Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Acta toxicológica argentina
On-line version ISSN 1851-3743
Acta toxicol. argent. vol.21 no.2 Ciudad Autónoma de Buenos Aires July/Dec. 2013
ARTICULOS
Acute silver toxicity to Cnesterodon decemmaculatus (Pisces: Poeciliidae) in a river with extreme water-quality characteristics
Toxicidad aguda de la plata sobre Cnesterodon decemmaculatus (Pisces: Poeciliidae) en un río con características extremas de calidad de agua
Casares, María Victoria1*; de Cabo, Laura I.1; Seoane, Rafael2,3; Natale, Oscar2
1 Bernardino Rivadavia National Museum of Natural History, Avenida Angel Gallardo 470 (C1405DJR), Buenos Aires, Argentina. Tel. 54 011 4982 4494; fax: 54 011 4982 0306.
2 National Water Institute, Autopista Ezeiza-Cañuelas, Tramo Jorge Newbery km 1.62 (1802) Ezeiza, Buenos Aires, Argentina.
3 Faculty of Engineering, University of Buenos Aires, Avenida Las Heras 2214 (C1127AAR), Buenos Aires, Argentina.
Recibido: 12 de abril de 2013 Aceptado: 13 de agosto de 2013
Abstract. A 96 h acute silver toxicity test was performed in order to determine silver toxicity (LC50) to a local fish species (Cnesterodon decemmaculatus) in a river with extreme water-quality characteristics (Pilcomayo River, South America) and evaluate a cross-fish-species extrapolation of the Biotic Ligand Model. The dissolved silver concentrations tested were 0.095, 0.148, 0.175 and 0.285 mg Ag L-1. The 96 h Ag LC50 calculated for C. decemmaculatus was 0.14 mg L-1 (0.18 - 0.10) and the value predicted by BLM for Pimephales promelas was 0.051 mg Ag L-1. Test water elevated hardness may have exerted some protective effect. High mean water pH may have exerted a major protective effect by reducing silver free ion form and causing silver precipitation. The mortality pattern observed in this toxicity test may lend some support to a relationship between gill silver accumulation and mortality. A cross-fish-species extrapolation of Ag BLM for P. promelas was not valid in Pilcomayo River water and experimental conditions of this toxicity test.
Keywords: Silver; Cnesterodon decemmaculatus; Biotic Ligand Model; Extrapolation.
Resumen. Con el objeto de determinar la toxicidad de la plata en un pez nativo (Cnesterodon decemmaculatus), se llevó a cabo un ensayo estático de toxicidad aguda a 96 horas en un agua natural con características de calidad de agua, extremas (río Pilcomayo, Sudamérica). Asimismo, se evaluó una posible extrapolación inter-especie del Modelo del Ligando Biótico en el agua experimental. La concentración inicial de plata en solución en los distintos tratamientos fue de 0,095; 0,148; 0,175 y 0,285 mg Ag L -1 . La CL50 a las 96 horas calculada para C. decemmaculatus fue de 0,14 (0.18 - 0.10) mg Ag L-1 y el valor predicho por el BLM para Pimephales promelas fue de 0,051 mg Ag L-1. La elevada dureza del agua experimental pudo haber tenido algún efecto protector frente a la toxicidad de la plata. El valor medio de pH del ensayo fue elevado y posiblemente tuvo un gran efecto protector por reducción de la forma iónica libre y precipitación del metal. El patrón de mortalidad observado en este ensayo de toxicidad apoyaría la relación causa-efecto entre acumulación de plata en las branquias y mortalidad. La extrapolación inter-especie del BLM para P. promelas no resultó válida en el agua del río Pilcomayo y en las condiciones experimentales de este ensayo.
Palabras clave: Plata; Cnesterodon decemmaculatus; Modelo de Ligando Biótico; Extrapolación.
