Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Acta toxicológica argentina
versión On-line ISSN 1851-3743
Acta toxicol. argent. vol.22 no.3 Ciudad Autónoma de Buenos Aires dic. 2014
ARTÍCULOS
Histo-cytological responses of the prolactin cells of the catfish Heteropneustes fossilis to cadmium exposure
Respuesta histológica y citológica frente a la exposición a cadmio de las células prolactínicas del pez gato Heteropneustes fossilis
Srivastav, A.K.1*; Rai, Rubi1; Mishra, D.2; Srivastav, S. K.1; Suzuki, Nobuo3
1Department of Zoology, D.D.U. Gorakhpur University, Gorakhpur 273 009, India.
2Department of Zoology, Government Girls P.G. College, Ghazipur, (U.P.), India.
3Noto Marine Laboratory, Institute of Nature and Environmental Technology, Kanazawa University, Ogi, Noto-cho, Ishikawa 927-0553, Japan.
Recibido: 31 de enero de 2014
Aceptado: 17 de julio de 2014
Abstract. Cadmium is an important metal for modern industrial processes and, being biologically non-essential, poses health hazards to the organisms. In this study we aimed to evaluate the effect of cadmium exposure on the histo-cytology of prolactin cells in the freshwater catfish, Heteropneustes (H.) fossilis. Fish were subjected to 288 mg/L (0.8 of 96 h LC50) and 72 mg/L (0.2 of 96 h LC50) of cadmium chloride for short-term and long-term, respectively. After sacrificing the fish, the blood was collected on 24, 48, 72 and 96 h in short-term and after 7, 14, 21, and 28 days in long-term experiment and analyzed for plasma calcium levels. Also, pituitary glands were fixed on these intervals. The plasma calcium levels of short-term cadmium exposed fish remain unchanged after 24 h. The levels exhibit a progressive decrease from 48 h onwards. The fish exposed to cadmium for 7 days exhibit a decrease in the plasma calcium level. Thereafter, the levels progressively decrease till the end of the experiment (28 days). The prolactin cells of the control fish exhibit structural resemblance to the description given for the prolactin cells of normal H. fossilis. No change in the histological structure and nuclear volume of prolactin cells of cadmium non-exposed fish has been noticed throughout the experiment. In cadmium treated fish, the prolactin cells remain unchanged till 14 days. On day 21, the nuclear volume of these cells exhibits an increase and the cells degranulate. These changes increased profoundly on day 28. In addition, vacuolization and cytolysis were also encountered on day 28 following cadmium treatment. It is concluded that cadmium affects the prolactin cells of the fish H. fossilis thus disturbing the ionic balance.
Keywords: Cadmium; Fish; Prolactin; Pituitary gland
Resumen. El cadmio es un metal importante para los procesos industriales modernos, siendo no esencial biológicamente, representa riesgos para la salud de organismos. En este estudio tratamos de evaluar el efecto de la exposición al cadmio por el aspecto histológico y citológico de células secretoras de prolactinas del pez gato de agua dulce Heteropneustes (H.) fossilis. Los peces fueron sometidos a una exposición de 288 mg/L (0,8 de 96 h CL50) and 72 mg/L (0,2 de 96 h CL50) de cloruro de cadmio por a corto y largo término respectivamente. Después del sacrificio de los peces, la sangre fue colectada, tomando muestras de 24, 48, 72 y 96 hs en el corto término y de 7, 14, 21 y 28 días en las sometidas a largo término, la cuales se analizaron para medir niveles de calcio. Además, las glándulas pituitarias fueron fijadas en esos intervalos El nivel plasmático de calcio en los experimentos de exposición a corto tiempo se mantuvo sin cambio tras 24 h. Los niveles exhibieron una caída progresiva a partir de las 48 hs. Los peces expuestos a cadmio por 7 días presentaron una disminución en el nivel plasmático de calcio. Después de esto, los niveles decayeron progresivamente hasta el fin del experimento (28 días). Las células prolactínicas de los peces controles mostraron semejanza estructural a la descripción dada para estas células normales en H. fossilis. No se observaron cambios en la estructura histológica y el volumen nuclear de las células prolactínicas de los peces no expuestos a cadmio a través de todo el experimento. En los peces tratados con cadmio las células prolactínicas se mantuvieron sin cambios hasta los 14 días. En el día 21, el volumen nuclear de esas células se incrementó y estas células presentaron desgranulación. Estos cambios aumentaron profundamente en las muestras del día 28. Adicionalmente en el día 28 posterior al tratamiento con cadmio se encontró vacuolización y citólisis. Se concluyó en que el Cadmo afecta las cñelupas prolactínicas de H fossilis, produciendo disturbios en el balance iónico.
