Introduction
The genus Baccharis L. is the richest in species within the Astereae tribe, comprises from 354 species (Müller, 2013) to 440 (Heiden et al, 2019) of exclusively American distribution. Zuloaga et al. (2019) reported 210 species for southern cone of South America (Argentina, southern Brazil, Chile, Paraguay, and Uruguay). In Argentina inhabit 99 species (Giuliano & Plos, 2014). The present contribution is about Baccharis notosergila Griseb., which is a woody shrub that inhabit in the Salado river basin situated in Buenos Aires province (Argentina). This region alternate cyclically periods of drought and floods, the land surface is nearly horizontal with predominance of saline-alkaline soils and a deficient drainage. This area is occupied by natural pastures and principally dedicated to livestock farming (Rodriguez & Jacobo, 2012). Baccharis notosergila not only reduce with its cover the area accessible to grazing, but produce strong competition for water, nutrients and light with the species of value of the natural grassland, generating a process of degradation that normally result in low productivity (Sione et al., 2006). Baccharis notosergila in the Salado river basin has shown to be resistant to mechanical and chemical control (Urdampilleta, 2019; Carbone et al, 2019). For the reasons mentioned above, this species is included in the project on problematic weeds, developed in the Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata (Argentina). In a previous paper, Carbone et al. (2019) analyzed the underground system in which was found a xylopodium with buds constituting a bud bank, either was detected in the radical system the accumulation of inulin as the principal reserve. According to Hayashi & Appezzato-da-Glória (2007) and Appezzatto-da-Glória & Cury (2011), the presence of these two traits allow for regeneration and survival through unfavourable environmental conditions. Carbone et al. (2019), attributed to subterranean system’s traits the capacity to sprout and develop new organs in the spring after mechanical and chemical control, and the accumulation of inulin reinforces the resprout and persistence of this shrub. Now, two questions arise: what is the role of aerial vegetative organs in the survival of this species?, and what is its reaction to the chemical control treatment?. Despite what has just been said, there is not a comprehensive study of aboveground system of B. notosergila. Therefore, the aims of this study were to investigate leaves and stems morphology, structure and histochemical features to ascertain whether a correlation exists between the leaves and stems traits and survival strategies, and to obtain some insight into the biological problems concerning the resistance to current control methods.
Materials and Methods
Plant materials
Plants of B. notosergila having fully developed leaves were collected during the spring of 20182020 in the “El Amanecer” establishment-farm situated at 57°37’ W, 35°15’S in Vieytes, Magdalena party (Pdo.), Buenos Aires province (Prov.), Argentina. The botanical material was identified and the vouchers were deposited in the herbarium of Facultad de Agronomía, Universidad Nacional de La Plata, and registered as: 17-XII-2018, F. Fernández & A. Carbone 1, 2, 3, 4 (LPAG); F. Fernández & A. Carbone 5, 15-X-2019, (LPAG); F. Fernández & A. Carbone 6 (specimen from half hill), 7 (specimen from alkaline-saline lowland), 8 (specimen from sweet lowland), 11-XI-2020 (LPAG). In addition, specimens having leaves from Instituto de Botánica Darwinion were consulted. ARGENTINA. Prov. Buenos Aires: Pdo. Berazategui, Hudson, 16-IV-1927, Burkart 1302 (SI) (image 15). Prov. Entre Ríos: Dpto. Concordia, 31-I-1927, Burkart 1122 (SI), Det. Cabrera (image 14). Dpto. Gualeguaychú, 10-IV-1960, Burkart 21988 & Gamerro (SI).
Optical microscopy
For anatomy study, mature leaves and stem fragments of different specimens were fixed in FAA (a solution of formaldehyde, glacial acetic acid, and 70% ethyl alcohol, Johansen, 1940), then stored in 70% ethanol. To analyze epidermis in surface view, the leaves were diaphanized using the method of Franklin (1945) modified, for that, the leaves were boiled in ethyl alcohol 96° for 20 min, allowed to cool were washed and bleached in 50% sodium hypochlorite (NaClO) for 2 h, washed twice and submerged in a solution of hydrogen peroxide + glacial acetic acid (1: 1 v/v) for 48 h, washed twice in distilled water, bleached again in 50% NaClO. At the completion of the bleaching process, five washes in distilled water were carried out to remove the NaClO, and samples were then transferred into a solution of chloral hydrate (5%) for 24-48 h. To complete the process, some leaves were washed and stained with safranin or Oil red “O”, on others was applied the peeling method to obtain the epidermis which were also stained. To analyze the structures, freehand cross-sections of stems, petiole and at leaf blade middle part of leaves were cut; the selected sections were bleached in 50% NaClO, washed thrice with distilled water, then a successive double staining was performed with Alcian blue and safranin (Luque et al., 1996), on others a metachromatic staining was performed using Toluidine blue “O” (0.05%) (O’Brien et al., 1964) or Cresyl brilliant blue (0.05%) (Pérez & Tomasi, 2002), and also a monochromatic staining was made using alcoholic solution of safranin (80%) (D’Ambrogio, 1986). The sections, clarified leaves, and epidermis were mounted in gelatin-glycerin on glass slides and sealed with nail polish.
The histochemical analysis was performed on freehand sections of leaf and stem samples. To test lipophilic substances an alcoholic solution of Oil red “O” was used (Gurr, 1971), the red color indicated positive test. Detection of phenolic compounds (tannins) was performed using ferric chloride (10%) and sodium carbonate (2%) (Zarlavsky, 2014), a green-blue color was a positive test. Starch was identified with Iodine-Potassium-Iodide (IKI) (Ruzin, 1999) a black-blue color indicate positive test. Resins were detected using a saturated solution of copper sulphate (Cosa et al., 2014) a color emerald green indicate positive test. Additionally, toluidine blue “O” was used to contrast polyphenols (Tapia-Torres et al., 2014), a color turquoise green was a positive test.
The leaf morphology and phyllotaxy type were evaluated through a visual inspection. To see details a Bausch & Lomb stereomicroscope was used. Photographs were taken with a digital camera, resolution 12 MP. Slides were analyzed with a Nikon E200 LED optical microscope and using micrometrics SE Premium software. The measurements of outer periclinal epidermal cell walls and cuticle thickness in micrometers (gm) were obtained by using ImageJ software (González, 2018). Terminology used by Metcalfe & Chalk (1988) was followed in this work. The stomata types were recognized using the manual of Ash et al. (1999). Trichomes were described considering the literary works (Ramayya, 1962; Freire et al, 2007; Tosoratto et al., 2016, and Budel et al., 2018).