INTRODUCTION
Silver, as many other heavy metals enters the aquatic ecosystems as a by-product of many industrial and mining processes. Silver ion is one of the most toxic forms of a heavy metal, surpassed only by mercury and thus, it has been assigned to the highest toxicity class, together with cadmium, chromium (VI), copper and mercury (Ratte 1999). The primary effect of silver is on sodium and chloride uptake and efflux. When present as silver nitrate, silver is one of the most acutely toxic metals to freshwater rainbow trout (Oncorhynchus mykiss) with 96-h LC50 values in the range of 6.5-13 μg L-1 in freshwaters that are generally low in organic matter content. Silver nitrate is highly toxic because it readily dissociates in water to yield ionic silver (Ag+), the most acutely toxic species of silver. Silver toxicity occurs in freshwater fishes as a result of Ag+ disabling first the carbonic anhydrase and later the enzyme Na+/K+ adenosinetriphosphatase (ATPase) at the gill, leading to a net loss of sodium and chloride (Morgan et al. 2004). As a result, blood volume decreases and blood viscosity and pressure increase leading to fish mortality (Kennedy 2011).
The Biotic Ligand Model (BLM) (Di Toro et al. 2001) has been proposed as a tool to evaluate quantitatively the manner in which water chemistry affects the speciation and biological availability of metals in aquatic systems (Paquin et al. 2002). This model was developed using geochemical equilibrium principles, and provides site-specific toxicity predictions (Clifford and McGeer 2009). It is implicitly assumed that BLMs can be extrapolated within taxonomically similar groups, i.e., that BLMs developed for P. promelas can be applied to ecotoxicity data for other fish species, that BLMs for D. magna and C. dubia can be applied to ecotoxicity data for other invertebrates (Schlekat et al. 2010). The basis for a crossspecies extrapolation is the assumption that the parameters, which describe interactions between cations (notably calcium, magnesium and protons), the toxic free metal ion (M n+) and the biotic ligand are similar across organisms, and that only intrinsic sensitivity varies among species (Schlekat et al. 2010).
A variety of water chemistry factors can protect against metal binding to the sites of action of toxicity either by cationic competition (sodium and protons in the case of silver), or by anionic complexation (e.g., hydroxide, (bi) carbonate, chloride, thiosulfate, sulfide, and most importantly dissolved organic matter), thereby preventing it binding to the toxicity sites (Wood 2008). One of the first and most well recognized of the modifying factors for metals is water hardness. However, it appears to be less important for silver toxicity (Kennedy 2011). Alkalinity, on the other hand, affects metal ionic species in water solution through their complexation with carbonates (Erickson et al. 1996; Lauren and McDonald 1986) and dissolved organic matter forms complexes with metals, which reduces the free form in the water, and therefore the amount of ionic metal available to bind to the gill sites (Matsuo et al. 2004).
Pilcomayo River water is characterized by high concentration of sulphates, chloride, calcium, magnesium, and total suspended solids. Moreover, most of the major ions mean concentration in Pilcomayo River water is at the 95th percentile compared to the 60 largest rivers in the world (data in Gaillardet et al. 1999). Toxic waste spills containing high concentrations of arsenic and heavy metals are released daily from the mining district of Potosí, in Bolivia into Pilcomayo River waters. Mining of the Cerro Rico de Potosí ores began in 1545 however, since the introduction of the Crushing- Grinding-Flotation method of mineral processing (1985) the chronic contamination of the Pilcomayo River water and sediments has increased steadily (Smolders et al. 2003).
Cnesterodon decemmaculatus (Pisces: Poeciliidae; Jenyns 1842) is an endemic member of the fish family Poeciliidae with extensive distribution in neotropical America. The species attains high densities in a large variety of water bodies within the entire La Plata River and other South American basins. Cnesterodon decemmaculatus is a small, viviparous, microomnivorous, benthic-pelagic, non-migratory fish. This species, highly tolerant to extreme environmental conditions, is additionally easy to handle and breed under laboratory conditions (Menni 2004). Thereby, several authors have used C. decemmaculatus in bioassays (de la Torre et al. 1997, 2002, 2005; Ferrari et al. 1998; Gómez et al. 1998).
