Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista industrial y agrícola de Tucumán
On-line version ISSN 1851-3018
Rev. ind. agric. Tucumán vol.90 no.1 Las Talitas June 2013
TRABAJO YA PUBLICADO
Effect of sugar cane trash blanketing on the development of microorganisms of agronomic and environmental interest
María L. Tortora*, Lucía Vera*, Noel Grellet Naval*, Juan Fernández de Ullivarri*, Patricia A. Digonzelli* and Eduardo R. Romero*
*Sección Caña de Azúcar, EEAOC. ltortora@eeaoc.org.ar
Abstract
The global sugar industry is progressively moving away from pre-harvest burning to a green-cane harvesting system. It is well known that when harvest residue is returned to the soil, nutrients and organic matter increase and soil structure is improved. However, the effect of trash blanketing on the development of different soil microorganisms has not been evaluated in Tucumán, Argentina. Hence, the aim of this study was to evaluate changes in microbial populations, especially those of agronomic and environmental interest, which occur under two management situations: with and without trash blanketing. Tests were performed at Finca San Genaro, located in eastern Tucumán (Dpto. Leales), using LCP 85-384 sugar cane variety at fourth ratoon age. This means that treatments started four years before sampling. During the 2011/2012 crop cycle, in June, July, November 2011 and May 2012, soil and different tissue samples from sugar cane roots and stems were microbiologically analyzed. Microorganisms were counted using different culture media: LB for mesophilic aerobic bacteria, PGA for fungi and yeasts, CA for Pseudomonas sp., and different N-free semisolid media for micro aerobic nitrogen fixing bacteria. It was observed that trash blanketing increased the number of yeast, fungus and Pseudomonas sp. populations in soil samples during high temperature seasons. Some of these isolated fungi showed ligninolytic activity and some Pseudomonas genus bacteria were able to solubilize phosphorus, thus indicating that these microorganisms may be involved in residue decomposition. Interestingly, trash blanketing also increased the number of nitrogen fixing bacteria associated with the plant root and stem tissues from June to February. The further development of trash degrading microorganisms and a better colonization of sugar cane tissues by nitrogen fixing bacteria could improve sugar cane crop growth and development.
Key words: Sugar cane field; Trash blanket; Microbial communities.
Resumen
Efecto del residuo agrícola de la cosecha en verde de la caña de azúcar en el desarrollo de microorganismos de importancia agrícola y ambiental
En la actualidad, la industria azucarera mundial tiende a reemplazar la quema del cañaveral previo a la cosecha, por el sistema de caña verde. Trabajos demuestran que cuando el residuo agrícola de cosecha (RAC) regresa al suelo, aporta nutrientes, materia orgánica y mejora su estructura. Sin embargo, el efecto del RAC en el desarrollo de microorganismos del suelo aún no ha sido evaluado en Tucumán, R. Argentina. Por esta razón, el objetivo de este trabajo fue evaluar los cambios que ocurren en el desarrollo de microorganismos de importancia agrícola y ambiental, en dos situaciones de manejo del suelo: con y sin mantenimiento de cobertura con RAC. Los ensayos se realizaron en el Dpto. Leales, Tucumán, utilizando la variedad LCP 85-384 en la edad de soca 4; es decir, los tratamientos se establecieron cuatro años antes del muestreo. Para el análisis microbiológico, se tomaron muestras de suelo y de diferentes tejidos durante los meses de junio, julio, noviembre 2011 y mayo 2012. El recuento de microorganismos
se realizó utilizando diferentes medios de cultivo: LB para aerobios mesófilos totales, APG para hongos y levaduras, AC para Pseudomonas sp. y medios de cultivo semisólidos libres de N2 para bacterias microaeróbicas fijadoras de nitrógeno. En forma general, se observó que la cobertura con RAC aumentó el número de hongos, levaduras y Pseudomonas sp. durante las épocas con temperaturas más altas. Algunos hongos presentaron actividad ligninolítica y algunas Pseudomonas sp. fueron capaces de solubilizar fósforo, lo que indica que estos microorganismos podrían estar involucrados en la descomposición del residuo. Fue interesante observar que la cobertura con RAC también incrementó el número de bacterias fijadoras de nitrógeno asociadas a raíces y tallos, de junio a febrero. El mayor desarrollo de microorganismos degradadores de materia orgánica y una mejor colonización de los tejidos por bacterias fijadoras de nitrógeno podrían mejorar el crecimiento y desarrollo del cañaveral.
