Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Acta toxicológica argentina
On-line version ISSN 1851-3743
Acta toxicol. argent. vol.14 no.2 Ciudad Autónoma de Buenos Aires Aug./Dec. 2006
Drinking water: problems related to water supply in Bahía Blanca, Argentina
Echenique Ricardo1, Giannuzzi Leda2 and Ferrari Luis3
1-Departamento Científico Ficología, Facultad de Ciencias Naturales y Museo (UNLP)* - Paseo del Bosque s/nº, 1900 La Plata, Argentina;
2-Cátedra de Toxicología y Química Legal, Facultad de Ciencias Exactas (UNLP) - 47 y 115, 1900 La Plata, Argentina;
3-Facultad de Ciencias Exactas. Universidad de Morón, Bs As., Argentina
Abstract: In April 2000, alterations in drinking water quality, such as turbidity and odors similar to those of organochloride pesticides, were detected at Bahía Blanca (Argentina). This fact was associated to reports of dermic reactions and respiratory problems in the population. Samples drawn from Paso de las Piedras reservoir, two water treatment plants of at inlet and outlet points and at several particular houses were analyzed. Total phytoplankton in the reservoir varied between 49440 and 84832 cells.ml-1, while dominant species was, Anabaena circinalis. Efficiency to remove microorganisms (ER) by the treatment plants considering cell number of total phytoplankton was analyzed. Bacteriological analysis, qualitative and quantitative studies of phytoplankton, pesticides, THM (trihalomethanes) and BTEX (benzene, toluene, ethylbenzene and xylene) analysis were carried out in samples of domestic supply and in the treatment plants. Even though samples were bacteriological potable, Anabaena circinalis and Microcystis aeruginosa algae were found at high concentrations in some cases. No pesticides were detected. THM and BTEX were below guideline values into recommendations for drinking water. Geosmin was responsible for detected odors. Besides, Copaene, another volatile metabolite, which on account in its structure could be considered a Geosmin precursor, was also detected. In Argentina, this was the first report of Cyanobacteria presence in drinking water.
Key words: Cyanobacteria; Phytoplankton; Blooms; Biogenic odour compounds.
Resumen: Agua potable: problemas en el abastecimiento de la ciudad de Bahía Blanca, Argentina. En abril de 2000, en Bahía Blanca (Argentina), se detectaron alteraciones en el agua de bebida, turbidez y olor semejante a "Gamexane". Esto coincidió con la aparición de problemas dérmicos y respiratorios en la población. Para determinar el origen del problema, se analizaron muestras, tomadas en el embalse Paso de las Piedras, en las plantas potabilizadoras y en varios domicilios particulares. En el embalse, la densidad celular del fitoplancton, fluctuó entre 49440 y 84832 cél/ml-1, muy por encima de los valores de referencia. Anabaena circinalis en altas concentraciones fue la especie dominante (48320 - 84032 células por mililitro). Se cuantificó la eficiencia de las plantas de tratamiento (algas-entrada/algas-salida). Anabaena circinalis y Microcystis aeruginosa fueron encontradas a la entrada y a la salida de las plantas, indicando una baja eficiencia de las plantas de tratamiento. Para el agua domiciliaria, se realizaron estudios fitoplanctonicos; análisis bacteriológicos; presencia de plaguicidas, trihalometanos y BTEX. Si bien, bacteriológicamente el agua era potable, se encontraron Cyanobacteria en algunos casos en concentraciones muy altas. La presencia de pesticidas resultó negativa. La media de los THM y de los valores de BTEX resultaron menores a valores guías para agua potable. Se detectó la presencia de Geosmina, resultando el responsable del fuerte olor. Asimismo, se halló otro metabolito volátil, Copaene, que por su estructura pudiera llegar a considerarse precursor de la Geosmina. Este es el primer reporte de la presencia de cianobacterias en aguas de consumo humano en Argentina.
Palabras clave: Florecimiento; Fitoplancton; Compuestos odoríferos; Cianobacterias.