Pimephales promelas (Pisces: Cyprinidae; Rafinesque 1820), one of the fish species for which BLM has been developed, is a temperate, holartic fresh water fish. As well as C. decemmaculatus, is quite tolerant to turbid, lowoxygenated water bodies, and can be found in muddy ponds and streams and in small rivers (http://www.fishbase.org/summary/Pimephales- promelas.html). There is one previous work on the toxicity of metals to C. decemmaculatus in Pilcomayo River water (Casares et al. 2012). The authors found that a crossfish- species extrapolation of the Cu-BLM was valid within the Pilcomayo River water quality characteristics and experimental conditions of their toxicity test. With respect to Ag BLM, more work with a wider range of species will be required to improve its predictive capability (Bianchini et al. 2002).
The aim of this study was to evaluate a crossfish- species extrapolation of the silver BLM in a river with extrem water-quality characteristics (Pilcomayo River water). In order to achieve this aim: (a) silver toxicity (96-h Ag LC50) to C. decemmaculatus was assessed in Pilcomayo River water, (b) BLM, version 2.2.3 was applied to predict acute silver toxicity to P. promelas (Ag LC50) under Pilcomayo River water quality characteristics and (c) the predicted Ag LC50 value for P. promelas was compared to the calculated for C. decemmaculatus.
MATERIALS AND METHODS
Study area
The Pilcomayo River in South America is a tributary to the large La Plata system. Its headwaters are located in Bolivia along the eastern flank of the central Andes at an elevation of approximately 5,200 m (Figure 1). The river flows in a southeasterly direction until reaching the Chaco Plains along Bolivia's southern border with Argentina. Its total length is 2,426 km and its basin covers an area of approximately 288,360 km2 (http://www.pilcomayo.net/web/).

Figure 1. Map of Pilcomayo River basin with the water sampling location (Misión La Paz, Argentina)
Water sampling and chemical analysis
Discrete water samples for chemical analysis were taken 10 cm below the water surface and in triplicate from the navigation channel, left and right shore of Pilcomayo River in Misión La Paz International bridge (22º 22' 45" S - 62º 31' 08" W; 254 meters over sea level) in September 2009 (Figure 1). Water discharge (Q), pH and water temperature (T) were determined in situ. Dissolved concentrations of calcium (Ca), magnesium (Mg), chloride (Cl), potassium (K), sodium (Na), sulphate (SO4), hardness (Hard), alkalinity (Alk), dissolved organic carbon (DOC), total suspended solids (TSS), total dissolved solids (TDS), total (T. Ag) and dissolved silver (D. Ag) concentrations were determined using Standard Methods test protocols (APHA, AWWA, WPCF 1992).
Toxicity test
Water for the toxicity test was collected in prerinsed 10-L polypropylene containers. Samples were immediately placed into coolers and transported by plane to the laboratory. Later, water was centrifuged (2000 rpm during 15 minutes) and filtered through 47 mm, 0,45μm pore glass-fibre filters (Whatman GF/C). Dissolved silver background concentration in Pilcomayo River water was below the method detection limit (ND).
Juvenile C. decemmaculatus were collected from a small pond, located in Reserva Natural Los Robles, Buenos Aires Province, Argentina (main chemical and physical parameters in Casares et al. 2012). Fish were kept at temperatures ranging from 20 to 24ºC and pH ranging from pH 7.1 to 7.5 in an aquarium supplied with a continuous flow of aerated de-chlorinated tap water for 30 days. During this period and posterior laboratory and test water (centrifuged and filtered Pilcomayo River water) acclimation the fish were fed with a daily ration of commercial fish food. Acclimation to test water was performed by adding small quantities of test water to the aquarium until most of the water volume corresponded to test water. One day before and during the experiment, fish were not fed.
Toxicity effect of silver on fish was tested in static systems (4L glass aquaria) with continuous artificial aeration, constant environmental temperature and natural laboratory photoperiod. Test water volume in each aquarium was 2L. Test silver concentrations were attained by spiking from stock solutions of 200 mg Ag L-1. The reagent-grade toxicants used to perform the stock solutions was AgNO3 (Merck analytical grade).