Scanning electrón microscopy (SEM)
It was performed using portions of stem and leaf blade. They were taken from fixed material, dehydrated in ethyl alcohol (100°) for 24 h. Then the samples were affixed on stubs by double-sided adhesive tape and were submitted to metallization with a fine and thin gold layer. Afterwards they were examined with a Philips 505 SEM, and micrographs were prepared. Center for Research and Development in Applied Sciences “Dr. Jorge J. Ronco” (CINDECA), National Council for Scientific and Technical Research (CONICET), National University of La Plata (UNLP).
Results
Leaf morphology
Leaves are deciduous and have an alternate arrangement. They are variable in shape and size. They are elliptic or elliptic-obovate, linear-elliptic and linear. The largest elliptic or elliptic-obovate frequently are situated in the basal part of the plant or stems, the linear-elliptic towards the middle part, and the linear leaves at the top of the stem or the upper branches of the plant. All leaves have acute-mucronate apex, decurrent base with petiole, and reticulate vascularization. The largest leaves have the serrate margins with 1-4(-10) teeth on each margin; 3.7-7 cm long and 0.5-1 cm wide. The median leaves have a few teeth or entire margins, 2.3-4.2 cm in length and 0.3-0.5 cm in width, and the smallest linear leaves have entire margins, measuring 1-2.5 cm long x 0.1-0.3 cm wide (Fig. 1).
Epidermal tissue in surface view by SEM
The leaves epidermis exhibit striate and massive cuticle and frequently waxes overlap the guard cells, partly obscuring them (Fig. 2A). Stem topography view by SEM reveals longitudinal ribs alternating with grooves, all covered by striate cuticle, and the stomata located in the depth of the grooves (Figs. 2 B; 8B).

Fig. 1: Leaf morphology. It shows the variability of B. notosergila leaf size and shapes. The large elliptic and elliptic-obovate leaves (3.7-7 cm length); with toothed margins, and it may be seen the reticulate venation. The median elliptic and linear-elliptic leaves (2.3-4.2 cm length), with toothed or entire margins. The small linear leaves (1-2.5 cm length), with entire margins. Scale: 2 cm.
Epidermal tissue in surface view by lightmicroscope
The epidermal cells on both leaf blade faces are polygonal with anticlinal cell walls straight to slightly wavy, and the cuticle is striated especially around the trichomes and stomata. Generally, the anticlinal cell walls are thin. Stomata are uniformly distributed over whole petiole and leaf blade surfaces and on the margins. They are randomly distributed, however, the stomata longitudinal axis is oriented following the longitudinal axis of the organ (Fig. 2A). Stomata are predominantly actinocytic, anomocytic, anomotetracytic, cyclocytic types and less frequently anisocytic. Each stoma shows in surface view the outer stomatal ledge, a polar thickening, and ellipsoidal stomatal aperture (pore) (Fig. 3A-E). The trichomes are found on both leaf blade surfaces, margins, and the petiole. The trichomes types are: (i) Biseriate capitate glandular with 2 basal cells and 6-8 cells which contains druses (Fig. 4A); (ii) Bulbiferous flagelliform glandular (bfg) type II, uniseriate and curved body with 3-7 (frequently 4) rounded cells, the terminal swollen spherical, and a translucent flagellum-like apical cell (Fig. 4B), less frequently are found bfg type I with straight body of 5-7(-8) cells and flagellum cell (Fig. 4D); (iii) Uniseriate capitate glandular trichome composed by one basal cell, 2-6(-8) stalk cells and a multicellular head (Fig. 4C). All the trichomes have basal cells cutinized, given positive reaction for lipophilic compounds, and are responsible for the secretion of oil droplets deposited over the leaf blade surface (Fig. 4D-F). The biseriate glandular trichomes can be seen forming a tuft themselves (Fig. 4A), but frequently appear clustered with bfg trichomes (Fig. 4B, D-G) in tufts and localized in a small epidermal depression. Either of the bfg trichomes can occur singly. It was observed that frequently two or three tufts appear connected by more elongated epidermal cells with cuticular thickened over the anticlinal cell walls (Fig. 4G). The uniseriate capitate trichomes are found in lower frequency and solitaries (Fig. 4C).
The caulinar axis exhibits epidermal cells tangentially elongated on the ribs with slightly thick anticlinal cell walls, and the surface covered by waxes; in the grooves there are numerous actinocytic stomata and trichome tufts (Figs. 3F; 4B).
Leaf structure
The petiole cross section next to attach the stem (basal part) shows a concave adaxial side forming two lobes (Fig. 5A). This cross-section exhibits quadrangular or rectangular epidermal cells, covered by cuticle. The angular collenchyma occurs in each lobe and some layers are found at middle vein level. The parenchyma is composed by rounded cells except the chlorenchyma which is formed by palisade parenchyma on the abaxial side, except at middle vein level. There are three main vascular bundles and some traces. The cross section in the middle part of the petiole length (Fig. 5B), exhibits a reduction of adaxial concavity; the collenchyma appears as one continuous subepidermal layer, increasing the layers at midvein level, and the chlorenchyma begin to extend to adaxial side. The petiole cross section at the base of leaf blade (distal part, Fig. 5C), shows deltoid outline, however at midvein level there are adaxial and abaxial prominences, and the palisade chlorenchyma is present on both sides except at middle vein level; the wings of the leaf blade begin to appear. Along the entire length of the petiole there are three main vascular bundles. Each vascular bundle has fiber caps on the xylem and phloem sides, and one to three secretory ducts next to phloem fiber cap, all surrounded by a parenchyma sheath (Fig. 5D).

Fig. 2: Leaf and stem surface view by SEM. A: Leaf epidermis with massive cuticle and waxes over the stomata. B: Stem showing ribs and grooves covered by striate cuticle. Scales= A: 50 pm; B: 5 pm.