Introducción
Cyanobacteria produce a diverse range of secondary metabolites including hepatotoxins, neurotoxins and cytotoxins. Several notable works deal with toxins and the species responsible for their production (1-3). Literature is mainly devoted to analyze cyanobacterial toxins in freshwater and the resulting acute or chronic poisoning, and even death, in animals (4-7), health problems and the impairment of water supply as well as water-based-activities (recreation, tourism, aquaculture). Human health incidents associated with cyanobacterial toxins, largely in freshwater, are discussed in several reviews (3, 6, 8 and 9). Incidents include gastroenteritis outbreaks and respiratory problems after contact or with ingestion of water containing toxic blooms or scum during drinking and recreation. Incidents were recorded after single or relatively short-term exposures to cyanobacterial blooms, scums and toxins. Laboratory assays with cobayos/Guinea pigs demonstrated that these toxins were teratogenic and promoters of liver tumors (6, 8, 10-12). Consequences of long term exposure to cyanobacterial toxins are under investigation in China, where a higher incidence of primary liver cancer among communities drinking untreated surface water is suspected to be associated with the ingestion of microcystin toxins (8). Phytoplanktonic communities respond to eutrophication with a loss of biodiversity followed by a dominance of blue-green algae and the occurrence of water blooms. Reports of toxic Cyanobacteria are available from at least 44 countries and from the Baltic and Caribbean Seas and Atlantic, Pacific and Indian Oceans (1, 2). In Argentina one of the most common taxa found in fresh water and reservoirs as consequences of eutrophication is Anabaena circinalis (12, 13). Pizzolon, 1996 (14) reported 16 continental water environments in Argentina under intoxication hazards due to Cyanobacteria, being the main responsible species of Microcystis and Anabaena genera. However, the occurrence of toxic cyanobacterial species in drinking water supply has not been sufficiently studied, due perhaps to the difficult identification of these organisms and toxins.
Farlow (1983) (15) stressed that blue-green algae were responsible for water tastes and odors generally described as muddy or earthy. A relationship between blue-green algae and off-flavors in water has shown that two metabolites, geosmin and methylisoborneol are the major contributors to unpalatable flavors in water (9, 16 and 17). Besides, additional substances like trihalomethanes (THM), benzene, toluene, ethylbenzene and xylenes (BTEX) may be present either due to Cyanobacteria decomposition or as metabolites.
In the present work we analyze an incident occurred in Bahía Blanca (Argentina) during April and May, 2000 where a strong odor was detected both in the dam area and in the city. This odor was similar to that produced by the commercial organochlorine pesticide and was related to the presence of great amount of particulate materials in the drinking water.
The objectives of the present work were
a. To identify and quantify algae, bacteria and compounds with toxicological interest found in samples drawn from the reservoir, the water treatment plant and tap water of the city of Bahía Blanca, Argentina.
b. To evaluate the presence of volatile biogenic compounds in the samples.
Material and Methods
Paso de las Piedras reservoir is located at the south of Buenos Aires Province, Argentina (61º12’ West and 38º 22’ South), with a surface of 27.52 Km2, provides drinking water to the cities of Bahía Blanca and Punta Alta, with a population of approximately 400.000 people. Water is treated in Grünbein (P1) and Planta Patagonia (P2) both located in the area. A total of 20 samples were collected from different points of the reservoir, the treatment plants and the distribution network. Samples were collected near the dam, at the input and output points of the treatment plants P1 and P2 and at 14 randomly selected particular houses of Bahía Blanca city.
1. Phytoplankton microscopical analysis
Qualitative studies of phytoplankton were performed on samples drawn from the reservoir and the treatment plants; with a 30 µm pore plankton net. For the quantitative analysis samples were obtained with van Dorn bottles from the reservoir and from treatment plants and directly from the inlet tap at each assigned home. Qualitative samples were analyzed "in vivo" with a photonic microscope Wild M20. Quantitative samples were fixed with 1% lugol solution and observed with an inverted microscope Carl Zeiss following Utermöhl’s (1958) methodology (18).