To define the range of silver concentrations to be employed in the bioassay a nominal concentration of 1 mg Ag L-1 was tested in an aquarium with 2L volume of Pilcomayo River water and 10 acclimated specimens of juvenile C. decemmacuatus for 96 h. Subsequently, the experimental design applied included different metal concentrations or treatments (T) with one control group (kept in test water and without metal addition). Fish (not sexed) taken from the acclimation tank were randomly distributed in the different experimental aquaria. Mean standard length of the specimens selected was 13.8 ± 1.2 mm and each aquarium contained 10 specimens. Metal concentration in the experimental aquaria was adjusted prior to the fish transfer. Survival was registered four times a day during 96 h. Water pH, conductivity and dissolved oxygen were measured with portable probes from HANNA (HANNA instruments, Inc. Woonsocket, RI, USA) daily. At the beginning of the toxicity test, water samples were collected into polypropylene conical tubes and acidified to pH<2 with concentrated nitric acid (reagent-grade) for metal analysis.
Silver in samples was measured by graphite furnace atomic absorption spectrophotometry Perkin Elmer AAnalyst 800 (Perkin Elmer, Inc. Waltham, MA, USA). Method detection limit was 0.008 mg L-1.
LC50 calculations
The median lethal concentration (LC50) at 24, 48, 72 and 96 hours (24-h LC50, 48-h LC50, 72-h LC50, 96-h LC50) were calculated using PROBIT method (Finney 1978) and the statistical program StatPlus (Analyst Soft Inc.).
Version 2.2.3 of the BLM Windows Interface (available at http://www.hydroqual.com/wr_ blm.html) was run in order to predict acute silver toxicity to P. promelas (toxicity mode). Pilcomayo River water quality parameters employed to run the BLM were temperature, pH, dissolved organic carbon, calcium, magnesium, sodium, potassium, sulphate, chloride and alkalinity.
Metal speciation
BLM (speciation mode) was also used to assess chemical speciation of the measured silver concentrations.
RESULTS
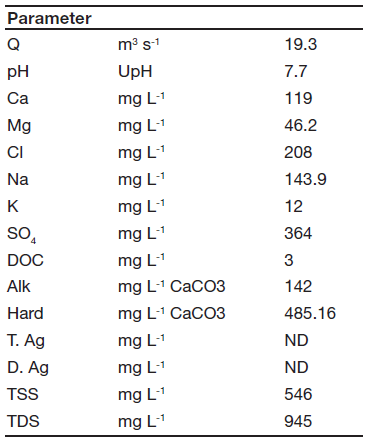
Water quality parameters of Pilcomayo River test water are shown in table 1.
Table 1. Test water main physicochemical parameters (data provided by Subsecretaría de Recursos Hídricos - Argentina). ND: not detected.
Toxicity test mean water pH and temperature were 8.3 ± 0.2 and 25.1 ± 1.2º C, respectively. Within the first few hours from the beginning of the toxicity test, a precipitate was observed. No mortality was observed in the control group. Initial exposure silver concentrations were 0.095 (T1), 0.148 (T2), 0.175 (T3) and 0.285 mg Ag L-1 (T4). Highest mortality was observed within the first five hours in all treatments.
The median lethal concentrations (LC50, mg L-1) at 24, 48, 72 and 96 h (24-h LC50, 48-h LC50, 72-h LC50, 96-h LC50) with their corresponding confidence intervals (quoted) calculated using PROBIT method and the 96-h Ag LC50 predicted by BLM for P. promelas in the toxicity test water are shown in table 2.
Table 2. The LC50 at 24, 48, 72 and 96 hours (24-h LC50, 48-h LC50, 72-h LC50, 96-h LC50) with their respective confidence intervals calculated for C. decemmaculatus and the predicted 96-h LC50 by BLM for P. promelas in the test water.

Figure 2 shows calculated silver toxicity to C. decemmaculatus compared with predicted silver toxicity (LC50, in μg Ag L-1) using the Biotic Ligand Model. As it can be observed BLM prediction was not accurate within a factor of 2.

Figure 2. Calculated silver toxicity to C. decemmaculatus compared with predicted silver toxicity (LC50, in μg Ag L-1) using the Biotic Ligand Model developed for P. promelas. The thicker line represents a 1:1 relationship. The thinner lines represents predictions within a factor of 2. The error bars represent 95% confidence intervals.
