Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Acta toxicológica argentina
versión On-line ISSN 1851-3743
Acta toxicol. argent. vol.18 no.1 Ciudad Autónoma de Buenos Aires jun. 2010
ARTÍCULOS ORIGINALES
Toxicity Of The Essential Oil Of The Cytral Chemotype Of Lippia Alba (Mill.) N. E. Brown
Olivero-Verbel, Jesús1*; Guerrero-Castilla, Angélica1; Stashenko, Elena2 *
1Environmental and Computational Chemistry Group. Faculty of Pharmaceutical Sciences. University of Cartagena, Campus of Zaragocilla, Cartagena, Colombia.
2Chromatography Laboratory, Research Centre for Biomolecules, CIBIMOL, CENIVAM, Industrial University of Santander, Bucaramanga, Colombia.
*Corresponding author: jesusolivero@yahoo.com - joliverov@unicartagena.edu.co
Abstract. The essential oil (EO) of Lippia alba (Mill.) N. E. Brown (Verbenaceae) has been traditionally used to treat several diseases. In this study, the acute toxic effects of the citral chemotype of L. alba EO were evaluated in mice. Animals were treated via intraperitoneal receiving the L. alba essential oil at doses between 50 and 2500 mg/kg, and the control group received sesame oil (vehicle). The EO induced dose-dependent neurotoxic effects at doses greater than 1000 mg/kg, including decreased locomotion, motor skills and muscle strength, hypotonia, dyspnea, kyphosis and convulsions. The EO was lethal at a dose of 2500 mg/kg. Animals receiving 1000 mg/kg were euthanized at the end of the treatment period and their blood and livers were collected for analysis. Mice exposed to L. alba EO presented signifcantly greater plasma alanine aminotransferase (ALT) activities than the control group. Liver histological changes included mild infammation, in particular, an increase in nuclear size. Compared to vehicle control group, changes in expression for selected genes were signifcant for FABP5, a fatty acid transport related gene. In summary, the intraperitoneal administration of L. alba EO (citral chemotype) causes neurological damage in mice at doses equal or greater than 1500 mg/kg, whereas at 1000 mg/kg, it generates mild liver damage. Therefore, the systemic use of this EO raises concerns about its safety.
Keywords: Essential oil; Lippia alba; Neurological damage; Acute toxicity.
Resumen. Toxicidad del aceite esencial de Lippia alba (Mill.) N. E. Brown quimiotipo citral. El aceite esencial (AE) de Lippia alba (Mill.) NE Brown (Verbenaceae) ha sido utilizado tradicionalmente para tratar varias enfermedades. En este estudio, los efectos tóxicos agudos del AE de Lippia alba quimiotipo citral fueron evaluados en ratones. Los animales fueron tratados por vía intraperitoneal recibiendo el AE en dosis entre 50 y 2500 mg/kg de peso, y el grupo control aceite de sésamo (vehículo). Dosis superiores a 1000 mg/kg del AE mostraron efectos neurotóxicos incluyendo disminución de la locomoción e hipotonía, disnea, cifosis y convulsiones. El AE fue letal a la dosis de 2500 mg/kg. Veinticuatro horas después de que los animales fueron tratados con 1000 mg/kg del AE se les realizó eutanasia y su sangre e hígado fueron recolectados para análisis. Los ratones expuestos al AE de L. alba, presentaron actividad alanina aminotransferasa (ALT) en plasma signifcativamente mayor que el grupo control. Dentro de los cambios histológicos hepáticos se incluyen infamación leve, en particular, un aumento del tamaño nuclear. En comparación con el grupo control, la expresión de genes seleccionados tuvo diferencias signifcativas para FABP5, un gen relacionado con el transporte de ácidos grasos. En conclusión, la administración intraperitoneal del AE de L. alba (quimiotipo citral) causa daños neurológicos en ratones a una dosis igual o superior a 1500 mg/kg, mientras que a 1000 mg/kg, genera daño hepático leve. Por lo tanto, el uso sistémico de este AE plantea preocupaciones en cuanto a su seguridad.
Palabras clave: Aceite esencial; Lippia alba; Daño neurológico; Toxicidad aguda.