Palabras clave: Cadmio; Peces; Prolactina; Glándula pituitaria.
Introduction
Cadmium is an important metal for modern industrial processes and being biologically non-essential poses health hazards to the organisms. The toxic effects of cadmium have been investigated in mammals (Jarup 2003; Johansen et al. 2006), amphibians (Othman et al. 2009; Sparling and Fellers 2009; Jia et al. 2010) and fish (Chan and Cheng 2003; Lacroix and Hontela 2004; Metz et al. 2005; Senger et al. 2006; Isani et al. 2009; Wu et al. 2009; Poleksic et al. 2010; Kalman et al. 2010; Brunelli et al. 2011). Fish, inhabiting a complex and diverse environment, are particularly more susceptible to environmental contamination as they are exposed to aquatic toxicants through their extensive and delicate respiratory surface of the gill (Wendelaar Bonga 1997). After entering into an animal’s physiological system, cadmium accumulates in various tissues (Hilmy et al. 1985; Rani 2000; Bervoets et al. 2001; Rashed 2001; Szebedinszky et al. 2001; Pillai et al. 2002) and causes serious damage to vital organs.
Although few reports are available regarding the effects of toxicants on the endocrine gland regulates calcium homeostasis in fish (Fu et al. 1989; Srivastav et al. 2002), the effects of cadmium is least studied in this group with regard to similar aspect. Hence, in this study we aimed to evaluate the effect of cadmium exposure on the histo-cytology of prolactin cells in the fish, Heteropneustes fossilis (Bloch, 1794) (Siluriformes, Heteropneustidae).
Materials and Methods
Live freshwater catfish H. fossilis (body weight 37-44 g) were collected and acclimatized for 15 days in plastic pools. The experiments were run for short-term (for 96h; by using 0.8 of 96h LC50 value of cadmium chloride i.e. 288 mg/L), and long-term (for 28 days; by using 0.2 of 96h LC50 value of cadmium chloride i.e.72 mg/L). Simultaneously, a control group was also used for comparison in both experiments. Fish were kept in groups of 10 in 30 L media. Six fish were killed on each time intervals from control and experimental groups in short-term (24, 48, 72, and 96h) and long-term (7, 14, 21, and 28 days) treatment and blood samples were collected by sectioning of the caudal peduncle. Plasma calcium levels were analyzed by Sigma kits. After collection of blood sample, the pituitary gland along with the brain were fixed in aqueous Bouin’s fluid and Bouin’s-Hollande for histological studies. Tissues were routinely processed in graded series of alcohol, cleared in xylene and embedded in paraffin wax. Serial sections were cut at 6-μm. The pituitaries were stained with Herlant tetrachrome and Heidenhain’s azan techniques.
Nuclear indices (maximal length and maximum width) of the prolactin cells (for each fish, 50 randomly nuclei were measured in those cells whose perimeter was clearly discernible in the plane of the section) were taken using ocular micrometer. The nuclear volume was calculated as -
volume = 4/3 π ab2
where ‘a’ is the major semiaxis and ‘b’ is the minor semiaxis. Student’s t test was used to test for significant differences between the control and experimental groups with P<0.05 being accepted as significant.
Results
The plasma calcium levels of the fish exposed to cadmium for 24 h exhibit no change. After 48 h of cadmium exposure, the level records a significant decrease. This response persisted until the end of the experiment (96 h) (Figure 1). The fish exposed to cadmium for 7 days exhibited a decrease in plasma calcium level. Thereafter, the levels progressively decrease until the end of the experiment (28 days; Figure 2).
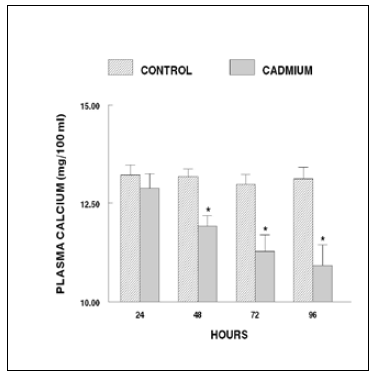
Figure 1. Plasma calcium levels of short-term cadmium chloride treated fish. Values are mean ± S.E. of six specimens. Asterisk indicates significant differences (P< 0.05) from control.