BLM speciation output can be observed in figure 3. Silver speciation in the four treatments was practically the same with 60% of the silver as AgCl and 30% as AgCl2. The free ion form represented the highest contribution (6%) amongst the remaining species group.

Figure 3. BLM speciation output for each treatment. Silver species are expressed as percentages of total dissolved silver concentration. Remaining species summeraizes the contribution of the following species: free ion, AgCl3, AgCl4, AgNO3, AgSH, Ag(SH)2, Torg Ag, AgOH and Ag(OH)2.
DISCUSSION
Pilcomayo River water characterizes by its high water hardness. The ameliorative effects of water hardness on copper toxicity have been well documented; whereas, its protective effect on silver toxicity seems to be not clear. For Paquin et al. (2002), when competing with silver at the biotic ligand, calcium tends to be of less importance than it appears to be for other metals. Bury et al. (1998) found that an increment on calcium concentration from 2 to 80 mg L-1 had a small influence on the 96-h LC50 for rainbow trout and P. promelas.
These authors reported a 96-h Ag LC50 for juvenile P. promelas of 0.008 mg L-1. Karen et al. (1999) reported that dissolved organic carbon was more important than hardness for predicting the toxicity of ionic silver in natural waters to O. mykiss, P. promelas, and D. magna. On the other hand, Erickson et al. (1998) reported a substantial decrease in silver toxicity to P. promelas (96-h LC50 increase from 0.005 to 0.012 mg L-1) when water hardness was increased from 48 to 249 mg L-1 CaCO3. Bielmyer et al. (2007) found that C. dubia and P. promelas were less sensitive to silver in waters with a combination of higher hardness and dissolved organic carbon. In water with a hardness of 225 mg L-1 CaCO3, these authors reported a 96-h LC50 for 7-days old P. promelas of 0.021 (0.019-0.023) mg L-1. Despite the opposite results reported, higher 96-h LC50 estimated for C. decemmaculatus may reflect some protective effect exerted by Pilcomayo River water elevated hardness.
High levels of sodium are thought to inhibit silver accumulation through competition; however, Bury and Wood (1999) found that one-third of the silver uptake continued in presence of a sodium channel and ATPase blockers or high levels of sodium in water. Erickson et al. (1998) reported no significant effects on silver toxicity to P. promelas when 2 meq L-1 of sodium sulphate were added to test water. This means that alternatively or additionally, multiple pathways exist for apical silver uptake at the gill cells. If multiple silver pathways are also present in C. decemmaculatus, the high levels of sodium in Pilcomayo River water did not exert a protective effect against silver toxicity.
Pilcomayo River dissolved organic matter levels are relatively low. Increased levels of organic carbon are expected to affect silver bioavailability through complexation. Bury et al. (1998) observed that an increase of 0.3 to 5.8 mg L-1 in dissolved organic carbon reduced acute silver toxicity to rainbow trout and P. promelas. Erickson et al. (1998) reported that increased levels of dissolved organic carbon (from 1 to 11 mg C L-1) significantly increased Ag LC50 for 30-day-old P. promelas. However, Rose-Janes and Playle (2000) highlighted the strong binding of Ag+ to trout gills and the relatively weaker binding of silver to dissolved organic matter. In the present contribution, dissolved organic carbon did not exert an important effect on silver speciation, since the predicted percentage of silver bound to dissolve organic matter was very low (0.52 %).
A net loss of chloride is also observed during silver exposure. The protective effect of high levels of chloride in water has been shown to be different among species. Some species rely on branchial chloride uptake, which, in some species, is not inhibited by silver exposure, and others depend on multiple chloride uptake pathways. Apparently, chloride uptake pathways in P. promelas are not inhibited by silver exposure (Bielmyer et al. 2008). Given that chloride uptake pathways in C. decemmaculatus are unknown, we cannot suggest that high chloride concentration in Pilcomayo River water could have exerted a protective effect against silver toxicity. External chloride may also exert a protective effect through complexation. In this regard, BLM speciation output for each of the silver concentrations tested showed that 90% of the silver was as AgCl and AgCl2. High chloride levels have been shown to protect O. mykiss against silver toxicity presumably by complexation to form AgCl, thereby reducing the concentration of Ag+. However, the formation of AgCl does not appear to substantially influence silver sensitivity for any other fish species tested (Bielmyer et al. 2008). Therefore, it remains to determine experimentally whether changes in silver speciation related to changes in chloride concentration reduce silver toxicity to C. decemmaculatus.
