammensetzung und Variation atherischer Ole von Pflanzen der venezolanischen Anden unter besonderer Berücksichtigung der Gattung Lepechinia WILLD.(Lamiaceae). Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Universitat Hamburg, Deutschland.
EGGERS, M. D., G.O RSINI & E. STAHL-BISKUP. 2001. Composition and chemical variation of the essential oil of Lepechinia salviaefolia (Kunth) Epl. from Venezuela. J. Essent. Oil Res. 13:1-4.
EPLING, C. 1938. Las labiadas de la Argentina. Extracto de la revista del Museo de La Plata, Tomo II, Sección Botánica, CONI. Buenos Aires. pp. 89-178.
FAHN, A. 2000. Structure and function of secretory cells. In HALLAHAN, D. L. & J. C. GRAY (eds.), Plant Trichomes. pp. 37. Academic Press: New York, NY, USA.
FARKA, P., M. HOLLÁ, J. TEKEL, S. MELLEN & T. VAVERKOVÁ. 2005. Composition of the essential oils from the flowers and leaves of Salvia sclarea L.(Lamiaceae) cultivated in Slovak Republic. J. Essent. Oil Res. 17:141-144.
FESTER, G. A., E. A. MARTINUZZI, J. A. RETAMAR & A. J. RICCHIARDI. 1961. Aceites esenciales de la República Argentina. Academia Nacional de Ciencias, Córdoba. 98.
FUSELLI, S. R., S. G. DE LA ROSA, M. J. EGUARAS & R. Fritz. 2008. Susceptibility of the honeybee bacterial pathogen Paenibacillus larvae to essential oils distilled from exotic and indigenous Argentinean plants. J. Essent. Oil Res. 20:464-470.
GLAS, J. J., B. C. SCHIMMEL, J. M. ALBA, R. ESCOBAR-BRAVO, R. C. SCHUURINK & M. R. KANT. 2012. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 13: 7077-17103.
GOPFERT, J. C., N. HEIL, J. CONRAD & O. SPRING. 2005. Cytological development and sesquiterpene lactone secretion in capitate glandular trichomes of sunflower. PlantBiol. 7:148-155.
HALLAHAN, D. L. 2000. Monoterpenoid biosynthesis in glandular trichomes of Labiate plants. Adv. Bot. Res. 31:77-121.
JUNKER, R. R., C. LOEWEL, R. GROSS, S. DOTTERL, A. KELLER & N. BLÜTHGEN. 2011. Composition of epiphytic bacterial communities differs on petals and leaves. Plant Biol. 13:918-924.
KENG, C. L. & L. P. SIONG. 2006. Morphological similarities and differences between the two varieties of Cats Whiskers (Orthosiphon stamineus Benth.) grown in Malaysia. Int. J. Bot. 2:1-6.
KESSLER, A. & I. T. BALDWIN. 2002. Plant responses to insect herbivory: the emerging molecular analysis. Ann. Rev. plant Biol. 53:299-328.
LAWRENCE, B. M. & J. K. MORTON. 1979. Volatile constituents of Lepechinia calycina. Phytochemestry 18:1887.
LI, B., P. D. CANTINO, R. G. OLMSTEAD, G. L. BRAMLEY, C. L. XIANG, Z. H. MA, Y. H. TAN & D. X. ZHANG. 2016. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Scientific reports, 6: 34343.
MAFFEI, M. & T. SACCO, 1987. Chemical and morphometrical comparison between two peppermint notomorphs. Planta med. 53:214-216.
MAFFEI, M., F CHIALVA & T. SACCO. 1989. Glandular trichomes and essential oils in developing peppermint leaves. New Phytol. 111:707-716.
MAFFEI, M. & A. CODIGNOLA. 1990. Photosynthesis, photorespiration and herbicide effect on terpene production in peppermint (Mentha piperita L.). J. Essent. Oil Res. 2:275-286.
MALAGÓN, O., R. VILA, J. IGLESIAS, T. ZARAGOZA & S. CAÑIGUERAL. 2003. Composition of the essential oils of four medicinal plants from Ecuador. Flavourfrag. J. 18:527-531.
MARÍN-LOAIZA. J. & C .CÉSPEDES. 2007. Compuestos volátiles de plantas. Origen, emisión, efectos, análisis y aplicaciones al agro. Rev. Fitotec. Mexicana 30:327-351.
MARZOUG, H. N. B., M. ROMDHANE, A. LEBRIHI, F. MATHIEU, F. COUDERC, M. ABDERRABA, M. L. KHOUJA & J. BOUAJILA. 2011. Eucalyptus oleosa essential oils: chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 16:1695-1709.
METCALFE, C. R. & L. CHALK. 1950. Anatomy of the Dicotyledons. At The Clarendon Press; Oxford,
MOLET, M., L. CHITTKA & N. E. RAINE. 2009. How floral odours are learned inside the bumblebee (Bombus terrestris) nest. Naturwissenschaften 96:213-219.
MOROCHO, V., M. L. TORO, L. CARTUCHE, D. GUAYA, E. VALAREZO, O. MALAGÓN & J. RAMÍREZ. 2017. Chemical Composition and Antimicrobial Activity of Essential Oil of Lepechinia radula Benth Epling. Rec. Nat. Prod. 11:57.