INTRODUCTION
Essential oils (EOs) are complex mixtures of volatile compounds with diverse chemicalstructure. They are composed of secondary metabolites that plants produce for their survival. EOs can be isolated from any part of aromatic plants and are widely used in cosmetics, food industry and pharmaceuticals (Stashenko 2009). Within aromatic species, Lippia alba (Mill.) N.E. Brown (Verbenaceae) has been commonly used in natural and traditional medicine in Latin America. L. alba is one of the most studied species within the Lippia genus, and several studies have described its use as a sedative, as well as for the treatment of digestive disorders (vomiting, fatulence, diarrhea, abdominal pain), respiratory infections (bronchitis, sore throat, fu, cough, cold), hypertension, anemia, and skin diseases (Hennebelle et al. 2008a). In Colombia, L. alba has been found to exist as three different chemotypes: carvona, citral/geranial and the mixed type carvone/citral. Other major components present in this EO include limonene, bicyclosesquiphellandrene, piperitenone, piperitone and β-bourbonene (Stashenko et al. 2004). Despite the fact that L. alba EO is currently used as either phytomedicine or food favouring, the knowledge about its mammalian toxicity is scarce. Although this is also true for many other EOs, the available literature has registered a wide toxicological profle for these substances, in particular, at high doses, and depending on their chemical composition. These include lipid peroxidation and hepatic damage (Sztajnkrycer et al. 2003; Akdogan et al. 2004), increases in kidneybody weight ratio (Odeyemi et al. 2009) and glutathione depletion (Lima et al. 2004), among others. However, it also should be stated that EOs have also been tested as protectors in different toxicity models such as those induced by cyclophosphamide (Rezvanfar et al. 2008) and carbon tetrachloride (Mansour et al. 2001). The aim of this work was to study the effects of the L. alba (citral chemotype) EO on a mice model of acute toxicity.
MATERIALS AND METHODS
L. alba EO (citral chemotype) was obtained by hydrodistillation from plants grown at the CENIVAM at the Industrial University of Santander, Bucaramanga, Colombia, and chemically characterized as described previously (Stashenko et al. 2004; Mesa-Arango et al. 2009; Olivero-Verbel et al. 2009). The species has been identifed by botanist José Luis Fernández at the Institute of Natural Sciences of the National University of Colombia (Bogotá). A voucher of L. alba has been deposited at the Institutes Herbarium with the number 480750. Female BALBc mice, aged 8 to 10 weeks, were purchased from the National Institute of Health (INS), Bogotá, Colombia, and allowed to acclimate for a minimum of 2 weeks before the start of the experiments. Groups of 10 mice were injected intraperitoneally with sesame oil (vehicle) or the citral chemotype of L. alba EO (50-2500 mg/kg), and their behaviour/ signs and symptoms were followed for a 24 h period. Preliminary experiments showed that doses greater than 2500 mg/kg were lethal within minutes. Mice were given ad libitum access to food and water, housed in polycarbonate cages, and maintained in a climate-controlled room with a 12:12-h lightdark cycle in an animal facility at the University of Cartagena, Cartagena, Colombia. Mice receiving the maximum dose, at which not behavioral changes were noticed after the exposure period, were euthanized with sodium pentobarbital. Portal blood was collected in sodium citrate, plasma isolated by centrifugation and alanine aminotransferase (ALT) and aspartate amino-transferase (AST) activities were measured spectrophotometrically using commercially available kits (Product Codes: ALT, 11568; AST, 11567, Biosystems, Barcelona, Spain). Liver was removed immediately after blood collection; a section was also immediately stored in RNA Later (Ambion, Austin, Texas, USA) for gene expression analysis. Another section was fxed with 10% buffered formalin, and subsequently embedded in paraffn for histological analysis. Sections were stained with hematoxylin and eosin using standard techniques. Sections were viewed without knowledge of the treatment group to which each animal had belonged.
Gene expression measurements were performed from RNA isolated from the liver using RNeasy Mini Kit (Quiagen, California, USA) as described by the manufacturer. cDNA was prepared from total mRNA using QuantiTect Reverse Transcription Kit (Qiagen Inc, Valencia, CA, USA). In this work we studied changes in mRNA expression on selected genes, representing different signaling pathways important in liver function, including oxidative stress defense mechanisms (superoxide dismutase, SOD), cholesterol metabolism (cholesterol 7a-hydroxylase, CYP7A1; sterol regulatory element binding protein, SREBP), fatty acid transport (FABP5), fatty acid metabolism (peroxisome proliferator-activated receptor alpha, PPARa) and cell cycle regulation (cyclin, CYCL). Changes in gene expression were determined using realtime RTPCR with β-actin as the reference gene. Each sample was run in duplicates on the LightCycler 1.5 (Roche Applied Science, Indianapolis, IN, USA).