Water pH is another water quality parameter that would affect silver toxicity through speciation. During the present toxicity test, mean water pH was 8.3. In this regard, Erickson et al. (1998) reported a reduction in silver toxicity with increasing pH. The 96-h LC50 at pH 8.6 was three times higher than at pH 7.2. In the present toxicity test, higher 96-h LC50 for C. decemmaculatus may reflect some protective effect against silver toxicity exerted by the elevated mean water pH.
Silver BLM LC50 prediction for P. promelas in Pilcomayo River water did not agree well with the LC50 calculated for C. decemmaculatus. Bielmyer et al. (2007) in their silver BLM validation study found that only 50% of the LC50 predicted values for P. promelas were accurate within a factor of two. Higher 96-h LC50 for C. decemmaculatus may show that this species is less sensitive to silver toxicity than other fish species. However, it must be taken into account that almost all of the studies previously cited on silver toxicity to P. promelas worked with younger life-stages of the species which are known to be more sensitive to pollutants. In this regard, Bielmyer et al. (2007) reported higher 96-h LC50 for P. promelas as fish aged and Grosell and co-authors (2002) reported higher sodium turnover in smaller animals compared with larger animals in fresh water. Bianchini et al. (2002) studied LC50 values based on total silver as a function of the body mass. According to their results, log LC50 progressively increased with log body weight, independent of the species considered. For the mean body weight of the animals tested in the present contribution, the relationship found by these authors predicted a lower LC50 (0.45 μg Ag/L) compared to the value calculated for C. decemmaculatus (140 μg Ag/L). This difference may be another indicator of a lower sensitivity of C. decemmaculatus to silver toxicity.
In the present contribution, a clear pattern of mortality was observed. Most of the deaths occurred within the first five hours. Due to the fact that silver precipitation was observed, is possible that fish were in contact with higher silver concentrations during the first minutes to hours of the toxicity test. We did not measure silver accumulation on the gills but this pattern of mortality would correspond to a peak and decline in gill silver accumulation observed in static toxicity tests. Nebeker et al. (1983); Erickson et al. (1998) and Morgan et al. (2004) attributed this pattern of silver accumulation to changes in silver bioavailability through complexation with the increasing organic matter. In the present contribution, silver concentrations tested were higher compared to other toxicity tests performed, therefore, changes in silver bioavailability may have occurred mainly by silver precipitation.
CONCLUSIONS
C. decemmaculatus higher Ag 96-h LC50 may reflect that this species is less sensitive to silver than other fish species. The mortality pattern observed suggests that toxicity can be attributed to the initial dissolved silver concentrations. The ameliorative effect of water hardness on silver toxicity is not clear; however, Pilcomayo River water elevated hardness may have exerted some protective effect. Dissolved organic carbon contribution in reducing silver bioavailabity was negligible. Elevated mean water pH may have exerted a major protective effect. More research is needed to determine the effects of high sodium and chloride concentrations on silver toxicity to C. decemmaculatus. The mortality pattern observed in this toxicity test may lend some support to a relationship between gill silver accumulation and mortality. A cross-fish-species extrapolation of the P. promelas Ag BLM was not valid in Pilcomayo River water and experimental conditions of this toxicity test.
Acknowledgements
This research was supported by grants of the University of Buenos Aires. The authors want to thank Subsecretaría de Recursos Hídricos-Argentina (SSRH) who kindly performed water sampling and monitoring operations at Misión La Paz. and provided Pilcomayo River water quality data. The authors also want to thank Mrs. Amalia González for the artwork and Dr. Sergio Goméz and Dr. Jimena González Naya for useful technical suggestions.