The leaf blade cross section exhibits the uniseriate epidermis formed by quadrangular or rectangular epidermal cells with periclinal cell walls thin or conspicuously thicker, always covered by a thin and striate cuticle (Fig. 6A, B). The stomata are localized above or at level with the surrounding epidermal cells, and the guard cells show conspicuous outer and inner stomatal ledges (Fig 6A, B). Internally the isobilateral mesophyll shows 2-3 layers of palisade parenchyma on both faces interrupted at middle vein level, but continuous in the margins (Fig. 6C), sometimes separated from the epidermis by collenchyma (Fig. 6D). In the center, there are 3-4 layers of colorless dense spongy parenchyma (Fig. 6C). The vascular system is composed by collateral vascular bundles each surrounded by a parenchyma sheath, and cross the spongy parenchyma (Fig. 6C). The xylem always is to the upper side. The middle vein exhibits in the center one collateral vascular bundle with fiber cap adjoining the xylem and one to three secretory ducts next to phloem, all surrounded by a parenchyma sheath adding several layers of thin-walled parenchyma cells that connect with two or three layers of angular collenchyma below the epidermis, on both sides (Fig. 6E). The schizogenous secretory ducts exhibits a single layered epithelium composed of 4-6 cells (Fig. 6D-E). In all materials analyzed the linear-elliptic and linear leaves have fiber cap only on the xylem side, and they have ample ducts, while in the largest elliptic or elliptic-obovate leaves the vascular bundles have fiber caps on xylem and phloem sides (similar occurs in the petiole), and the duct exhibit a reduced size (Fig. 6F). The teeth found on apex and margins of the leaves present hydathode structure, each tooth shows three vascular bundles which converge approaching the tooth end, the epithema is poorly developed and there is an open stoma at the tooth apex.
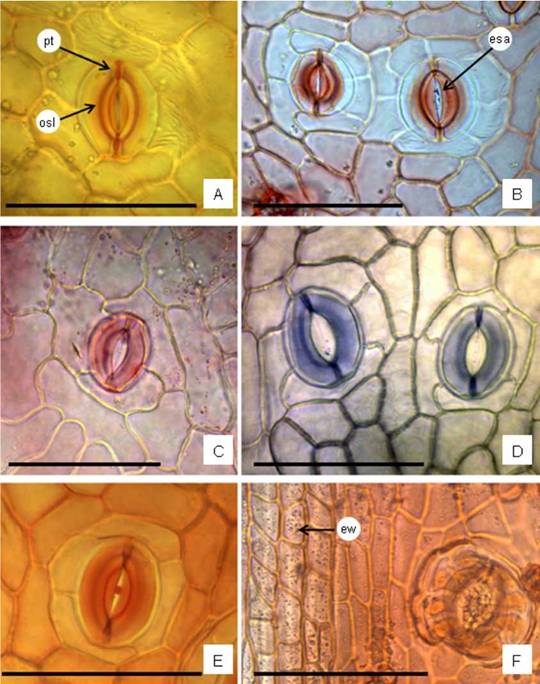
Fig. 3: Leaf and stem surface view by light microscope. A-E: Leaf. A: Anomocytic stoma, it exhibits the outer stomata ledge and polar thickening; cuticular striaes are notable on subsidiary cells. B: Two actinocytic stomata. C: Anisocytic. D: Anomocytic stoma on the left and anomotetracytic on the right. E: Ciclocytic. F: Epidermis of stem in surface view showing rectangular epidermal cells with anticlinal cell walls straight and thickened; epicuticular waxes visible as points on the surface, and an actinocytic stoma. Abbreviations= esa: ellipsoidal stomata aperture; ew: epicuticular waxes; pt: polar thickening; osl: outer stomata ledge. Staining= A, B, E, F: oil red “O”; C: safranin; D: toluidine blue “O”. Scales= A-F: 100 pm.
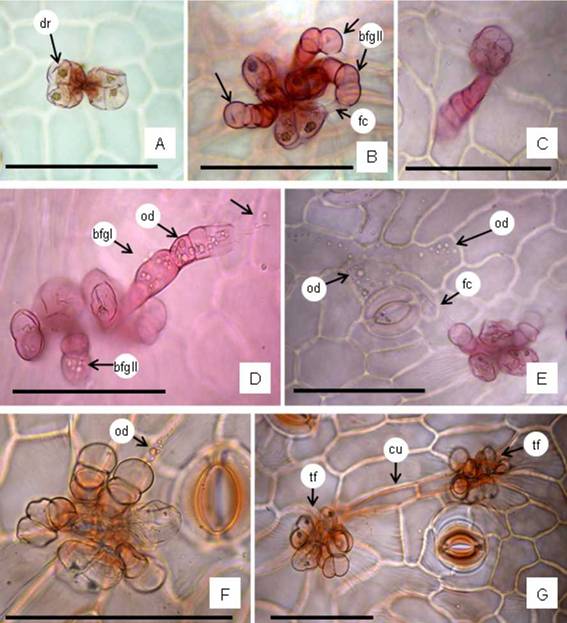
Fig. 4: Trichome features. A: A tuft with two biseriate capitates glandular trichomes showing druses in cells. B: A tuft showing three biseriate capitate trichomes clustered with three bulbiferous flagelliform glandular (bfg) type II (arrows). C: Uniseriate capitate trichome, solitary, rarely was seen in large leaves. D: A tuft showing biseriate capitate trichomes clustered with bfg type II, and one bfg type I (straight) with numerous oil droplets inner the trichome cells (arrows). E: A tuft showing the secretion of oil droplets and deposited on the epidermal cells surface. F: A tuft exhibiting oil droplets in the flagellum cell. G: Two tufts connected by elongated epidermal cells with cuticular thickened on the anticlinal epidermal cell walls. Abbreviations= bfgI: bulbiferous flagelliform glandular straight (type I); bfgII: bfg curved (type II); cu: cuticle; dr: druses; fc: flagellum cell; hd: head; od; oil droplets; st: stalk cells; tf: tuft. Staining= A-E: safranin; F, G: oil red “O”. Scales: A-G= 100 pm.