Ethical considerations
The study was conducted according to the declaration of Helsinki (Villar 1988), and following national scientifc and ethical guidelines established in Law 84 of December 27, 1989, Chapter VI; as well as those in the Resolution 8430 of 1993, issued by the Colombian Ministry of Health.
Statistical analysis
All data were expressed as means ± SE. A two-tailed t-test was used to compare vehicle vs. EO-treated groups. P values <0.05 were considered statistically signifcant. All statistics were performed using Prism software (version 5.0a).
RESULTS
Mice treated with vehicle control or doses of L. alba lower than 1000 mg/kg did not experience any signs or symptoms of toxicity during the 24 h surveillance. However, doses equal or greater than 1500 mg/kg induced several dosedependent neurological and motor defcits (Table 1), mostly characterized by decreased locomotion and muscle strength, hypotonia, dyspnea, kyphosis and seizures. A greater dose (2500 mg/kg) was lethal for all mice within 24 h exposure (Figure 1), although 91% of them died during the frst 12 hours. As 1000 mg/kg of L. alba EO did not cause any observable change in normal behaviour, this dose was chosen to study the effects on liver function. ALT activities in the 1000 mg/kg group was signifcantly higher than in the control group (P <0.05), whereas AST activities did not change with the treatment (Figure 2). Although the ALT values in the EO-treated group were near 40% greater than in the control group, this increase in ALT is far from the threshold value used to defne potential liver injury (Navarro and Senior 2006). Compared to the vehicle group, mice treated with 1000 mg/kg of L. alba EO did not show signifcant histological liver lesions (Figure 3), with the exception of some minor infammation, mostly characterized by increase in nuclear size, and few scattered polymorphonuclearmonocytic infltrations. Gene expression analysis of liver was performed on vehicle and 1000 mg/kg L. alba EO-treated mice after 24 h exposure and the results are presented in Figure 4. Treatment with EO signifcantly downregulated gene expression of FABP5 in the liver. On the other hand, SREBP was also downregulated, whereas SOD, PPAR-a, CYP7A1 and CYCL, were up-regulated in the EO-treated group when average values were compared to control, although these changes in mRNA expression were not statistically signifcant.
Table 1. Characteristic signs and survival of BALB/c at different doses of Lippia alba essential oil.

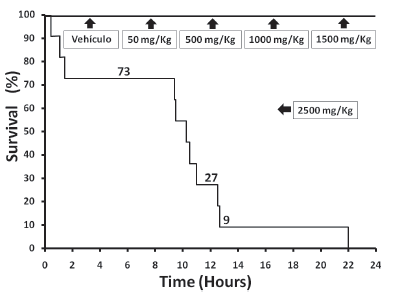
Figure 1. Survival of the different groups of mice exposed to vehicle (sesame oil) or L. alba EO.

Figure 2. Plasma ALT (A) and AST (B) activities in vehicle or 1000 mg/kg L. alba EO-treated mice. *Signifcantly different from control (P<0.05).

Figure 3. H&E staining of liver sections from vehicle- or 1000 mg/kg L. alba EO-treated mice. Vehicle-treated mice showed normal liver histology, whereas L. alba exposed group presented scattered foci of enlarged hepatocyte nucleus.

Figure 4. Liver mRNA expression of selected genes after mice exposure to vehicle or 1000 mg/kg L. alba EO. *Signifcantly different from control (P<0.05).