Palabras clave: Cañaveral; Cobertura; Comunidades microbianas.
![]()
Introduction
Sugar cane has high agricultural and economic importance in Tucumán, Argentina, and 217.000 ha are currently planted with this crop (Romero et al., 2009). However, it is well known that continuous sugar cane planting in fields and the burning of crop residues reduce concentrations of soil organic matter, which results in soil structural degradation. In an attempt to improve soil health, the practice of pre-harvest burning is being gradually replaced with green-cane harvesting practices all around the world.
During green sugar cane harvesting, 7 to 30 tons of dry matter, depending on the variety and mainly on field productivity level, remains on the field. Residue management implies preserving it on the soil as mulching (trash blanketing), mixing it with the most superficial soil layers, or removing it mechanically (Digonzelli et al., 2007; Digonzelli et al., 2009). It has been demonstrated that when harvest residue is returned to the soil, contents of nutrients and organic matter increase and soil structure is improved. This management system allows the return of an important quantity of vegetable residues to the soil, favoring nutrient recycling, reducing both water and wind erosion, diminishing soil water evaporation, increasing infiltration and allowing a better conservation of soil moisture, while also reducing soil temperature in topsoil profile and favoring meso and microflora proliferation (Braunack and Ainslie, 2001; Scandaliaris et al., 2002; Thorburn et al., 2004; Digonzelli et al., 2007; Núñez and Spaans, 2007; Romero et al., 2007; Sanzano et al., 2009; Digonzelli et al., 2011a; Digonzelli et al., 2011b).
A sensitive indicator of soil organic matter dynamics is microbial biomass, because it changes relatively rapidly with C supply alterations and differences are detected before they can be measured by total organic matter content (Gregorich et al., 1997; Sparling, 1997). However, soil quality is strongly influenced by microorganism mediated processes,
and measurement of microbial biomass gives no indication of microbial activity. Considering that until now there have not been any local studies showing the effect of sugar cane
trash blanketing on the development of different soil microorganisms, this study aimed to evaluate changes in microbial populations, especially those of agronomic and environmental interest, which are produced under two different situations: sugar cane management with and without trash blanketing.
Materials and methods
The trials were conducted in a commercial field located at San Genaro (Dept. Leales), Tucumán, Argentina (27º 14'18" LS and 65º 12' 57" LW). This site is in the dry-subhumid saline depressed plain region, characterized by an annual mean temperature of 19ºC and a mean annual rainfall of 750 mm to 800 mm. There is also a moderate water deficit from August to October, which is a climatic limitation for sugarcane growing. Soils are non saline typic Haplustol, with silty loam texture in surface and clay loam at deeper levels, moderately well drained.
The evaluated variety was the main cultivar in Tucumán, LCP 85-384, which is planted in 76.65% of the whole sugarcane area (Ostengo et al., 2012). The trial was planted in 2007 and kept under conventional agronomic management practices: post-emergence herbicides were used for weed control, as well as nitrogen (urea) for fertilization (applied at a 115 kg/ha rate), and irrigation was not supplied.
Evaluated treatments at the site included: i) harvest residues left on the soil surface, and ii) removal of residues with a fork. To ensure that soil quality was influenced by trash blanket, plots were kept under green cane system four years before sampling. Therefore, microbiological analyses were performed during the 2011/2012 crop cycle in June,
July, November 2011 and May 2012, on samples of cane at its fourth ratoon age. The experimental design was completely randomized with four replications. Experimental plots consisted of five 1.6 m rows which were 10 m long.