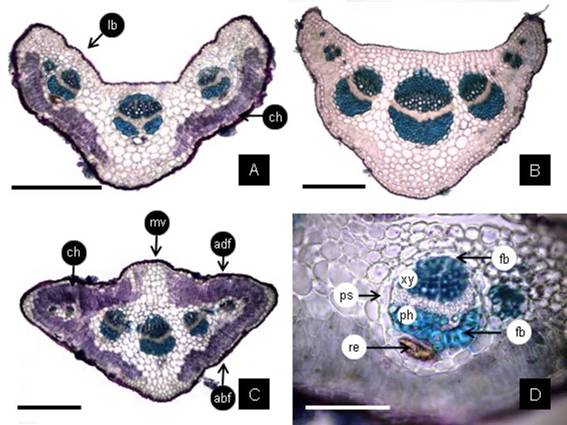
Fig. 5: Petiole cross section (CS). A: The CS near the insertion point on the stem is bilobed with chlorenchyma on abaxial side except at middle vein; there are three main vascular bundles. B: The CS in middle of the petiole length shows a reduction of lobes. C: The CS at base of the leaf-blade has a deltoid outline beginning to develop the wings of the leaf-blade with chlorenchyma on both faces, except at middle vein. D: detail of one vascular bundle showing caps of fibers on the xylem and phloem sides, and on the phloem side one duct; all surrounded by the parenchyma sheath. Abbreviations= abf: abaxial face; adf: adaxial face; ch: chlorenchyma; fb: fibers; Ib: lobe; mv: middle vein; ph, phloem; ps: parenchyma sheath; re: resins; xy, xylem. Scales= A-C: 300 pm; D: 100 pm.
The histochemical tests showed that some epidermal cells are filled with tanniniferous substances, the guard cells of stomata also contain tannins and starch, and these compounds are abundant in the mesophyll (Fig. 7A, B). In the mesophyll, especially in the palisade parenchyma cells there are many chloroplasts, oil bodies (Fig. 6B), and small prismatic crystals (Fig. 7C). In the epithelial cells of ducts were identified oil droplets of volatile oils (Fig. 6E) and in the cavity were obtained positive reactions for resins and polyphenols (Fig. 7D).
Comparing leaves structures collected from plants inhabiting on half hill, alkaline-saline lowland and sweet lowland in the studied area
The leaves from half hill exhibit in surface view, thickened anticlinal epidermal cell walls, and striated cuticle; on the teeth the epidermal cells shows thicker cell walls. In cross section both epidermis, adaxial and abaxial, show conspicuous cellulosic thicker on the cell walls; in the periclinal epidermal cell walls the thickness ranged between 5.6-20.6 pm with a mean value of 14.2 pm (Fig. 6B). The leaves from alkaline-saline soils and from sweet lowland show in surface view, thin anticlinal epidermal cell walls, and slightly striated cuticle. In cross section, the periclinal epidermal cell walls thickness ranged between 0.9-3.6 pm with a mean value of 2.3 pm and 1.7 pm, respectively (Fig. 6A). The cuticle in all leaves is thin (0.4-2.7 pm) with a mean value of 1.2-1.6 pm (Fig. 6A, B). The stomata in the leaves from alkaline-saline and sweet lowland are localized somewhat elevated above to the other epidermal cells (Fig. 6A) while in the leaves from half hill they appeared localized at level (Fig. 6B). The leaves from half hill shows the palisade parenchyma with abundant oil bodies (Fig. 6B), compared to leaves from alkaline-saline and sweet lowland.
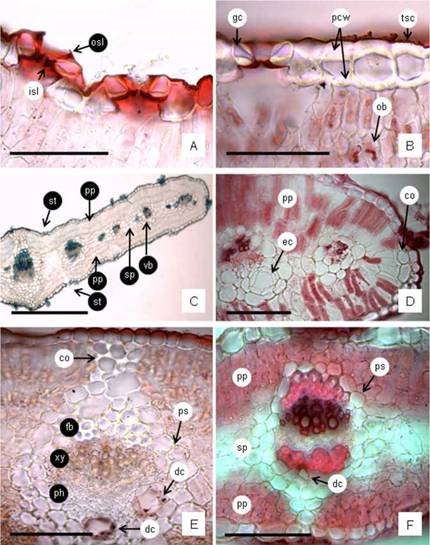
Fig. 6: Leaf blade cross section (CS). A: epidermal cells with thin cell walls and thin cuticle, the stomata slightly above the other epidermal cells, and the stoma guard cells with the cuticle forming conspicuous stomatal ledges. B: epidermal cells with thick periclinal cell walls, thin and striate cuticle, and stomata guard cells at level. Also may be seen the oil bodies in the mesophyll. C: Leaf-blade CS showing the prominences at middle vein level, the uniseriate epidermis, stomata and trichomes on sides, isobilateral mesophyll and vascular bundles (vb) across the spongy parenchyma. D: a detail of the obtuse margins showing the palisade parenchyma continuous, separated from epidermis by some collenchyma cells; and marginal vascular bundles, one with an ample duct. E: middle vein CS of a linear-elliptic leaf showing the collateral vb with fibers on the xylem side and two ample ducts on the phloem side containing volatile oils in the epithelial cells, all surrounded by a parenchyma sheath and its extension towards the angular collenchyma cells. F: middle vein CS of an large eNiptic-obovate leaf showing the collateral vb with fibers on both sides and one reduced duct on the phloem side, all surrounded by a parenchyma sheath and its extension to under epidermal collenchyma tissue. Abbreviations= co: collenchyma cells; dc: duct; ec: epithelial cells; fb: fibers; gc: guard cells; isl: inner stomatal ledge; ob: oil bodies; osl: outer stomatal ledge; pcw: periclinal cell walls; ph: phloem; pp: palisade parenchyma; ps: parenchyma sheath; sp: spongy parenchyma; st: stomata; tsc: thin and striate cuticle; vb: vascular bundle; xy: xylem. Scales: A, B, D-F: 100 pm; C: 500 pm.