2. Physicochemical and bacteriological analy-sis of drinking water
Physicochemical and bacteriological analyses were performed on 18 samples drawn from particular houses. Standard Methods (19) were used to quantify nitrates, nitrites, chlorides and sulfates. Bacteriological analysis was carried out on 1 ml sample. Total heterotrophic mesophilic bacteria counts were determined by the pour plate procedure on nutritive agar; samples were incubated at 37ºC for 48 hrs. (Method 9215B). Total coliforms and thermo-resistant coliforms were quantified by the Most Probable Number (MPN) procedure according to the Methods 9221B and 9221C (Standard Methods, 1998) (19). The presence of pathogenic microorganisms like Escherichia coli and Pseudomonas aeruginosa (Standard Methods, 1998) (19) were also analyzed.
3. Detection of organochlorine, organophos-phate and carbamate pesticides
These compounds were detected according to Moffat and Clarke’s (1986) (20) and Ferrari (1985)(21). Pesticides were determined by extraction methods using Extrelut columns (Merck). A 20 ml sample was acidified to pH 4.0 with a saturated solution of tartaric acid. Sample was transferred to the column and settled for 15 min., and then was eluted with 40 ml petroleum ether and 40 ml petroleum ether:ethyl ether mixture (50:50). The eluted liquid was concentrated in a rotative evaporator. The residue was dissolved in 0.5 ml ethyl ether:absolute ethanol (50:50). Quali-quantitative identification was carried out on steady phase HPTLC chromatoplates of silicagel 60 with a fluorescence indicator (Merck). The mobile phase was ciclohexane:n-hexane:chloroform:acetone (40:40:10:10). Standards like Parathion, Heptachlor, dichlorodiphenyltrichloroethane (DDT), diclorodiphenylvinyl phosphate (DDVP), carbofuran of known concentration (10 µl-1) were also seeded. Detection was performed first with UV light and then, organochlorine pesticides were developed with 0,2% diphenylamine in absolute ethanol. Afterwards carbamic pesticides were developed with 1N sodium hydroxide solution and then with a saturated solution of nitrobenzene diazonio tetrafluorborate in ethylenglycol:ethanol (1:9). The last developer used was a solution of 0.5% P/V palladium chloride in 10% hydrochloric acid. Chromogenic reactants were applied in sequence.
4. Detection and quantifi-cation of volatile biogenic compounds
Samples for chemical analysis were obtained with 250 ml dark glass flasks, previously washed with sulphochromic solution, rinsed three times with distilled water and later on, three times with bidistilled water. Samples were immediately refrigerated at 2 - 4 ºC. Solid phase microextraction fibers (SPME) Supelco (100 mm polydimethylsiloxane) were dipped in water samples and extracted with magnetic agitation for 20 min. Identification and quantification were accomplished by Gas Chromatography with a Shimadzu GC17A equipment with D135 J&W capillary column and a Shimadzu QP5000 mass detector. The chromatographic conditions were: injector temperature 150ºC; initial oven temperature 40ºC maintained for 2 minutes, 10ºC/min ramp until 250ºC, 25ºC/min ramp until 300ºC and then maintained for 2 minutes.
Results and Discussion
1. Paso de Las Piedras reservoir.
Table 1 shows quantitative analysis of phytoplankton of 2 water samples from Paso de Las Piedras reservoir. Cell density of total phytoplankton varied between 49440 and 84832 cells.ml-1. Anabaena circinalis, the dominant species reached very high concentrations, between 48320 and 84032 cells.ml-1. Other algae were scarce with low count values.
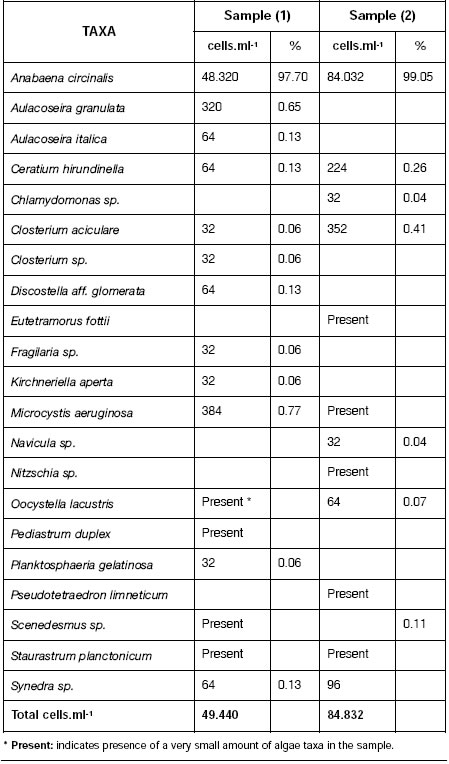
Table 1: Algae present in samples (1) and (2) of Paso de las Piedras reservoir. Values are expresed in cells per mililiter and percentage.