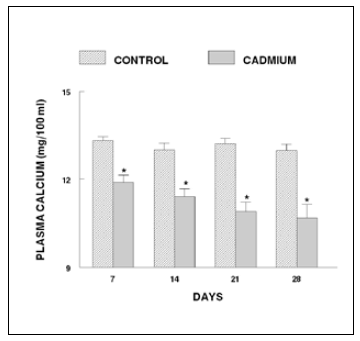
Figure 2. Plasma calcium levels of long-term cadmium chloride treated fish. Values are mean ± S.E. of six specimens. Asterisk indicates significant differences (P< 0.05) from control.
In control fish, the prolactin cells possess indistinct cell boundaries (Figure 3). However, the nuclei are distinct with dense chromatin granules. The cytoplasm is scanty and azocarminophilic and erythrosinophilic in nature. No change in the histological structure and nuclear volume of prolactin cells of cadmium exposed fish has been noticed throughout the short term experiment. In cadmium treated fish the prolactin cells remain unchanged till 14 days. On day 21, the nuclear volume of these cells exhibited a significant increase (Figure 4) and the cells degranulated (Figure 5). These changes increased profoundly on day 28. In addition, vacuolization and cytolysis were also encountered on day 28 following cadmium treatment (Figure 6).
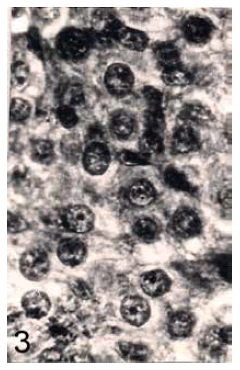
Figure 3. Prolactin cells of control fish. Herlant tetrachrome x 800.
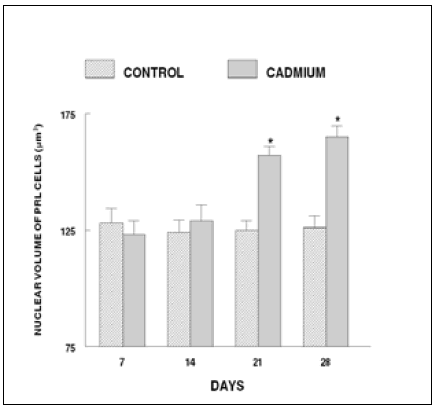
Figure 4. Nuclear volume of prolactin cells of long-term cadmium chloride exposed fish. Each value represents mean ± S.E. of six specimens. Asterisk indicates significant differences (P< 0.05) from control.

Figure 5. Prolactin cells of 21 days cadmium chloride treated fish exhibiting degranulation. Herlant tetrachrome x 800.
Figure 6. Prolactin cells of 28 days cadmium chloride exposed Heteropneustes fossils showing vacuolization (arrow) and cytolysis. (broken arrow). Herlant tetrachrome x 800.
Discussion
Cadmium exposed fish exhibited degranulation and increased nuclear volume of prolactin cells. Prolactin cell activity in response to toxicants has been reported by James and Wigham (1986), Fu et al. (1989), Mishra et al. (2008, 2011) and Srivastav et al. (2010). The present study is in agreement with the observations of earlier investigators who have reported hyperactivity of prolactin cells after exposure of fish to toxicants- cadmium (Fu et al. 1989), metacid (Mishra et al. 2008), cypermethrin (Mishra et al. 2011) and deltamethrin (Srivastav et al. 2010). However, James and Wigham (1986) noticed no effect on prolactin cell activity following cadmium injection to rainbow trout. The reports of Meredith et al. (1999), Thangavel et al. (2005, 2010) and Ramesh et al. (2009) supports the observations of this study as elevated levels of prolactin in fish has been recorded after exposure to toxicants. Moreover, it has also been reported that lead exposure to fish Catla catla caused a decrease in calcium and phosphate content of bones (Palaniappan et al. 2010). Bone demineralization has been reported in cadmium exposed carp (Koyama and Itazawa 1977; Muramoto 1981).