Each treatment was sampled with four replicates that were processed separately. Soil and sugar cane root and stem tissue samples were collected from different spots in each row. For the analysis of soil and tissue microbial communities, viable plate counts on selective media (Alef and Nannipieri, 1995) were used to estimate populations of culturable bacteria, fungi and yeasts. Microorganisms were counted using different solid culture media: Luria Bertani (LB) for mesophilic aerobic bacteria, potato glucose agar (PGA) for fungi and yeasts, and cetrimide agar (CA) for Pseudomonas sp. Different semisolid culture media were used for enumeration and isolation of micro aerobic nitrogen fixing bacteria: N2 free malate (NFb) for Azospirillum sp., LGI-P for Gluconoacetobacter sp. and Pantoea sp., and JMV for Burkholderia sp. Some bacteria that grew in CA solid medium were selected to evaluate phosphate solubilization activity, by plating them on NBRIP medium (Nautiyal, 1999). Positive results were indicated by the formation of a Clear halo around the colonies. For the isolation of microorganisms from sugar cane trash, residues were cut into 2 cm long fragments that were washed three times with sterile distilled water, and others that were sterilized with EtOH 70% (v/v) during 1 min, and then with NaClO 3% (v/v) during 3 min. After drying fragments with sterile papers, they were placed on Petri dishes containing PGA solid medium, supplemented with guaiacol 0.1% (v/v) to test ligninolytic activity. Brown dark halos surrounding fungi and yeast colonies indicated ligninolytic activity. Different fungi with ligninolytic activities were used as positive controls.
Results were analyzed with the Statistix program (Analytical Software, 1996). The LSD test was used to determine the arithmetic mean (significance level: 0.05) and the analysis of variance test (ANOVA) was performed to evaluate data dispersion with respect to the mean value.
Results and discussion
In general, we observed that when harvest residues were left on soil surface, changes in microbial population were induced depending on the season of the year. During cold weather months, trash blanket reduced the number of soil microorganisms, especially mesophilic aerobic bacteria, fungi and yeasts (Figure 1).

Figure 1. Count of culturable microorganisms in soil samples collected in different seasons. (- - -) and ( ------ ) lines represent the content of microorganisms in soil samples from fields without trash blanket (WTB), and from fields with trash blanket (TB), respectively. Mesophilic aerobic bacteria were counted on LB solid medium, fungi and yeasts on PGA solid medium, and Pseudomonas genus bacteria on CA solid medium.
This could be explained by the fact that during cold weather months, trash blanket keeps soil temperature lower as compared to bare soil (Chapman et al., 2001; Digonzelli et al., 2009; Morandini et al., 2009; Digonzelli et al., 2011b). A different situation was observed in November. In this case, when soil temperatures tended to be equal, trash blanket increased the number of fungi, yeasts and Pseudomonas genus bacteria (Figure 1). These microorganisms were probably involved in residue decomposition. In June, after sugar cane harvest, stalk samples were taken from different plots to analyze the content of different endophytic microorganism populations, especially those having nitrogen fixing activities (Figure 2).
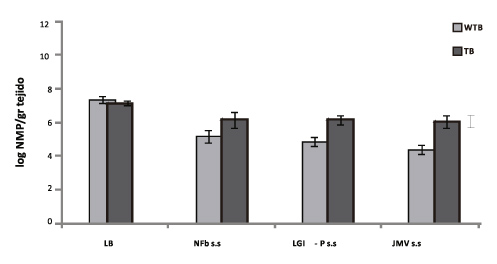
Figure 2. Count of different endophytic microorganisms in stalk samples collected in June 2011. Black and grey bars represent microorganism populations present in stalk samples from fields without trash blanket (WTB), and with trash blanket (TB), respectively. Mesophilic aerobic bacteria were counted on LB solid medium, and microaerobic nitrogen fixing bacteria were counted on different semisolid medium: Azospirillum sp. on NFb, Gluconoacetobacter and Pantoea on LGI-P, and Burkholderia sp. on JMV. Different letters indicate significant differences at PM0.05.
As observed in Figure 2, when residues were preserved on the soil as a cover, stalk endophytic colonization by nitrogen fixing bacteria was favored, in contrast to what happened with stalks collected from fields without a trash blanket. These results are of fundamental importance, if we consider that sugar cane crops have high nitrogen requirements and that endophytic nitrogen fixing microorganisms provide plants with a great proportion of the nitrogen they need to grow and develop (Boddey et al., 2003).
Similar results were observed when comparing nitrogen fixing microorganism populations found in sugar cane stems from fields with and without trash blanket (Figure 3).
Finally, we analyzed the effect of residue preservation on the development of different rhizosphere microorganisms associated with roots of sugar cane plants growing on soils
with and without trash blanket (Figure 4).