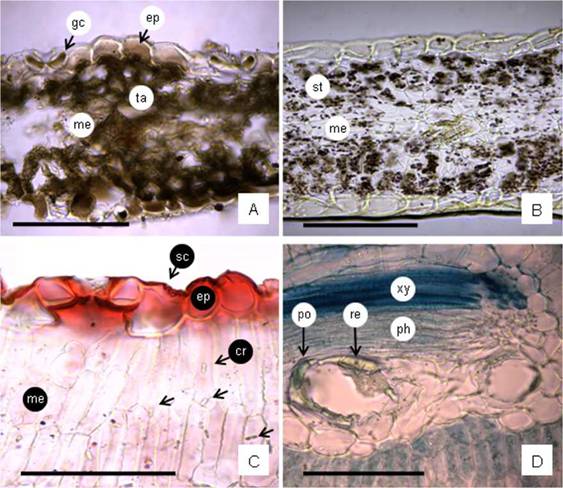
Fig. 7: Leaf histochemical tests and crystals. A: Leaf blade cross-section (CS) showing presence of tannins in epidermal cells, guard cells and mesophyll cells (test FeCl3). B: leaf blade CS showing starch grains in mesophyll cells (test IKI). C: Leaf blade CS showing thin and striate cuticle (test oil red “O”); small crystals in parenchyma cells (arrows). D: Vascular bundle showing two secretory ducts containing polyphenols and resins (tests toluidina blue “O” and copper sulphate have been made). Abbreviations= cr: crystals; ep: epidermis; gc: guard cells; me: mesophyll; ph: phloem; po: polyphenols; re: resins; sc: striate cuticle; st: starch grains; ta: tannins; xy: xylem. Scales= A-D: 100 pm.
Stem structure
A young stem of 1-2 mm diameter, in cross-section, reveals a wavy outline with eight ribs and altemating grooves (Fig. 8A). The epidermis is unilayered and consists of quadrangular or rectangular cells. The external periclinal walls of the cells are thick and impregnated with lipids and are covered by a cuticle of 1.2-3 pm thick. Stomata are localized in the grooves and are elevated above the adjacent epidermal cells (Fig. 8B). In the outer cortex, two to six layers of angular collenchyma are present beneath the ribs. Between the collenchyma, three to four layers of palisade chlorenchyma occur in the grooves. The inner cortex comprises two or three layers of polygonal parenchyma cells, and the innermost layer is the endodermis surrounding the vascular cylinder. In the cortex, a few vascular bundles corresponding to foliar traces are present. They each contain xylem internally and phloem externally with a cap of phloem fibers and a secretory duct adjacent to the fibers. The vascular bundle is surrounded by a parenchyma sheath with cell walls impregnated with lipophilic substances. The endodermis is formed by rectangular cells with thin cell walls impregnated with suberin, obscuring the Casparian strips. In the vascular cylinder, fiber clusters are found adjoining the phloem, and also fibers are in the secondary phloem. The secretory ducts with a uniseriate epithelium formed by 4-10 cells occur in front of the phloem. The secondary xylem has fibers as the predominant component and tannins are occasionally found in the secondary xylem. The center of the stem is filled with the pith formed by thin-walled parenchyma cells. In the perimedullary cells, calcium oxalate crystals are observed (Fig. 8B).

Fig. 8: Stem outline (1-2 mm diam.) and tissues in cross section. A: stem outline showing eight lobes or ribs. B: Stem tissues: ch, chlorenchyma; co, collenchyma; en, endodermis; ep, epidermis; fb, fiber clusters; ft, foliar trace; ph, secondary phloem; phf, secondary phloem fibers; pt: pith; dc, duct; st, stomata; xy, secondary xylem. Scales= A: 1mm; B: 400 pm.

Fig. 9: Stem (3-4 mm diam.) in cross section. A: secondary bark (periderm) developed under the epidermis; cork cells contain tannins (test ClFe3). B: This photograph shows cutin in the epidermis; lipophilic substances in cork cell walls and in the endodermis cell walls (test oil red “O”); in the cortex may be seen isodiametric parenchyma cells. C: Secondary phloem with fibers and starch grains (test IKI). D: pith with crystals. Abbreviations= ck: cork; cp: cortical parenchyma; en: endodermis; ep: epidermis; fb: fiber cluster; Is: lipophilic substances; pe: periderm; phf: fibers in the secondary phloem; pt: pith; st: starch grains; sty: styloids; ta: tannins. Scales= A-D: 100 pm.
A caulinar axis of 3-4 mm diameter exhibits rounded outline and similar structure as was described above, however the periderm (cork + cork cambium + phelloderm) is developed below the epidermis (Fig. 9A, B). The cork cells contain tannins (Fig. 9A). The cork cell walls are impregnated by suberin (Fig. 9B). In the cortex, the cortical parenchyma exhibits rounded cells, and the most inner cell layer, the endodermis, also shows cell walls impregnated with lipophilic substances (Fig. 9B). Internally, the parenchyma of the secondary phloem contains starch grains (Fig. 9C). In the medullary rays and pith there are abundant crystals which have very thin tabular shape that according to the focal distance may be confused with raphides (Fig. 9D).
Discussion
Leaf morphology. The leaves arrangement and features found in B. notosergila accord well with description of Baccharis leaves reported by Heiden et al. (2009). The petiole is not common in Baccharis leaves, however it has been found in several species (e. g., Souza et al., 2011; Jasinski et al., 2014; Bobek et al, 2016; Tosoratto et al., 2016). Respect to the variability of leaf margins dentate or entire, is common trait for the genus (Budel & Duarte, 2008; Oliveira et al., 2011; Souza et al, 2011). Baccharis notosergila has leaves generally with reduced foliar expansion and stay on the plant about five months in spring and beginning summer. This is the best period to do photosynthesis. The character deciduous of the leaves is a favorable feature to the economy of water without affect its physiology, because it can continuous doing photosynthesis with its numerous younger green stems which have active palisade chlorenchyma. It would be an adaptation to the environment (Roth, 1984). On the other hand, the deciduous, small and narrow leaves would be unfavorable to penetration of herbicide treatments.
Hydathodes. Teeth found in leaves are hydathodes. The structure has been found in Asteraceae and in some species of Baccharis (Smiljanic, 1969; Lersten & Curtis, 1985). The function is to discharge water (guttation), however, it would be interesting to do a more comprehensive study about the structure and function in B. notosergila.
Epidermal cells and cuticle. The polygonal epidermal cell shapes in surface view found in Baccharis notosergila accords well with the genus pattern (Ariza Espinar, 1973), while the anticlinal cell walls straight to slightly wavy also have been reported for some species (Cortadi et al, 1999; Freire et al, 2007; Budel & Duarte, 2008; Oliveira et al, 2011; Souza et al, 2011; Bobek et al, 2015). The cuticle of B. notosergila in surface view appears massive and striated in leaves and stems, but it is always thin. These cuticle traits coincide with the most cases reported in the literature (Ariza Espinar, 1973; Cortadi et al, 1999; Budel et al, 2003; Budel & Duarte, 2007; Petenatti et al, 2007; Jasinski et al., 2014; Bobek et al., 2015, 2016; Budel et al, 2018).