References
1. American Public Health Association, American Water Works Association, Water Pollution Control Federation (APHA, AWWA, WPCF). Standard Methods for the examination of water and wastewater. 20sth ed. Washington DC: APHA, AWWA, WPCF, 1992. [ Links ]
2. Bianchini A., Grosell M., Gregory S.M., Wood C.M. Acute Silver Toxicity in Aquatic Animals Is a Function of Sodium Uptake Rate. Environ. Sci. Technol. 2002;36,1763-1766. [ Links ]
3. Bielmyer G.K., Grosell M., Paquin P.R., Mathews R., Wu K.B. Validation study of the acute Biotic Ligand Model for silver. Environ Toxicol Chem. 2007;26(10):2241-2246. [ Links ]
4. Bielmyer G.K., Brix K.V., Grosell M. Is Cl- protection against silver toxicity due to chemical speciation? Aquat Toxicol. 2008;87:81-87. [ Links ]
5. Bury N.R., Galvez F., Wood C.M. Effects of chloride, calcium, and dissolved organic carbon on silver toxicity: comparison between rainbow trout and fathead minnows. Environ Toxicol Chem. 1999;18:56-62. [ Links ]
6. Bury N.R. and Wood C.M. Mechanism of branchial apical silver uptake by rainbow trout is via the proton-coupled Na+ channel. Am. J. Physiol. 1999;277:R1385-R1391. [ Links ]
7. Casares M.V., de Cabo L.I.; Seoane R., Natale O., Castro Rios M., Weigandt C., F. de Iorio A. Measured copper toxicity to Cnesterodon decemmaculatus (Pisces: Poeciliidae) and predicted by Bitic Ligand Model in Pilcomayo River water: a step for a cross-fish-species extrapolation [on line]. J Toxicol [Accessed Nov2012]. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3317198/pdf/JT2012- 849315.pdf [ Links ]
8. Clifford M. and McGeer J.C. Development of a biotic ligand model for the acute toxicity of zinc to Daphnia pulex in soft waters. Aquat Toxicol. 2009;91:26-32. [ Links ]
9. de la Torre F.R., Demichelis S.O., Ferrari L., Salibián A. Toxicity of Reconquista river water: bioassays with juvenile Cnesterodon decemmaculatus. Bull Environ Contam Toxicol. 1997;58:558-565. [ Links ]
10. de la Torre F.R., Ferrari L., Salibián A. Freshwater pollution biomarker: response of brain acetylcholinesterase activity in two fish species. Comp Biochem Physiol Part C. 2002;131:271-280. [ Links ]
11. de la Torre F.R., Ferrari L., Salibián A. Biomarkers of a native fish species (Cnesterodon decemmaculatus): application to the toxicity assessment of the water of a periurban polluted river of Argentina. Chemosphere. 2005; 59:577-583. [ Links ]
12. Di Toro D.M., Allen H.E., Bergman H.L., Meyer J.S., Paquin P.R., Santore R.C. A biotic ligand model of the acute toxicity of metals I. Technical basis. Environ Toxicol Chem. 2001; 20: 2383-2396. [ Links ]
13. Erickson R.J., Benoit D.A., Mattson V.R., Nelson H., Leonard E.N. The effects of water chemistry on the toxicity of copper to fathead minnows. Environ Toxicol Chem. 1996;15:181-193. [ Links ]
14. Erickson R.J., Brooke L.T., Kahl M.D., Venter F.V., Harting S.L., Markee T.P., Spehar R.L. Effects of laboratory test conditions on the toxicity of silver to aquatic organisms. Environ Toxicol Chem. 1998;17:572-578. [ Links ]
15. Ferrari L., García M.E., de la Torre F.R., Demichelis S.O. Evaluación Ecotoxicológica del agua de un río urbano mediante bioensayos con especies nativas. Rev. Museo Argentino de Ciencias Naturales. Vol. Extra Nueva Serie. 1998;148:1-16. [ Links ]
16. Finney D.J. Statistical method in biological assay. London: Charles Griffin, 1978. [ Links ]
17. Gaillardet J., Dupré B., Louvat P., Allègre C. J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology. 1999;159:3-30. [ Links ]