Prolactin has been implicated to produce hypercalcemia in various species of fishes (Pang et al. 1978; Wendelaar Bonga and Flik 1982; Flik et al. 1986; 1994; Wendelaar Bonga and Pang 1991) mainly by controlling the gill epithelium permeability (Dharmamba and Maetz 1972; Clark and Bern 1980; Wendelaar Bonga et al. 1983). In teleosts, prolactin has been reported to control ion uptake mechanisms (Pickford and Phillips 1959; Sakamoto and McCormick 2006). Sakamoto and McCormick (2006) have suggested that prolactin has the ability to control proliferation, differentiation and necrosis/ apoptosis of chloride cells. In the present study an increased activity of prolactin cells in cadmium treated H. fossilis is indicative of possible action of prolactin on gills, kidney and bones to restore the ionic content of blood.
References
1. Bervoets L., Blust R., Verheyen R. Accumulation of metals in the tissues of three spined stickleback (gasterosteus aculeatus) from natural fresh waters. Ecotoxicology & Environmental Safety. 2001;48:117-127. [ Links ]
2. Brunelli E., Mauceri A., Maisano M., Bernabo I., Giannetto A., De Domenico E., Corapi B., Tripepi S., Fasulo S. Ultrastructural and immunohistochemical investigation on the gills of the teleost, Thalassoma pavo L., exposed to cadmium. Acta Histochemica. 2011;113:201-213. [ Links ]
3. Chan P.K., Cheng S.H. Cadmium-induced ectopic apoptosis in zebrafish embryos. Archives of Toxicology. 2003;77:69-79. [ Links ]
4. Clark W., Bern H.A. Comparative endocrinology of prolactin. In: Hormonal Proteins and Peptides, vol. 8 (Li, C.H., ed.), Academic Press, New York; 1980. p. 105-197. [ Links ]
5. Dharmamba M., Maetz J. Effects of hypophysectomy and prolactin on the sodium balance of Tilapia mossambica in freshwater. General& Comarative Endocrinology. 1972;19:175-183. [ Links ]
6. Flik G., Fenwick J.C., Kolar Z., Mayer-Gostan N., Wendelaar Bonga S.E. Effects of ovine prolactin on calcium uptake and distribution in the freshwater cichlid teleost fish, Oreochromis mossambicus. American Journal of Physiology. 1986;250:161-166. [ Links ]
7. Flik G., Rentier-Delrue F. Wendelaar Bonga S.E. Calcitropic effects of recombinant prolactins in Oreochromis mossambicus. American Journal of Physiology. 1994;266:1302-1308. [ Links ]
8. Fu H., Lock R.A.C. Wendelaar Bonga S.E. Effect of cadmium on prolactin cell activity and plasmna electrolytes in the freshwater teleost Oreochromis mossambicus. Aquatic Toxicology. 1989;14:295-306. [ Links ]
9. Hilmy A.M., Shabana M.B., Daabees A.Y. Bioaccumulation of cadmium: toxicity in Mugil cephalus. Comparative Biochemistry & Physiology. 1985;81:39-144. [ Links ]
10. Isani G., Andreani G., Cocchioni F., Fedeli D., Carpene E., Falcioni G. Cadmium accumulation and biochemical responses in Sparus aurata following sublethal cadmium exposure. Ecotoxicology & Environmental Safety. 2009;72:224-230. [ Links ]
11. James V.A., Wigham T. The effects of cadmium on prolactin cell activity and plasma cortisol levels in the rainbow trout (Salmo gairdneri). Aquatic Toxicology. 1986;8:273-280. [ Links ]
12. Jarup L. Hazards of heavy metal contamination. British Medical Bulletin. 2003;68:167-182. [ Links ]
13. Jia X., Shi C., Liu X. Effects of cadmium on oxidative stress and metallothioneim of liver of frog, Rana nigromaculata. Shengtai Xuebao/ Acta Ecologica Sinica. 2010;30:416-420. [ Links ]
14. Johansen P., Mulvad G., Pedersen H.S., Hansen J.C. Riget F. Accumulation of cadmium in livers and kidneys in Greenlanders. Science of the Total Environment. 2006;372:58-63. [ Links ]
15. Kalman J., Riba I., DelValls T.A., Blasco J. Comparative toxicity of cadmium in the commercial fish species Sparus aurata and Solea senegalensis. Ecotoxicology & Environmental Safety. 2010;73:306-311. [ Links ]