Figure 3. Count of culturable microorganisms on stem samples collected in different seasons. (- - -) and ( ------ ) lines represent microorganism contents in stem samples collected from fields without trash blanket (WTB), and fields with trash blanket (TB), respectively. Mesophilic aerobic bacteria were counted on LB solid medium, fungi and yeasts on PGA solid medium, and nitrogen fixing bacteria on NFb semisolid medium.
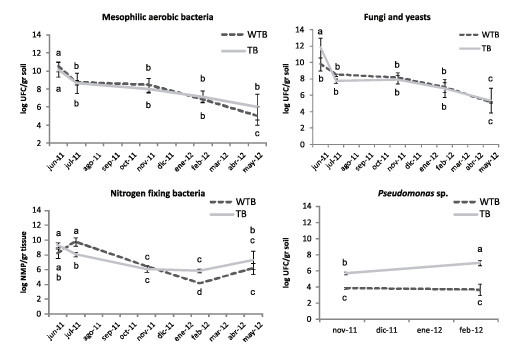
Figure 4. Count of rhizosphere microorganisms in samples collected in different seasons. (- - -) and ( ------ ) lines represent rhizosphere microorganism populations in sugar cane root samples collected from fields without trash blanket (WTB), and fields with trash blanket (TB), respectively. Mesophilic aerobic bacteria were counted on LB solid medium, fungi and yeasts on PGA solid medium, Pseudomonas sp. on CA solid medium, and nitrogen fixing bacteria on NFb semisolid medium.
In June, we observed that plant roots in fields under the trash blanket were colonized by a great number of fungi, yeasts and nitrogen fixing bacteria, in comparison with roots of sugarcane kept in bare soils. Although from July to November the number of rhizosphere microorganisms was not statistically different between treatments, we observed a significant increase in Pseudomonas genus bacteria, whose number remained high both in November and May. This result coincided with what was observed in soil samples
analyzed in the same season of the year (Figure 1).
Some bacteria that grew on CA solid medium were tested for their ability to solubilize phosphorus, by plating them on NBRIP medium. Five bacteria isolated from soil samples and three from stalk samples were able to solubilize phosphorus, as evidenced by clear halos surrounding the bacterial colonies (Figure 5). However, the ability to solubilize phosphorus depended on the origin of the strains: after seven days of incubation, isolates from soil samples had a better performance in solubilizing phosphate than those from stalks (Figure 5). This could be explained by the fact that endophytic bacteria live in a protected environment inside the host plant, and have all the nutrients they need for survival. Therefore, bacterial mechanisms for phosphate solubilization must be less efficient than in the rhizospheric environment. Although soil phosphate content was not statistically different between treatments, we observed that isolates from trash blanketed fields solubilized tricalcium phosphate better than isolates from soil of fields without residue cover.
Isolation of fungi and yeasts from sterile and non sterile sugar cane residues was performed on PGA solid medium (Figure 6). After seven days of incubation at 30ºC and under both sterile and non sterile conditions, we observed a very low diversity of fungi and yeasts, indicating that there are only a few species that were able to survive and which remained in sugar cane residues.

Figure 5. Phosphate solubilization activity of different strains isolated from soil and sugar cane samples collected in fields with (TB) and without (WTB) trash blanket.
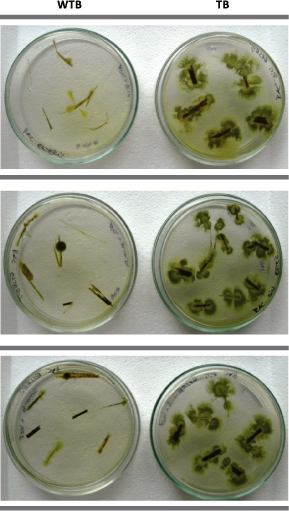
Figure 6. Fungus and yeast growth on PGA solid medium from sterile and non sterile trash fragments, after seven days of incubation at 30ºC.