The epidermal cells and cuticle features may be attributed to environmental factors. Roth (1984), reported that when the foliar epidermis is exposed greater luminosity and reduced humidity develop characters of the “sun leaves”, such as the straight epidermal anticlinal cell walls. Nughes et al. (2013) found the principal factor producing the “sun leaves” is the high light intensity, whereas they suggested the humidity is related to the stomata number and size. In B. notosergila, the linear leaves predominantly found in the high part of the plants, show the most reduced size and present epidermal anticlinal cell walls slightly thickened, also the epidermal periclinal cell walls conspicuously thickened were found in elliptic-obovate leaves collected in half hill. These features may be adaptations to more exposed localization to weather factors such as wind, high light intensity, because straight and thick periclinal cell walls tend to decrease the excessive loss of water (Fahn & Cutler, 1992), and prevent cell collapse due to dehydration (Cosa & Dottori, 2010). The striated cuticle would be a response to the environment as prevent rapid dehydration (Fahn & Cutler, 1992). The massive aspect found in the cuticle would be produce by waxes deposited over the cuticle (Barthlott et al., 1998). Cuticle and epicuticular waxes also constitute the outermost defensive barrier of plant leaves against pathogens because their repellents properties (Barthlott et al., 1998; Stenglein et al., 2005). In cross sections the cuticle was found thin, however the chemical composition of the cuticle is of a great importance limiting transpiration, and thin cuticles are often effective in preventing excessive cuticular transpiration (Merida et al., 1981). The chemical weed control is more difficult in those species which have less foliar area and thicker cuticle and epicuticular waxes (Westwood et al., 1997; Dall’Armellina & Zimdahl, 1989; Carbone, 2015), whereas a thin cuticle could be an advantage for the penetration of the herbicide, and therefore, increase its absorption into the inner tissues (Santier & Chamel, 1992), however in this case the epicuticular waxes and volatile oils over the leaves and stem surfaces could be a barrier to penetration the herbicides or phytosanitary treatments applied.
Stomata types. Actinocytic, anisocytic, anomocytic, anomo-tetracytic and cyclocytic types of stomata were found in B. notosergila. According to Metcalfe & Chalk (1950), the Asteraceae family may present anomocytic and anisocytic stomata, being the former predominant. These two types have been also reported in the genus by Ariza Espinar (1973), and were described for most species of Baccharis (Cortadi et al, 1999; Budel et al., 2004; Budel & Duarte 2007, 2008, 2010; Petenatti et al. 2007; Souza et al., 2011; Tosoratto et al., 2016). However, cyclocytic was previously referred for B. notosergila (Freire et al, 2005, 2007), and several authors mentioned tetracytic type (e. g., Freire et al. 2007; Bobek et al., 2015, 2016; Budel et al., 2018; Ornellas et al., 2019). The actinocytic stomata type was mentioned by Freire et al. (2007) for 11 species of Baccharis, and Pereira et al. (2014) for B. milleflora DC. On the other hand, different stomata types in the same epidermis of one species of Baccharis have been previously encountered (Pereira et al.; 2014; Budel et al., 2015; Bobek et al., 2015, 2016; Budel et al., 2018; Ornellas et al., 2019).
Stomata distribution. Baccharis notosergila presents amphistomaty, and the stomata localized at level or elevated above the epidermal cells around them. Both stomata characteristics have been reported in Baccharis by different authors (Metcalfe & Chalk, 1950; Budel et al., 2003, 2004; Budel & Duarte 2007, 2008, 2010; Petenatti et al., 2007; Jasinski et al., 2014; Bobek et al., 2015, 2016; Ornellas et al., 2019). Metcalfe & Chalk (1950) demonstrated that a significant number of plant species have amphistomatic leaves. Mott et al. (1982) suggested that the presence of stomata on both sides of the leaf has an adaptive significance, being a derived character and ecologically correlated with the increased leaf conductance of carbon dioxide in plants living in open areas and full sun environments or with high light intensity, with seasonal variations of water availability in the soil. Mott & Michaelson (1991) established that high light intensity produce amphistomatic leaves in Ambrosia cordifolia (A. Gray) W. W. Payne (Asteraceae), and increase leaf thickness, photosynthetic capacity, and maximum stomatal conductance, because the presence of stomata on adaxial and abaxial sides would be reducing diffusional limitations to photosynthesis. Amphistomatic leaves are common of arid environment with high solar radiation and also it would be a manner to increase photosintetic process, because increase gas exchange proving more efficient when compared to hypostomatic leaves (Roth, 1984; Parkhurst, 1978; Arambarri et al., 2011; Carbone, 2015). Liesenfeld et al. (2019), analyzed morphology and anatomy traits of leaves in 34 species of Asteraceae inhabiting in extreme environmental conditions, and they also reported among the characteristics adaptive to xeric environments, the amphistomatic leaves.
Mesophyll. The isobilateral mesophyll found in B. notosergila has been reported for many Baccharis species (e. g., Oliveira & Bastos, 1998; Budel & Duarte, 2007, 2008, 2010; Petenatti et al., 2007; Jasinski et al., 2014; Barreto et al, 2015; Bobek et al., 2015, 2016; Tosoratto et al., 2016; Budel et al., 2018). Metcalfe & Chalk (1950) noted the relationships among presence of adaxial stomata in plants with isobilateral organization of palisade mesophyll. The correlation among amphistomatic leaves and isobilateral mesophyll was also found by Arambarri et al. (2011). Nughes et al. (2013) studied the behavior of Celtis ehrenbergiana (Klotzsch) Liebm., plants growing under temperate and wet weather with available water, and the leaves exposed to a high light intensity were amphistomatic with isobilateral or homogeneous (palisade parenchyma) mesophyll, whereas the leaves expanded under low light intensity were hypostomatic with dorsiventral mesophyll. Ornellas et al. (2019) had similar results studying six species of Baccharis inhabiting in the high-altitude grasslands, they found the occurrence of stomata and the mesophyll organization seem to be correlated, the amphistomatic leaves tend to be isobilateral.