DISCUSSION
L. alba has an important therapeutic background in traditional medicine. Different types of non volatile compounds isolated from this plant have shown antioxidant, neurosedative (Zétola et al. 2002; Hennebelle et al. 2008b), anticonvulsant (Neto et al. 2009) and antimicrobial (Oliveira et al. 2006) properties, whereas its EO has been shown to be antifungal (Mesa-Arango et al. 2009). However, up to now few studies have shown the in vivo toxic effects of its EO in eukaryotic species (Olivero-Verbel et al. 2009; Sena-Filho et al. 2009). The present experimental study found that a high acute dose (>1000 mg/kg) of L. alba EO (citral chemotype) causes signs and symptoms of severe neurological damage, and also that a dose of 2500 mg/kg was lethal in a 24 h period. A previous report (Vale et al. 1999) used elevated plus maze, open feld and rota rod tests to demonstrate that mice exposed to 50-200 mg/kg L. alba EO (all three chemotypes) developed behavioral changes, which varied according to the chemotype used in the assays. The mechanisms by which this happens are not clear, though effects on the central nervous system may play a role, as it has been observed for citral, (Yang et al. 2009) one of the major components of the L. alba EO, probably as a result of retinoic acid defciency (Zhang et al. 2009). On the other hand, it has also been reported that the ethanolic non-volatile fraction of L. alba presents sedative and myorelaxant effects (Zetola et al. 2002). The level of ALT activity refects damage to hepatocytes and it is considered to be a highly sensitive and fairly specifc biomarker of hepatotoxicity (Ozer et al. 2008). Results reported here showed that the essential oiltreated group presented a small increase in ALT activity, suggesting that this EO has little hepatotoxicity, observation correlated to the mild histological effects observed. Moreover, this negligible increase in plasma ALT activity level could also be associated with other organ toxicities, especially considering that no changes were observed in AST activity. This is the frst report on the effects of L. alba EO on gene expression in mice liver. Gene expression analysis revealed that among several targets, only FABP5 was signifcantly down-regulated by 1000 mg/kg EO. It has been suggested that the expression of FABP5 in liver parenchymal cells facilitates lipid uptake, transport, and metabolism as a response to the uptake of dietary lipids (Boord 2002; Glatz 2002). Therefore, downregulation of this gene could derive from decreased food intake, as a result of altered taste and smell senses, due to the volatile components of the EO, or as well as a consequence of central depression. In average, SREBP was also downregulated in the EO-exposed group, although this event was not signifcant. However, some action on cholesterol metabolism cannot be completely neglected. On the other hand, the lack of signifcant effects of the EO on the expression of genes related to cholesterol metabolism (CYP7A1), fatty acid metabolism (PPARa), cell cycle regulation (CYCL) and oxidative stress (SOD), might indicate that the liver is not a primary target for the EO. Moreover, the fact that no signifcant change in SOD RNA expression was produced by L. alba EO was not surprising, taking into account that this species has shown antioxidant properties (Aran and Nur 2009). Alternatively, it is important to state that, as also seen in the mortality study, the response to the EO in some animals is highly variable, and this could indeed impact the signifcance obtained in the comparisons between groups. This observation adds uncertainty to the toxic effects associated with the acute exposure of the L. alba EO to mice, suggesting that care must be taken when evaluating possible health risks in humans.
CONCLUSION
High doses of the L. alba essential oil (citral chemotype) induced severe neurological and motor damage in mice. A dose with non observable neurological effects (1000 mg/kg) produced a mild liver infammation and down-regulation of liver FABP5, a gene involved in lipid uptake, transport and metabolism.
Acknowledgements
The authors wish to thank Colciencias, Bogotá, and the University of Cartagena, Cartagena (Colombia) for their fnancial support (Grants RC 432-2004 and 110749326186-2009), as well as to Barbara Arroyo for her expertise.
REFERENCES
1. Akdogan M., Ozguner M., Aydin G., Gokalp O. Investigation of biochemical and histopathological effects of Mentha piperita Labiatae and Mentha spicata Labiatae on liver tissue in rats. Hum Exp Toxicol. 2004;23:21-28. [ Links ]
2. Aran N., Nur H. In vitro antioxidant activity of methanolic leaves and fowers extracts of Lippia alba. Res J Med Medical Sci. 2009;4:107-110. [ Links ]