When analyzing trash composition, we observed that it consisted of approximately 35% cellulose, 40% hemicellulose and 25% lignin. Many microorganisms are known to be able to degrade and utilize cellulose and hemicellulose as carbon and energy sources, but only a much smaller group of filamentous fungi has the ability to break down lignin, the most recalcitrant component of plant cell walls (Sanchez, 2008). Considering this, representative fungi and yeasts observed above were plated on PGA solid medium upplemented with guaiacol 0.1 % (v/v), in order to analyze their ligninolytic activities. Three fungal strains with proven ligninolytic activities were used as positive controls.
Only one of the tested strain isolates from the sterile trash sample (named as sterile trash strain 2) exhibited a small brown halo during the incubation period (Figure 7). Unlike
control strains, the slow growth of this fungal strain could serve as an explanation for the small halo observed around the colony (Figure 7).
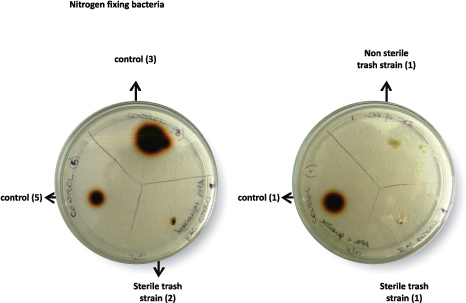
Figure 7. Ligninolytic activity of fungal strains isolated from sterile and non sterile trash fragments, after seven days of incubation at 30ºC. Tests were performed on PGA solid medium, supplemented with guaiacol 0.1% (v/v).
Conclusions
We conclude that when sugar cane harvest residues remain over the soil, they have strong effects on microbial communities in the soil and in different sugar cane tissues, but that is restricted to high temperature months. Our results indicate that trash blanket promotes the growth of soil and rhizosphere microorganisms, some belonging to the Pseudomonas genus, which have the ability to solubilize phosphorus. Trash blanket also increases the number of rhizosphere and endophytic nitrogen fixing bacteria capable
of colonizing inner sugar cane stalk and stem tissues. A higher amount of nitrogen and available phosphorus could promote a better plant growth under nutrient-limited conditions.
The great number of soil fungi and yeasts recorded when residues were kept over the soil could serve as an explanation for the high residue decomposition rates observed
during high temperature months. This hypothesis correlates with the ligninolytic activity observed in some fungal strain isolates from sterile trash fragments.
Acknowledgments
We thank Dr. Carlos Gusils for providing unrestricted access to all of the laboratory reagents and equipment under his charge.
Cited references
1. Alef, K. Y. and P. Nannipieri. 1995. Methods in applied Soil Microbiology and Biochemistry. Academic Press Inc., NY, USA. [ Links ]
2. Boddey, R. M.; S. Urquiaga; B. J. R. Alves and V. Reis. 2003. Endophytic nitrogen fixation in sugarcane: present knowledge and future applications. Plant and Soil 252 (1): 139-149. [ Links ]
3. Braunack, M. and H. Ainslie. 2001. Trash blanket and soil. physical properties: Mackay experience. Proc. Aust. Soc. Sugar Cane Technol. 23: 154-160. [ Links ]
4. Chapman, L. S.; P. L. Larsen and J. Jackson. 2001. Trash conservation increases cane yield in the Mackay District. Proc. Aust. Soc. Sugar Cane Technol. 23: 176-184. [ Links ]
5. Digonzelli, P. A.; J. Férnandez de Ullivarri; E. R. Romero; J. Giardina; L. Alonso; S. Casen; J. Tonatto; M. F. Leggio Neme and J. Scandaliaris. 2009. Assessment of two sugarcane management systems: with or without post-green cane-harvest residue retention. In: Proc. ISSCT Agronomy Workshop, 8, Uberlandia, Brasil, pp. 27-28. [ Links ]
6. Digonzelli, P. A.; E. R. Romero; L. Alonso; J. Férnandez de Ullivarri; H. Rojas Quinteros; S. Fajre and J. Scandaliaris. 2011a. Assessing a sustainable sugarcane production system in Tucumán, Argentina. Part 1: Dynamics of sugarcane harvest residue (trash) decomposition. Rev. Ind. y Agríc. de Tucumán 88 (1): 1-12. [ Links ]