16. Koyama J. Itazawa Y. Effects of oral administration of cadmium on fish. I. Analyt i - cal results of the blood and bones. Bulletin of the Japanese Society for the Science of Fish. 1977;43:523-526. [ Links ]
17. Lacroix A., Hontela A. A comparative assessment of the adrenotoxic effects of cadmium in two teleost species, rainbow trout, Oncorhynchus mykiss, and yellow perch, Perca flavescens. Aquatic Toxicology. 2004;67:13-21. [ Links ]
18. Meredith H.O., Richman N.H., Collier J.T., Seale A.P., Riley L.G., Ball C.H., Shimoda S.K., Stetson M.H., Grau E.G. Pesticide effects on prolactin release from the rostral pars distalis and their effects on growth in the tilapia. In: Environmental Toxicology and Risk Assessment: Standardization of Biomarkers for Endocrine Disruption and Environmental Assessment, 8th Volume, Henshel DS, Black MC, Harrass MC (eds), American Society for Testing and Materials (ASTM), STP 1364, West Conshohocken, PA; 1999. p. 239-253. [ Links ]
19. Metz C.J., Treble R.G., Krone P.H. Accumulation and elimination of cadmium in larval stage zebrafish following acute exposure. Ecotoxicology & Environmental Safety. 2005;66:44-48. [ Links ]
20. Mishra D., Rai R., Srivastav S.K., Srivastav A.K. Histological alterations in the prolactin cells of a teleost Heteropneustes fossilis after exposure to cypermethrin. Environmental Toxicology. 2011;26:359-363. [ Links ]
21. Mishra D., Srivastav S.K., Srivastav A.K. Effects of insecticide cypermethrin on plasma calcium and ultimobranchial gland of the teleost Heteropneustes fossilis. Ecotoxicology& Environmental Safety. 2005;60:193-197. [ Links ]
22. Mishra D., Srivastav S.K., Suzuki N. Srivastav A.K. Prolactin cells of a teleost Heteropneustes fossilis intoxicated with metacid-50. International Journal of Biological & Chemical Sciences. 2008;2:339-345. [ Links ]
23. Muramoto S. Vertebral column damage and decrease of calcium concentrations in fish exposed experimentally to cadmium. Environmental Pollution (Ser. A). 1981;24:125-133. [ Links ]
24. Othman M.S., Khonsue W., Kitana J., Thirakhupt K., Robson M.G., Kitana N. Cadmium accumulation in two populations of rice frogs (Fejervarya limnocharis) naturally exposed to different environmental cadmium levels. Bulletin of Environmental Contamination & Toxicology. 2009;83:703-707. [ Links ]
25. Palaniappan P.R., Krishnakumar N., Vadivelu M., Vijayasundaram V. The study of the changes in the biochemical and mineral contents of bones of Catla catla due to lead intoxication. Environmental Toxicology. 2010;25:61-67. [ Links ]
26. Pang P.K.T., Schreibman M.P., Balbontin F. Pang R.K. Prolactin and pituitary control of calcium regulation in the killifish, Fundulus heteroclitus. General & Comparative Endocrinology. 1978;36:306-316. [ Links ]
27. Pickford G.E., Phillips J.G. Prolactin, a factor promoting survival of hypophysectomized killifish in freshwater. Science. 1959;130: 454-455. [ Links ]
28. Pillai A., Laxmi Priya P.N., Gupta S. Effects of combined exposure to lead and cadmium on pituitary membrane of female rats. Archives of Toxicology. 2002;76:671-675. [ Links ]
29. Poleksic V., Lenhardt M., Jaric I., Djordjevic D., Gacic Z., Cvijanovic G. Raskovic B. Liver, gills, and skin histopathology and heavy metal content of the Danube starlet (Acipenser ruthenus Linneaus, 1758). Environmental & Toxicological Chemistry. 2010;29:515-521. [ Links ]
30. Ramesh M., Saravanan M., Kavitha C. Hormonal responses of the fish, Cyprinus carpio, to environmental lead exposure. African Journal of Biotechnology. 2009;17:4154-4158. [ Links ]