Australian water authorities have adopted a three level alert system based on blue green algae cell counts in water. At level 1 (500-toxic, water must be declared unsafe for human 2000 cells.ml-1) water authorities are alerted and consumption. At level 3, above 15000-20000 sampling increased. At level 2 (2000-15000 cells.ml-1 of blue-green algae, a persistent bloom, cells.ml-1) toxicity testing is carried out, water fil-consultation between health and water supply tration plant operators warned and if algae are authorities is required to ensure safe domestic and recreational water supplies, and warnings are issued (6).
2. Water treatment plants (P1 and P2) Table 2 shows phytoplanktonic densities of inlet and outlet samples of treatment plants, P1 and P2. At the inlets, Microcystis aeruginosa had a high proportion (90.5 and 88.9%, for P1 and P2 respectively) followed by Chroomonas sp. (5.2 and 4.0%) and Anabaena circinalis (2.1 and 2.2%). At the outlets, Microcystis aeruginosa abundance slightly decreased to 49.0 and 87%, while Anabaena circinalis increased to 50.5 and 12.0%, for P1 and P2 respectively. Plant efficiency to remove microorganisms (ER) was analyzed considering its capacity to retain algae cells according to the following equation:
ER= ((cells)e - (cells)s))/(cells)e
where (cells)e and (cells)s are the cell counts for each algae species at the plant inlet and outlet respectively.
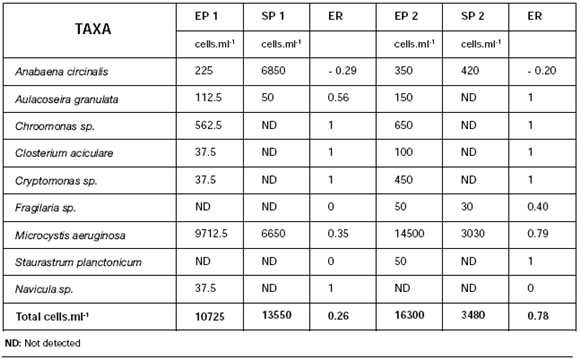
Table 2: Phytoplankton in treatment plants at the inlet of plant 1 (EP1), outlet of plant 1 (SP1), inlet of plant 2 (EP2) and outlet of plant 2 (SP2). ER: efficiency to remove microorganisms.
When the plant works properly, outlet cell count should be zero (no detected) and ER should equal 1. If plant does not retain the cells, inlet and outlet counts will be the same and ER will be zero. If the number of cells increases, ER will be negative and the absolute value will increase as cell count increases at the outlet. Table 2 shows ER values for both plants and each type of analyzed algae.
An ER= -29 for Anabaena circinalis in Plant 1 indicates that plant conditions favored the growth of this specie. An ER= 0.35 for Microcystis aeruginosa indicates an operative efficiency of 35%. For Plant 2, an ER of -0.2 for Anabaena circinalis also indicates that this plant is not effective in reducing the abundance of this cyanobacterium; on the contrary, Microcystis aeruginosa ER is 0.79 which means an efficiency of 79%.
For the rest of the algae, ER was 1 corresponding to 100% efficiency for both plants. ER values for total algal number indicate that Plant 1 efficiency was -26%, revealing a proliferation of algae during the purification process, whereas Plant 2, showing an ER=78%, resulted more effective.