Trichomes. Glandular trichomes were found in aerial vegetative organs of B. notosergila: (i) Biseriate capitate glandular, (ii) Bulbiferous flagelliform glandular type II, and rarely type I, that accords well with description provided byTosoratto et al. (2016) and Budel et al. (2018), and (iii) Uniseriate capitate glandular which was previously reported by Petenatti et al. (2007) for B. sagittalis (Less.) DC. and B. triangularis Hauman. The biseriate capitate and flagelliform trichomes have been found in nearly all Baccharis species. They have been cited for the genus by Ariza Espinar (1973) who namely “pilóse nests”, and have been reported for B. notosergila by Freire et al. (2007) and Heiden et al. (2009), and in other species of Baccharis (e. g., Cortadi et al., 1999; Budel & Duarte, 2007, 2008; Budel et al., 2003, 2004, 2012, 2015, 2018; Freire et al., 2005; Petenatti et al., 2007; Souza et al., 2011; Jasinski et al., 2014; Pereira et al, 2014; Barreto et al., 2015; Bobek et al., 2015, 2016). For many years, the flagelliform trichomes were described as non-glandular trichome multicellular with a whip-like apical cell with different characteristics (e. g., Ramayya, 1962; Ariza Espinar, 1973; Freire et al., 2007). However, Budel et al. (2012, 2015) reported positive test for lipophilic compounds in basal cells. Tosoratto et al. (2016) studying B. salicifolia described the flagelliform trichomes as glandular bulbiferous flagelliform. Budel et al. (2018) described and illustrated the secreted substances in the body and apical cell of the flagelliform trichomes, and they suggested that these flagelliform glandular trichomes have two functional properties for protection and secretion. They also reported two forms of flagelliform trichomes, type I and type II, with straight and curved body, respectively. Ornellas et al. (2019) also cited the glandular flagelliform trichomes in other species of Baccharis. The lipophilic substances secreted by glandular trichomes and deposited on the leaves form an oily layer that increase the impermeability and reduce transpiration (Haberlandt, 1928). In this way, trichomes help prevent leaf overheating and water loss by transpiration process (Johnson, 1975; Ehleringer & Mooney, 1978; Fahn & Cutler, 1992; García et al., 2008). Glandular trichomes are an important source of essential oils having different uses, although many of these substances have evolved to provide the plant with protection against herbivores and pathogens (Glas et al., 2012). Trichomes may also complement the chemical defense of a plant by possessing internal secretion of phenolics, alkaloids and other repellent substances (Levin, 1973; Delbon et al., 2012). Minteguiaga (2019) studying species of Baccharis found the oil droplets secreted by trichomes are essential oils that have bioactive properties (e. g., garrapaticide, insecticide, fungicide) for example in B. dracunculifolia DC., and B. tridentata Vahl.
Fibers and endodermis. Baccharis notosergila showed scarce fibers in the leaves, frequently reduced to xylem side in the vascular bundles. However, they were abundant in the secondary xylem of the stem. Also, the stem showed the endodermis with Casparian strips surrounding the vascular system. These stem characteristics were also found by Tosorato et al. (2016) in B. salicifolia Nutt. According to Fahn & Cutler (1992) the presence of fibers and endodermis are xeromorphic traits with the function to protect tissues of the dehydration.
Oil bodies. These lipid bodies were identified in the leaf mesophyll of B. notosergila. They were mentioned for the genus by Budel et al. (2018) and Ornellas et al. (2019). According to Pihakaski et al. (1987), there is a seasonal fluctuation in storage lipids, it would be increasing in the growing period. It may be seen large lipid bodies in summer but several small spherules in winter. Lersten et al. (2006) referred the presence of oil bodies in leaf mesophyll cells of many Angiosperms, and indicated the Asteraceae as one of the families in which the highest number of species have lipid bodies. Gidda et al. (2016) reported that environmental conditions such as dark and extreme temperatures (heat and cold) induce oil body formation, so they also suggested leaf oil bodies function in stress response. The function of the leaf oil bodies still are under study, however, Shimada et al. (2014) showed that the leaf oil bodies function as subcellular factories for the production of a novel stable phytoalexin (antimicrobial compounds that are synthesized after stresses) in response to fungal infection and senescence. Shimada et al. (2018) reported that oil bodies are lipid storage compartments that occur primarily in seeds and senescing leaves, and concluded the oil bodies have multiple functions, in seeds, seedlings and in leaf. In the leaves, the energy accumulated in oil bodies has a defensive role against fungal infection because they produce antifungal compounds.
Secretory ducts and chemical substances secreted. The secretory ducts were found in leaves and stems of B. notosergila. They were previously found in the roots of B. notosergila by Carbone et al. (2019). In cross sections of leaves, frequently, there is one duct associated to each vascular bundle, but may be seen two or three at middle vein level and coincidently in the main vascular bundle of the petiole. The presence of more than one duct was previously documented by Budel et al. (2018) and reported by Ornellas et al. (2019) for B. platypoda DC. and B. stylosa Gardner. It has been established for Asteraceae the principal substances secreted are volatile oils (Budel et al., 2012; Jasinski et al., 2014). The secretory ducts in B. notosergila have given positive reaction for resins, polyphenols, and oils. It was also found in B. notosergila subterranean system by Carbone et al. (2019), and it is in agreement with Ariza Espinar (1973) who reported that the secretory ducts may be releasing other chemical components such as tannins and resins besides essential oils. The resins could protect the plant against insects as occur in coniferous (Johnson & Croteau, 1987). Cobos et al. (2001) analyzed the composition of the essential oils in aerial organs of B. notosergila, and they found 32 constituents and a-pinene, limonene, P-caryophyllene, and the spathulenol as the major components. Budel et al. (2018) and Minteguiaga (2019) indicated that Baccharis species produce volatile oils that mainly contain sesquiterpenes and monoterpenes. Internal secretion of terpens and other secondary metabolites (e. g., flavonoids, tannins, resins) would help protect the plant from herbivores and pathogens, and contributing to the water balance (Fahn & Cutler, 1992; Delbon et al., 2012; Tosoratto et al., 2016). Essential oils components are produced in most plant organs and they are stored in secretory structures (glandular trichomes and internal ducts). Chemically essential oils are complex mixture which have biology effects (e.g., antimicrobial, analgesic, antioxidants, sedative, antiinflamatory, and mutagenic, phototoxic and citotoxic) (Budel & Duarte, 2008; Minteguiaga, 2019). In Baccharis has been isolated and identified more than 500 compounds, the most frequent would be essential oils, terpenoids, and flavonoids (Minteguiaga, 2019).