3. Boord J.B., Fazio S., Linton M.F. Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis. Curr Opin Li-pidol. 2002;13:141-147. [ Links ]
4. Glatz J.F., Luiken J.J., Van Bilsen M., Van der Vusse G.J. Cellular lipid binding proteins as facilitators and regulators of lipid metabolism. Mol Cell Biochem. 2002;239:3–7. [ Links ]
5. Hennebelle T., Sahpaz S., Gressier B., Joseph H., Bailleul F. Antioxidant and neurosedative properties of polyphenols and iridoids from Lippia alba. Phytother Res. 2008a;22:256-258. [ Links ]
6. Hennebelle T., Sahpaz S., Joseph H., Bailleul F. Ethnopharmacology of Lippia alba. J Ethnopharmacol. 2008b;16:211-222. [ Links ]
7. Lima C.F., Carvalho F., Fernandes E., Bastos M.L., Santos-Gomes P.C., Fernandes-Ferreira M., Pereira-Wilson C. Evaluation of toxic/protective effects of the essential oil of Salvia offcinalis on freshly isolated rat hepatocytes. Toxicol In Vitro. 2004;18:457-465. [ Links ]
8. Mansour M.A., Ginawi O.T., El-Hadiyah T., El-Khatib A.S., Al-Shabanah O.A., Al-Sawaf H.A. Effects of volatile oil constituents of Nigella sativa on carbón tetrachloride-induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol. 2001;110:239-25. [ Links ]
9. Mesa-Arango A.C., Montiel-Ramos J., Zapata B., Durán C, Betancur-Galvis L., Stashenko E. Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mili.) N.E. Brown: composition, cytotoxicity and antifungal activity. Mem Inst Oswaldo Cruz. 2009;104:878-884. [ Links ]
10. Navarro V.J., Sénior J.R.N. Drug-related hepatotoxicity. Engl J Med. 2006;354:731-739. [ Links ]
11. Neto A.C., Netto J.C., Pereira P.S., Pereira A.M., Taleb-Contini S.H., Franga S.C, Marques M.O., Beleboni R.O. The role of polar phytocomplexes on anticonvulsant effects of leaf extracts of Lippia alba (Mili.) N.E. Brown chemotypes. J Pharm Pharmacol. 2009;61:933-939. [ Links ]
12. Odeyemi O., Yakubu M.T., Masika P.J., Afolayan A.J. Toxicological evaluation of the essential oil from Mentha longifolia L. subsp. capensis leaves in rats. J Med Food. 2009;12:669-674. [ Links ]
13. Oliveira D.R., Leitáo G.G., Santos S.S., Bizzo H.R., Lopes D., Alviano C.S., Alviano D.S., Leitáo S.G. Ethnopharmacological study of two Lippia species from Oriximiná, Brazil J Ethnopharmacol. 2006;108:103-108. [ Links ]
14. Olivero-Verbel J., Guette-Fernandez J., Stashenko H. Acute toxicity against Aríem/a franciscana of essential oils isolated from plants of the genus Lippia and Piper collected in Colombia. Bol Latinoam Caribe Plant Med Aromát. 2009;8(5):419-427. [ Links ]
15. Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194-205. [ Links ]
16. Rezvanfar M., Sadrkhanlou R., Ahmadi A., Shojaei-Sadee H., Rezvanfar M., Mohammadirad A., Salehnia A., Abdollahi M., Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum Exp Toxicol. 2008;27:901-910. [ Links ]
17. Sena-Filho J.G., Duringer J., Souza I., Da Cunha E., Craig A.M., Silva M., Barbosa-Filho J., Xavier H. Phytochemistry and acute toxicity from the roots of Lippia alba. Pharmaceut Biol. 2009;47:142-145. [ Links ]
18. Stashenko E., Jaramillo B.E., Martinez J.R. Analysis of volatile secondary metabolites from Colombian Xylopia aromatica (Lamarck) by different extraction and headspace methods and gas chromatography. J Chromatogr A. 2004;1025:93-103. [ Links ]
19. Stashenko E. Propiedades y caracterización de los aceites esenciales. En: Aceites esenciales. División de publicaciones UIS - Universidad Industrial de Santander, Bucaramanga, Colombia, 2009. p. 85-124. [ Links ]
20. Sztajnkrycer M.D., Otten E.J., Bond G.R., Lindsell C.J., Goetz R.J. Mitigation of pennyroyal oil hepatotoxicity in the mouse. Acad Emerg Med. 2003;10:1024-1028. [ Links ]
21. Vale T.G., Matos F.J., De Lima T.C., Viana G.S. Behavioral effects of essential oils from Lippia alba (Mill.) N.E. Brown chemotypes. J Ethnopharmacol. 1999;67:127-133. [ Links ]
22. Villar J. Recomendaciones de la declaración de Helsinki sobre la investigación clínica y guías principales en el cuidado y uso de animales de laboratorio. Med Clin. 1988;91:702-703. [ Links ]
23. Yang Z., Xi J., Li J., Qu W. Biphasic effect of citral, a favoring and scenting agent, on spatial learning and memory in rats. Pharmacol Biochem Behav. 2009;93:391-396. [ Links ]
24. Zetola M., De Lima T.C.M., Sonaglio D. CNS activities of liquid and spraydried extracts from Lippia alba-Verbenaceae (Brazilian false melissa). J Ethnopharmacol. 2002;82:207–215. [ Links ]
25. Zhang M., Wan C., Ji B., Zhang Z., Zhu H., Tian N., La Y., Huang K., Jiang L., He G., Gao L., Zhao X., Shi Y., Huang G., Feng G., He L. Proteome alteration of U251 human astrocytoma cell after inhibiting retinoic acid synthesis. Mol Cell Biochem. 2009;323:185-193. [ Links ]