Since water treatment by flocculation and sedimentation, followed by sand filtration, is supposed to remove live cyanobacterial cells and debris, the increase in cell counts of algae with potential toxicity (Anabaena circinalis and Microcystis aeruginosa) evidence that the Plants were not working properly. Table 3 shows values of volatile compounds THM (chloroform, bromoform and dibromochlorometane) and BTEX in analyzed samples. Both values (THM and BTEX) were below guideline levels for surface water (Código Alimentario Argentino, 1996) (22). Besides, organochlorine, organophosphorate and carbamate pesticides were not detected in any sample (they were detection limits of 5 µg, 0.1 µg., 20 ng. respectively).
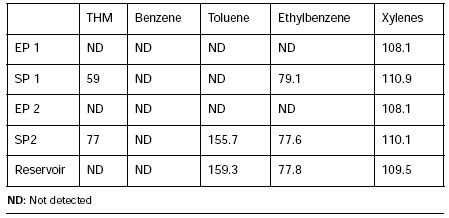
Table 3: THM (total concentration) and BTEX (µg.l-1) in water samples drawn at the inlet (E) and outlet (S) of treatment plants P1 and P2
3. Drinking water
3.1. Bacteriological and Physicochemical Assays Bacteriological counts of domestic water samples indicated that total heterotrophic microorganisms were below 500 UFC.ml-1, while total Coliforms and fecals were below 2NMP.100ml-1. Escherichia coli and Pseudomonas aeruginosa were not detected in 100 ml samples. Thus, analyzed samples were bacteriologically potable (Código Alimentario Argentino, 1996) (22).
Physicochemical analysis indicated that nitrates, nitrites, sulfates and chlorides levels were below the allowed values (Código Alimentario Argentino, 1996) (22). Even though samples were bacteriologically and physicochemically potable analysis of phytoplankton were performed.
3.2. Phytoplankton analysis
Although reservoir water is treated in the plants for drinking water supply to the population, a high algae concentration was found in plant outlets. Thus, identification and quantification of Cyano-bacteria in drinking water was carried out at 14 particular houses. Table 4 shows algae counts of in 14 samples of domestic water. Predominant algae were Anabaena circinalis and/or Microcystis aeruginosa, representing between 52 and 99.8% of the total cells number. In one sample, the Bacillariophyceae Stephanodiscus sp and Aulacoseira granulata were predominate taxa. The ratio Anabaena circinalis / Microcystis aeruginosa was analyzed as a function of total algae (Fig. 1). All but 2 samples showed ratios above 1. Thus, Anabaena circinalis was the predominant species in domestic water supply. Besides, a distribution of algae type as a function of total algae counts could be determined. When short fragments of trichomes or single cells of Anabaena circinalis were recorded in tap water total cells number were below 5000 cells.ml-1, whereas cells numbers above 5000 cells.ml-1 were due to the presence of fragmented colonies of Microcystis aeruginosa. To regulations regarding drinking water supply are in practice in Buenos Aires Province, Argentina.
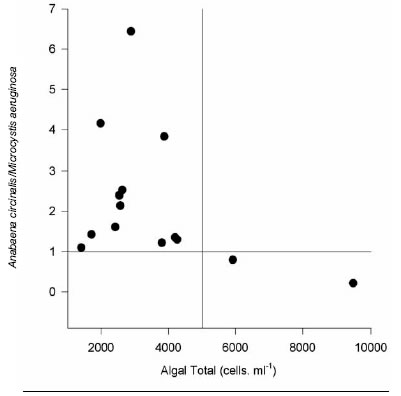
Fig 1: Relationship between total algae counts and Anabaena circinalis / Microcystis aeruginosa ratio of 14 drinking water samples
Table 4: Algae in drinking water of Bahía Blanca city (cells.ml-1).
Argentine Food Code indicates that domestic drinking water supply should not contain any amount of substances or strange particles of biological, organic, inorganic or radioactive origin that makes water a health hazard. Moreover, Argentine Food Code indicates that water should have an acceptable flavor and should be almost colorless, odorless, transparent and clear. On the other hand, Law 11820 of Buenos Aires Province (23), indicates that phytoplankton should be absent in drinking water. Both regulations mention directly or indirectly the presence of algae but they don’t regulate the occurrence of neither algal toxins nor cyanotoxins.
Cyanobacteria blooms coincided with the odor events perceived by the population. This fact led to the analysis of volatile biogenic compounds in waters of the affected area.