7. Digonzelli, P. A.; J. Scandaliaris; M. J. Tonatto; A. Giardina; S. D. Casen; M. F. Leggio Neme y E. R. Romero. 2007. La caña verde: un aporte a la sustentabilidad de la producción de caña de azúcar. II- Alternativas y equipos para el manejo del cañaveral sin quema. Avance Agroind. 28 (4): 16-20. [ Links ]
8. Digonzelli, P. A.; M. J. Tonatto; E. R. Romero; G. A. Sanzano; J. Fernández de Ullivarri; J. A. Giardina and J. Scandaliaris. 2011b. Assessing a sustainable sugar cane production system in Tucumán, Argentina. Part 2: soil water and thermal regime, stalk population dynamics and sugarcane production. Rev. Ind. y Agríc. de Tucumán 88 (2): 1-12. [ Links ]
9. Gregorich, E. G; M. R. Carter; J. W. Doran; C. E. Pankhurst and L. M. Dwyer. 1997. Soil quality for crop production and ecosystem health. In: Gregorich, E. G. and M. R Carter (eds.), Biological attributes of soil quality, Elsevier Science Publishers, Amsterdam, the Netherlands, pp. 81-113. [ Links ]
10. Morandini, M.; C. Hernández; H. Rojas Quinteros y G. A. Sanzano. 2009. Efecto de la conservación de residuos de cosecha de la caña de azúcar en la temperatura de un suelo Argiudol típico de la Llanura Chacopampeana sub húmeda-húmeda (Tucumán-Argentina). Rev. Ind. y Agríc. de Tucumán 86 (1): 15-23. [ Links ]
11. Nautiyal, C. S. 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 170: 265-270. [ Links ]
12. Núñez, O. and E. Spaans. 2007. Evaluation of greencane harvesting and crop management with trash blanket. In: Proc. ISSCT Congress, 26, Durban, South Africa, pp. 131-142. [ Links ]
13. Ostengo, S.; M. A. Espinosa; M. B. García; N. Delgado y M. I. Cuenya. 2012. Distribución varietal del cultivo de la caña de azúcar y aplicación de otras tecnologías en la provincia de Tucumán. Relevamiento de la campaña 2010/2011. Gac. Agroindustrial EEAOC (76). [ Links ]
14. Romero, E. R.; P. A. Digonzelli; L. Alonso; J. Fernández de Ullivarri; G. A. Sanzano; J. Scandaliaris y H. Rojas Quinteros. 2007. La caña verde: un aporte a la sustentabilidad de la producción de caña de azúcar. I- Consideraciones generales. Avance Agroind. 28 (4): 11-15. [ Links ]
15. Romero, E. R.; J. Scandaliaris; P. A. Digonzelli; M. F. Leggio Neme; J. A. Giardina; J. Fernández de Ullivarri; S. D. Casen; M. J. Tonatto y L. Alonso. 2009. La caña de azúcar. Características y morfología. In: Romero, E. R.; P. A. Digonzelli y J. Scandaliaris (eds.), Manual del Cañero, EEAOC, Tucumán, R. Argentina, pp. 15-22. [ Links ]
16. Sanchez, C. 2008. Lignocellulosic residues. Biodegradation and bioconversion by fungi. Biotechnol. Adv. 27 (2): 185-194. [ Links ]
17. Sanzano, G. A.; F. A. Sosa; C. F. Hernández; M. Morandini; H. Rojas Quinteros; J. I. Romero and P. A. Digonzelli. 2009. Evaluación de la erosión hídrica en caña de azúcar con y sin cobertura de maloja. Avance Agroind. 30 (3): 16-18. [ Links ]
18. Scandaliaris, J.; F. Pérez Zamora; M. Rufino; E. R. Romero y M. Morandini. 2002. La cosecha en verde como estrategia para disminuir el impacto ambiental de la caña de azúcar. Avance Agroind. 23 (1): 14-17. [ Links ]
19. Sparling, G. P. 1997. Biological indicators of soil health. In: Pankhurst, C.; B. M. Double and V. Gupta (eds.), Soil microbial, biomass activity and nutrient cycling as indicators of soil health. CAB International, Wallingford, pp. 97-119. [ Links ]
20. Thorburn, P. J.; H. L. Horan and J. S. Biggs. 2004. The impact of trash management on sugar cane production and nitrogen management: a simulation study. Proc. Aust. Soc. Sugar Cane Technol. (CD ROM) 26: 12. [ Links ]