MOYANO, F., A. COCUCCI & A. SÉRSIC. 2003. Accessory pollen adhesive from glandular trichomes on the anthers of Leonurus sibiricus L. (Lamiaceae). Plant Biol. 5:411-418.
MUHLEMANN, J. K. ,A. KLEMPIEN & N. DUDAREVA. 2014. Floral volátiles: from biosynthesis to function. Plant, cellEnviro. 37:1936-1949.
R DEVELOPMENT CORE TEAM. 2009. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Available in: http:// www.R-project.org.
REHMAN, R., M. A.HANIF, Z. MUSHTAQ, B. MOCHONA & X. QI. 2016. Biosynthetic Factories of Essential Oils: The Aromatic Plants. Nat. Prod. Chem. Res. 4:4
ROHLOFF, J. 1999. Monoterpene composition of essential oil from peppermint (Menthax piperita L.) with regard to leaf position using solid-phase microextraction and gas chromatography/mass spectrometry analysis. J. Agrie. Food Chem. 47:3782-3786.
RUIZ, C., C. DÍAZ & R. ROJAS. 2015. Composición química de aceites esenciales de 10 plantas aromáticas peruanas. Rev. Soc. Química Perú 81:81-94.
SCHULZ, G. & E. STAHL-BISKUP. 1991. Essential oils and glycosidic bound volatiles from leaves, stems, flowers and roots of Hyssopus officinatis L. (Lamiaceae). Flavour Fragr. J. 6:69-73.
SPRING, O. & U. BIENERT. 1987. Capitate glandular hairs from sunflower leaves: development, distribution and sesquiterpene lactone content. J. Plant Physiol. 130: 441-448.
STASHENKO, E. E., M. CERVANTES, Y. COMBARIZA, H. FUENTES & J. R. MARTÍNEZ. 1999. HRGC/FID and HRGC/MSD analysis of the secondary metabolites obtained by different extraction methods from Lepechinia schiedeana, and in vitro evaluation of its antioxidant activity. J. Separation Sci. 22:343-349.
STESEVIÓ, D., M. BOZOVIÓ, V. TADIÓ, D. RANCIÓ & Z. D. STEVANOVIC. 2016. Plant-part anatomy related composition of essential oils and phenolic compounds in Chaerophyllum coloratum, a Balkan endemic species. Flora 220:37-51.
TOUATI, B., H. CHOGRANI, I. HASSEN, M. BOUSSAID, L. TOUMI & N. B. BRAHIM. 2011. Chemical composition of the leaf and flower essential oils of Tunisian Lavandula dentata L.(Lamiaceae). Chem. Biodivers. 8:1560-1569.
TURNER, G. W., J. GERSHENZON & R. B. CROTEAU. 2000. Development of peltate glandular trichomes of peppermint. Plant physiol. 124:665-680. VALAREZO, E., A. CASTILLO, D. GUAYA, V. MOROCHO & O. MALAGÓN. 2012. Chemical composition of essential oils of two species of the Lamiaceae family: Scutellaria volubilis and Lepechinia paniculata from Loja, Ecuador. J. Essent. Oil Res. 24:31-37.
VALENZUELA, L., V. ROSER, S. CANIGUERAL & T. ADZET. 1992. The essential oil of Sphacele chamaedryoides. PlantaMed. 58:273-274. VALIOLLAHI, M. R., M. GHOLAMI, A. R. NAMJOO, Y. RAHIMIAN & A. RAFIEE. 2014. Effect of using Sumac (Rhus coriaria L.) and Ajwain (Trachyspermum copticum) powders on performance and intestinal microbial population in broiler chicks. ROAVS. 4:545-549.
VELASCO-NEGUERUELA, A., M. J. PÉREZ-ALONSO, J. L. ESTEBAN, C. A. GUZMÁN, J. A. ZYGADLO & L. ARIZA-ESPINAR. 1994. Essential oil of Lepechinia floribunda (Benth.) Epl. J. Essent Oil Res. 6:539-540.
VITURRO, C. I., A. MOLINA, M. JUÁREZ & M. ELECHOSA. 2002. Aceite esencial de Lepechinia floribunda de tres regiones del centro de Argentina. 1er. Congreso Latinoamericano de Fitoquímica y 4ta. Reunión de la Sociedad Latinoamericana de Fitoquímica. Buenos Aires, Argentina.
WERKER, E., U. RAVID & E. PUTIEVSKY. 1985. Glandular hairs and their secretions in the vegetative and reproductive organs of Salvia sclarea and S. dominica. Israel J. Bot. 34:239-252.
ZANONI, T. A. & R. P. ADAMS. 1991. Essential oils of plants from Hispaniola: 5. the volatile leaf oil of Lepechinia uvbanii (briq.) epling (lamiaceae). FlavourFragr. 6:75-77.
ZAR, J. H. 1999. Biostatistical analysis. 4th ed. Prentice Hall, New Jersey.
Instituto Multidisciplinario de Biología Vegetal, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de Córdoba (UNC), (5000) Córdoba, Argentina. Telefax (54) 351-433-1056/2104
Instituto de Ciencia y Tecnología de los Alimentos (ICTA), Facultad de Ciencias Exactas Físicas y Naturales, Universidad Nacional de Córdoba (UNC), Argentina
*juliacamina@yahoo.com.ar - 54-351-4331056/2104