3.3. Detection of volatile biogenic compounds and pesticides.
Numerous sources contribute to the origin of biogenic odor compounds found in surface freshwaters. It is well known that Cyanobacteria produce a variety of volatile organic compounds, many of which could contribute to the deterioration of water quality. There is wide evidence to associate blue-green algae as an important source of taste and odors in water and aquatic life. In the present work, the strong odor similar to organochlorine pesticide led to analyze volatile compounds and to investigate the presence of organochlorine, organophosphate and carbamate pesticides.
Trihalomethanes and BTEX values were below guideline levels for drinking water (Law 11820, Buenos Aires Province) (23). Besides, organochlorine, organophosphorate and carbamate pesticides were not detected in any of 14 drinking water samples for the applied methodology.
In all samples, either from the reservoir, the treatment plants and drinking water, the volatile metabolite geosmin was detected in a concentration of 1 µg.l-1. This compound is produced by actinomycetes (24). Geosmin is identified as the compound that most frequently leads to off-fla-vors in water. Persson (1980) (25) stressed that very little amounts are required to cause a problem. In addition to tastes and odors caused by geosmin it is possible that the combination of various metabolic or degradation products of blue-green algae may interact to impair water quality (16, 17) found that geosmin was the main responsible for the noxious odor detected in the potable water coming from Cruz de Piedra reservoir in San Luis, Argentina.
In the present work other substance similar to Copaene was also detected by mass spectroscopy. Copaene (tricyclo 4.4.0.02,7 dec-3-ene,1,7-dimethyl-8-(1-methylethyl)-steroisom) (CAS Nº: 3856-25-5) was identified by Gas-Chromatography-Mass Spectrometry (GC/MS). Both compounds, Geosmin and Copaene, are potent odorous agents causing serious organoleptic problems. Geosmin is the most frequently found compound in pure or mixture cultures and in surface waters (16, 26, 17). Geosmin is structurally a terpenoid (2x5+2) C; while Copaene is a sesquiterpene (3x5C) isolated from different essential oils of vascular plants. No reports were found regarding Copaene isolation from extracts of blue-green algae or actinomycetes cultures. As Copaene was detected together with Geosmin the possibility of a metabolic relationship between both compounds arise, and thus Copaene can be postulated as a precursor of Geosmin.
Considering that Geosmin has been isolated from pure cultures of algae, it is improbable that Geosmin is generated by extracellular degradation. It seems more likely to attribute its origin to a metabolic way of oxidative nature in which Geosmin is the final product and dimethyl ketone is a possible secondary product (Fig. 2). Thus, if Copaene is a Geosmin precursor its early detection would become a very important tool for handling water quality problems related to Geosmin formation.

Fig 2: Relationship between total algae counts and Anabaena circinalis / Microcystis aeruginosa ratio of 14 drinking water samples
Conclussions
Results showed that the strong off-odor events detected in domestic water supply Bahía Blanca city, were due to Geosmin, a volatile compound produced by Anabaena circinalis. This species was recorded in all analyzed samples, in some of them at high concentrations. Bacteriological, pesticide, THM and BTEX analysis were below to guideline values. Based on both the present work and the analysis of drinking water regulations of Buenos Aires Province, we suggest considering the incorporation of aspects regarding the presence of Cyanobacteria due to their health hazard implications.
Acknowledgements
We would like to thanks to Lic. José María Guerrero for the critical review of the manuscript.