31. Rani A.U. Cadmium-induced bioaccumulation in the selected tissues of a freshwater teleost, Oreochromis mossambicus (tilapia). Annalsof the New York Academy of Sciences. 2000;919:318-320. [ Links ]
32. Rashed M.N. Cadmium and lead levels in fish (Tilapia nilotica) tissues as biological indicator for lake water pollution. Environmenatl Monitoting & Assessment. 2001;68:75-89. [ Links ]
33. Sakamoto T., McCormick S.D. Prolactin and growth hormone in fish osmoregulation. General & Comparative Endocrinology. 2006;147:24-30. [ Links ]
34. Senger M.R., Rosenberg D.B., Rico E.P., de Bem Arizi M., Dias R.D. Bogo M.R., Bonan C.D. In vitro effect of zinc and cadmium on acetylcholinesterase and ectonucleotidase activities in zebrafish (Danio rerio) brain. Toxicology. 2006;20:954-958. [ Links ]
35. Sparling D.W., Fellers G.M. Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environmental Toxicology & Chemistry. 2009;28:1698-1703. [ Links ]
36. Srivastav A.K., Srivastava S.K., Mishra D., Srivastav S., Srivastav S.K. Ultimobranchial gland of freshwater catfish, Heteropneustes fossilis in response to deltamethrin treatment. Bulletin of Environmental Contamination & Toxicology. 2002;68:584-591. [ Links ]
37. Srivastav A.K., Srivastava S.K., Mishra D., Srivastav S.K., Suzuki N. Effects of deltamethrin on serum calcium and corpuscles of Stannius of freshwater catfish, Heteropneustes fossilis. Toxicological & Environmental Chemistry. 2009;91:761-772. [ Links ]
38. Srivastav A.K., Srivastava S.K., Tripathi S., Mishra D., Srivastav S.K. Chlorpyrifos based commercial formulation: Alterations in corpuscles of Stannius of catfish. Internatonal Journal of Environmenatl Health. 2010;4:323-332. [ Links ]
39. Srivastav A.K., Srivastava, S.K., Mishra, D., Srivastav, S.K. Deltamethrin-induced alterations in serum calcium and prolactin cells of a freshwater teleost, Heteropneustes fossilis. Toxicological & Environmental Chemistry. 2010;92:1857-1864. [ Links ]
40. Szebedinszky C., McGeer J.C., McDonald D.G., Wood C.M. Effects of chronic Cd exposure via the diet or water on internal organspecific distribution and subsequent gill Cd uptake kinetics in juvenile rainbow trout (Oncorhynchus mykiss). Environmental Toxicology & Chemistry. 2001;20: 597-607. [ Links ]
41. Thangavel P., Sumathiral K., Karthikeyan S., Ramaswamy M. Endocrine response of the freshwater teleost, Sarotherodon mossambicus (Peters) to dimecron exposure. Chemosphere. 2005;61:1083-1092. [ Links ]
42. Thangavel P., Sumathiral K., Maheshwari S., Rita S., Ramaswamy M. Hormone profile of an edible, freshwater teleost, Sarotherodon mossambicus (Peters) under endosulfan toxicity. Pesticide Biochemistry & Physiology. 2010;97:229-234. [ Links ]
43. Wendelaar Bonga S.E. Flik G. Prolactin and calcium metabolism in a teleost fish Sarotherodon mossambicus. In: Comparative Endocrinology of Calcium Regulation, Oguro, C. and Pang, P.K.T., eds. Jpn. Sci. Soc. Press, Tokyo, 1982. p. 19-26. [ Links ]
44. Wendelaar Bonga S.E. Pang P.K.T. Control of calcium regulating hormones in the vertebrates: Parathyroid hormone, calcitonin, prolactin and stanniocalcin. International Review of Cytology. 1991;128:139-213. [ Links ]
45. Wendelaar Bonga S.E. The stress response in fish. Physiological Review. 1997;77:591-625. [ Links ]
46. Wendelaar Bonga S.E., Lowik C.J.M., Van der Meij J.C.A. Effects of Mg2+ and Ca2+ on branchial osmotic water permeability and prolactin secretion in the teleost fish Sarotherodon mossambicus. General & Comparative Endocrinology. 1983;52:222-231. [ Links ]
47. Wu S.M., Jong K.J., Lee Y.J. Relationships among metallothionein, cadmium accumulation and cadmium tolerance in three species of fish. Bulletin of Environmental Contamination & Toxicology. 2009;76:595-600. [ Links ]