Phenolic compounds. Tannins were identified in the epidermal tissue and mesophyll of leaves. In stem in the secondary protection tissues, and were also found in the secondary xylem which is in agreement with the localization reported by Carbone et al. (2019) in the xylopodium of B. notosergila. Similar results were reported in different species of the genus by Tosoratto et al. (2016), Budel et al. (2018), and Ornellas et al. (2019). Tannins are phenolic compounds of high molecular weight, and may be found in all organs of plants. The presence of tannins is an adaptation to the high incidence of sunshine to protect against oxidative stress affecting the photosynthesis (Hassanpour et al., 2011). The sites of tannin production were the large cells located in the leaf mesophyll, plants can accumulate phenolic compounds in the leaf epidermis and mesophyll tissues, where its protective feature became evident against the UV radiation damage (Hassanpour et al., 2011). Del Valle et al. (2020) established the UV radiation increased the concentration of phenolic compounds (tannins, flavonoids, anthocianins) suggesting a photoprotective role against UV light. On the other hand, secondary metabolites of phenolic nature play an important role in the defensive response, e. g., phytoalexins who behave as inducer or elicitors of plant defense mechanisms (Ebel, 1986; Boller, 1989; Stone, 1989). Currently, research suggests that phytoalexins are a biochemical mechanism possessed by plants for disease tolerance and pathogen attacks (Ryan, 1987).
By other way, during the fieldwork was found a reduction of Stipa population around the B. notosergila plants (M. Oyhamburu & F. Fernández, personal communication, October 20, 2020). This is a new line to research, on the basis that numerous compounds of phenolic nature act as allelopathic agents inhibiting the germination and growth of other species around plants (Rice, 1984). Although there are few reports the Baccharis species allelopathic effects, Dias et al. (2017) probed an allelopathic potential of phenolic compounds using an ethanolic and aqueous extracts from aerial parts of Baccharis spp.
Starch grains. These were identified in the guard cells of stomata, in the chlorenchyma of leaves and stems, in the last also in the secondary phloem. However, this polysaccharide was not detected in subterranean organs (Carbone et al., 2019). We did not find starch grains in the parenchyma sheath surrounding the vascular bundles as was cited by Budel et al. (2018). Thus, we believe the starch grains presence and quantity is variable in relation with the period of the year in which the leaves and aerial stems are collected.
Calcium oxalate crystals (CaOx). Crystals were found in the mesophyll of leaves, in pith and medullary rays of stems, and in the head cells of the glandular capitate trichomes of B. notosergila. Carbone et al. (2019) recorded abundant styloids in medullary rays of the xylopodium and roots of the same species. These crystal types and others were revealed for the genus by previous authors, coincidentally in the mesophyll of leaves, and the perimedullary region of stems (Cortadi et al., 1999; Budel et al., 2003, 2004; Petenatti et al., 2007; Budel & Duarte, 2010; Jasinski et al., 2014; Bobek et al., 2015, 2016; Budel et al., 2015, 2018). Many reasons have been written about the formation and functions of CaOx in plants, such as the functions attributed to the calcification process include the elimination of oxalate in those plants unable to metabolize it, protection against herbivores (Molano-Flores, 2001), being a source of calcium reserve (Volk et al., 2002), the detoxification of heavy methals (Nakata, 2003). Franceschi & Nakata (2005) communicated the crystals are formed in specific shapes and sizes, and genetic regulation of CaOx formation is indicated by constancy of crystal morphology within species. The same authors, also mentioned as the major functions of CaOx crystals in plants include high-capacity calcium regulation, and protection against herbivores. Apóstolo (2005) reported high pH values in the soil promote formation of crystals in plants, and Garcia et al. (2008) indicated the presence of crystals in leaf mesophyll would be associated to saline/alkaline soils. In B. notosergila, we have seen small crystals in mesophyll cells of leaves collected in sweet lowland and half hill, but not observed in the leaves from alkaline-saline soils. On the basis of these data we infer that there might be several factors promoting the formation of calcium oxalate crystals.
Conclusions
This study allows us to achieve an interpretation about the adaptive characteristics of B. notosergila which shows higher stress tolerance and competitive ability. A number of meso-xeromorphic characters are found in B. notosergila, such as small, narrow and deciduous leaves; the hydathodes presence;
epidermal cells with straight cell walls; massive and striate cuticle; glandular trichomes secreting oily substances; stomata at level or above the other epidermal cells; presence of tannins in leaves and stem tissues; oily bodies in mesophyll cells; a moderate quantity of fibers; presence of endodermis with Casparian strips; amphistomatic leaves and isobilateral mesophyll. Among them the epidermal tissue, cuticle and epicuticular waxes covered by an oily layer, and presence of tannins would be a physical and chemical barrier for dehydration as well as the penetration of the herbicides applied for its control. The mentioned characteristics adding the abundant lipophilic substances, the presence of fibers and endodermis in stems would be producing tolerance to lowland areas and the alternate of drought and flooding periods which are common in the studied region. The stomata localized at level or slightly elevated and scarce sclerenchyma are mesomorphic traits corresponding to a luminous temperate weather in which B. notosergila inhabit, and the combination amphistomatic leaves with isobilateral mesophyll promote more efficient photosynthesis process. As a matter of fact, leaves are present during spring-summer, with favorable environmental conditions contributing plants produce as many resources as possible to storage in its deep root system, ensuring the survival during the unfavorable period, and providing the capacity to sprout and resprout from the bud-bank (xylopodium), restoring the plant aerial parts in the following spring.
Author contributions
AVC provided the global research ideas and goals, and with FEF provided the resources and fieldwork data and interpretation. MPH, SMMA and AMA carried out the laboratory research. AMA, AVC and MPH prepared the first manuscript. All authors have read and contributed to write the final manuscript.
Acknowledgements
We thank the technicians Mario Sánchez and Mariela Theiller of CINDECA-CONICET-UNLP for the electron microscope service.












 uBio
uBio 