18. Goméz S., Villar C., Bonetto C. Zinc toxicity in the fish Cnesterodon decemmaculatus in the Paraná River and Río de La Plata Estuary. Environ Pollut. 1998;99:159-165. [ Links ]
19. Grosell M., Nielsen C., Bianchini A. Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol Part C. 2002;133:287-303. [ Links ]
20. Karen D.J., Ownby D.R., Forsythe B.L., Bills T.P., La Point T.W., Cobb G.B., Klaine S.J. Influence of water quality on silver toxicity to rainbow trout (Oncorhynchus mykiss), fathead minnows (Pimephales promelas), and water fleas (Daphnia magna) Environ Toxicol Chem. 1999;18(1):63-70. [ Links ]
21. Kennedy C.J. The toxicology of metals in fishes. In: Encyclopedia of fish physiology: from genome to environment. Farrell A. P. (ed). San Diego: Academic Press 3 2011: p. 2061- 2068. [ Links ]
22. Lauren D.J., McDonald D.G. Influence of water hardness, pH, and alkalinity on the mechanisms of copper toxicity in juvenile rainbow trout, Salmo gairdneri. Can J Fish Aquat Sci. 1986;43:1488-1496. [ Links ]
23. Matsuo A.Y.O., Playle R.C., Val A.L., Wood C.M. Physiological action of dissolved organic matter in rainbow trout in the presence and absence of copper: sodium uptake kinetics and unidirectional flux rates in hard and soft water. Aquat Toxicol. 2004; 70: 63-81. [ Links ]
24. Menni R.C. Monografías del Museo Argentino de Ciencias Naturales No 5 2004 p.50- 53. [ Links ]
25. Morgan T.P., Grosell M., Gilmour K.M., Playle R., Wood C.M. Time course analysis of the mechanism by which silver inhibits active Na+ and Cl- uptake in gills of rainbow trout. Am J Physiol Regul Comp Physiol. [ Links ]
26. Nebeker A.V., McAuliffe C.K., Mshar R., Stevens D.G. Toxicity of silver to steelhead and rainbow trout, fathead minnows and Daphnia magna. Environ. Toxicol. Chem. 1983;2:95-104. [ Links ]
27. Paquin P.R., Gorsuch J.W., Apte S., Batley G.E., Bowles K.C., Campbell P.G.C., Delos C.G., Di Toro D.M., Dwyer R.L., Galvez F., Gensemer R.W., Goss G.G., Hogstrand C., Janssen C.R., McGeer J.C., Naddy R.B., Playle R.C., Santore R.C., Schneider U., Stubblefield W.A., Wood C.M., Wu K.B. The biotic ligand model: a historical overview. Comp Biochem Physiol Part C. 2002;133:3-35. [ Links ]
28. Ratte H.T. Bioaccumulation and toxicity of silver compounds: a review. Annual Review. Environ Toxicol Chem. 1999;18(1):89-108. [ Links ]
29. Rose-Janes N.G. and Playle R.C. Protection by two complexing agents, thiosulphate and dissolved organic matter, against the physiological effects of silver nitrate to rainbow trout (Oncorhynchus mykiss) in ion-poor water. Aquat Toxicol. 2000;51:1-18. [ Links ]
30. Santore R.C., Mathew R., Paquin P.R., Di- Toro D. Application of the biotic ligand model to predicting zinc toxicity to rainbow trout, fathead minnow, and Daphnia magna. Comp Biochem Physiol Part C. 2002;133: 271-285. [ Links ]
31. Schlekat E.C., Van Genderen E., De Schamphelaere K.A.C., Antunes P.M.C., Rogevich E.C., Stubblefield W.A. Cross-species extrapolation of chronic nickel Biotic Ligand Model. Sci Total Environ. 2010;408: 6148-6157. [ Links ]
32. Smolders A.J.P., Lock R.A.C., Van der Velde G., Medina Hoyos R.I., Roelofs J.G. Effects of Mining Activities on Heavy Metal Concentrations in Water, Sediment, and Macroinvertebrates in Different Reaches of the Pilcomayo River, South America. Arch. Environ Contam Toxicol. 2003;44:314-323. [ Links ]
33. Wood C.M. The biotic ligand model: Using physiology, geochemistry, and modeling to predict metal toxicity. Comp Biochem Physiol Part C. 2008;148: (4) 468-469. [ Links ]