References
1. Carmichael W.W. (1989) Characterization Pharmacology and Therapeutics in Natural Toxins. C. A. Ownby and G. V. Odell. Eds. Pergamon, Oxford. p 3- 16. [ Links ]
2. Codd G.A. (1995) Cyanobacterial toxins: occurrence, properties and biological significance. Wat. Sci. Tech. 32, 149-156. [ Links ]
3. Falconer I.R. (1993) Algal toxin in seafood and drinking water. Academic Press, Londres, 165 pp. [ Links ]
4. Francis, G. (1978) Poisonous Australian Lake. Nature 444, 11-12. [ Links ]
5. Carmichael W. W. (1996). Liver failure and human deaths at a haemodialysis center in brazil microcystins as a mayor contributing factors. Harmful Algae News, 15, 11. [ Links ]
6 . Carmicheal W.W. & Falconer I.R. (1993) Diseases related to freshwater blue-green algal toxins, and control measures. In: Algal toxins in seafood and drinking water Falconer I.R. (Ed.). Academic Press, p 187-209. [ Links ]
7. García De Emiliani M.O. & Emiliani F. (1997) Mortandad de ganado y aves silvestres asociada con una floración de Anabaena spiroides Kleb. Natura Neotropicalis 28, 150-157. [ Links ]8. Falconer I.R. (1996) Potential impact on human health of toxic cyanobacterial. Phycologia 35, 6-11. [ Links ] 9. González D., Echenique R.O. & Silva, H.J. (2001) Toxicidad y producción de metabolitos volátiles en Cyanophyta o algas verde-azules. Revista: Acta Toxicol. Argent. 9(2), 68-81. [ Links ]
10. Codd G.A. (1994) Blue-green algal toxins: water-borne hazard to health. In: Water and public health. Golding, A.M.B.; Noah N. and Stanwell -Smith (Ed.), p271-278. [ Links ]
11. Carmichael W.W. (1994) The toxins of Cyanobacteria. Scientific American 270, 78-86. [ Links ]
12. Echenique R.O. (1999) Cyanophyta toxicas, antecedentes y estudios actuales en la República Argentina. Notas Botánicas de la Soc. Arg. Bot, 3-7. [ Links ]
13. Echenique R.O. & González D.M. (1998) Las cianofitas: microalgas causantes de toxicidad. Rev. Museo 2, 77-80. [ Links ]
14. Pizzolon L. (1996) Importancia de las cianobacterias como factor de toxicidad en las aguas continentales. Interciencia 21, 239-245. [ Links ]
15. Farlow W.G. (1983) Relation of certain forms of algae to disagreeable tastes and odours. Science 2, 333-336. [ Links ]
16. Slater G.P. & Blok V. (1983). Volatile compounds of the Cyanophyceae. A review. Wat. Sci. Thechnol. 15, 181-190. [ Links ]
17. Silva J.H., Luco J.M., González D.M. y Baudino O.M. (1995) Detección de compuestos biogénicos-volátiles en un lago eutrófico de San Luis-Argentina. Acta Toxicol. Argent. 3, 38-42. [ Links ]
18. Utermöhl, H. (1958.) Zur Vervolkommung der quantitative Phytoplankton Methodik. Mitt. Int. Verein. Limnol. 9, 1-38. [ Links ]
19. American Public Health Association (APHA) (1998) Standard Methods for the Examination of Water and Wastewater. 20th.Edition., Washington, 9 pp. [ Links ]
20. Clarke's. Isolation and identification of drugs. (1986). 2nd Edition. Moffat A Ed. The Pharmaceutical Press, London, 70 pp. [ Links ]
21. Ferrari L.A. (1985) Cromatografía zonal y relevado secuencial diferenciado. Biblioteca Roberts Alcorta. Poder Judicial, 2 16-21. [ Links ]
22. Código Alimentario Argentino. (1996). Capítulo XII, Bebidas hídricas, aguas y aguas gasificadas. De La Canale y Asociados, Buenos Aires: p1-2. [ Links ]
23. Ley 11820. Marco regulatorio para la prestación de los servicios públicos de provisión de agua potable y desagües cloacales en la Provincia de Buenos Aires, Anexo A: "Normas de calidad para el agua potable"; Tabla 4: "Parámetros biológicos complementarios, Parámetros valor guía": [ Links ]
24. Gerber N.N. (1968) A volatile metabolite of actinomycetes, 2 methylisoborneol. J. Antibiot. 22, 508-509. [ Links ]
25. Persson, L. (1980) Muddy odour in fish from hyperthrophic waters. Dev. Hydrobiol. 2, 203-207. [ Links ]
26. Jüttner, F. (1988) Biochemistry of biogenic off-flavour compounds in surface waters. Wat. Sci. Tech. 20, 107-116. [ Links ]














