Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Acta toxicológica argentina
On-line version ISSN 1851-3743
Acta toxicol. argent. vol.26 no.2 Ciudad Autónoma de Buenos Aires Sept. 2018
ARTÍCULOS ORIGINALES
Toxic, cytotoxic and genotoxic potential of synthetics food flavorings
Potencial tóxico, citotóxico y genotóxico de saborizantes sintéticos de alimentos
Barros Rocha, Romário; Peron, Ana Paula*; Silva Santos, Fabelina Karollyne; Mendes Marques, Márcia Maria; Silva Sousa, Maria Eduarda; de Oliveira, Valtânia Ana; do Nascimento, Andressa Larissa
Laboratório de Citogenética e Mutagênese (LaCM). Curso de Ciências Biológicas. Campus Senador HelvÍdio Nunes de Barros (CSHNB), Universidade Federal do PiauÍ (UFPI). Rua CÍcero Duarte, 940. 64600-000. Picos, PiauÍ, Brazil.
Recibido: 21 de junio de 2018
Aceptado: 10 de septiembre de 2018
Abstract
Food flavorings in general are few studied for the toxicological aspect. This condition justifies toxicity, cytotoxicity and genotoxicity assessments of the substances. In the present study, the toxicity of banana, cherry and hazelnut flavorings was evaluated in meristematic cells of roots of Allium cepa, in pure form (as marketed) and in the concentrations of 12.5; 25 and 50%, after 24 and 48 hours of exposure. Toxic potential of these food additives was also evaluated against Artemia salina nauplii at concentrations of 0.78; 1.56; 3.12; 6.25; 12.5; 25 and 50%, after 24 hours of exposure. The three additives, in all treatments and times of analysis considered, caused significant inhibition of cell division in A. cepa, however did not cause cellular alterations to the evaluated meristems. These food flavorings also caused significant mortality to micro crustaceans with LC50<100 µg/mL. From this, under the conditions of mentioned analyzes, cherry, banana and hazelnut flavorings induced significant toxicity and cytotoxicity to the bioassays used.
Keywords: Aroma and flavor; Potential toxicicity; Allium cepa; Artemia salina.
Resumen
En general, los aspectos toxicológicos de los saborizantes de los alimentos son poco estudiados. Esta condición justifica las evaluaciones de toxicidad, citotoxicidad y genotoxicidad de estas sustancias. En el presente estudio, se evaluó la toxicidad de los aromas de plátano, cereza y avellana en células meristemáticas de raÍces de Allium cepa, en forma pura (según comercializa) y en concentraciones de 12.5; 25 y 50%, después de 24 y 48 horas de exposición. El potencial tóxico de estos aditivos alimentarios también se evaluó frente a nauplios de Artemia salina a concentraciones de 0,78; 1,56; 3.12; 6.25; 12.5; 25 y 50%, después de 24 horas de exposición. Los tres aditivos, en todos los tratamientos y tiempos de análisis considerados, causaron inhibición significativa de la división celular en A. cepa, sin embargo, no causaron alteraciones celulares a los meristemos evaluados. Estos saborizantes alimentarios también causaron una mortalidad significativa a microcrustáceos con LC50 <100 µg/ mL. A partir de esto, bajo las condiciones de los análisis descriptos, los aromatizantes de cereza, plátano y avellana indujeron toxicidad significativa y citotoxicidad para los bioensayos utilizados.
Palabras clave: Aroma y sabor; Potencial tóxico; Allium cepa; Artemia salina.
Introduction
Food additives or micro-ingredients have become mandatory in modern food, mainly because of their ability to maintain a long-time quality of food marketed in supermarkets (Xu et al. 2013; Sales et al. 2017). Among these substances, aroma and flavor additives are of particular relevance because they give or enhance aroma and flavor to the most varied types of processed foods (Adami and Condi 2016).
The aroma and flavor additives substances have a complex chemical composition, consisting of diluents, antioxidants, defoamers, preservatives, emulsifiers, stabilizers, acidity regulators, flavor enhancers, anti-wetting agents, anti-caking agents, dyes and extraction and processing solvents (Xu et al. 2013; Sales et al. 2017). The chemical formulation of the semicro-ingredients is approved for use worldwide by the Flavor Extract Manufacturers Association (FEMA), Food and Agriculture Organization (FAO) and in Brazil, by the National Health Surveillance Agency (ANVISA) (Agência Nacional de Vigilância Sanitária 2007; Carvalho et al. 2017). According to the Codex Alimentarius (2009), the general chemical formulation of flavor and flavor additives are standardized worldwide.
Although authorized, most of the flavorings used in the industry do not have established Acceptable Daily Intake (ADI), since so far there are few surveys of toxicological assessments on these food additives (Xu et al. 2013; Koca et al. 2015; Moura et al. 2016). Thus, it is relevant to carry out studies that determine the toxic, cytotoxic and genotoxic potential of flavor micro- ingredients (Koca et al. 2015; Sales et al. 2017). In this way pointed out that the toxicological evaluation, at systemic and cellular levels, of these additives is extremely important in terms of promoting food security of the population and providing a basis for the development or modification of food security strategies of surveillance agencies (Moura et al. 2016).
The root meristematic zone of Allium cepa L. (onion) is an efficient test organism for the evaluation of toxicity at cellular level (Herrero et al. 2012). This organism has excellent kinetic properties of proliferation, large chromosomes in reduced number (2n = 16), which facilitates the detection of chromosomal aberrations and abnormalities in the mitotic pindle (Tabrez et al. 2011; Gomes et al. 2013). It also allows the verification of changes in cell division or mitotic index when exposed to chemical compounds with potential cytotoxicaction (Neves et al. 2014). A. cepa system is very effective for initial assessment of cytotoxicity and genotoxicity of chemical compounds or for validation of these conditions after conducting research in other bioassays (Neves et al. 2014; Marques et al. 2015).
Another relevant test organism for the initial assessment of the toxicity of chemical compounds is Artemia salina (Anostraca) (Rosa et al. 2016). Nauplii of this species are used as biological test to evaluate the toxic potential of useful natural and/or synthetic substances (Paredes et al., 2016). The lethality of this organism has been used to identify biological responses, in which variables such as death or life are the only ones involved (Meyer et al. 1982; Paredes et al. 2016).
Thus, the present study aimed to evaluate, through different treatments or concentrations, the toxic, cytotoxic and genotoxic potentials of synthetic flavoring additives of cherry, banana and hazelnut, to the root meristem cells of A. cepa, in Vero cells and A. salina nauplii. These flavorings were chosen for analysis because they are widely used in the food industry in the preparation of processed sweet foods, and there is no scientific literature evaluating the toxicity of these flavorings.
Material and methods
Obtaining flavorings and determination of treatments for toxicity assessment
Aroma and flavor additives, nature identical synthetic, commercially available in non-greasy liquid form of cherry, banana and hazelnut were obtained from a food additive manufacturing industry located in the city of São Paulo, State of São Paulo, Brazil, specialized in the domestic and international marketing of food additives. In the test A. cepa, the roots of the bulbs were exposed to each flavoring from the following treatments: pure flavoring (without dilution) and flavoring dissolved in distilled water in the concentrations of 12.5; 25 and 50%. In the tests MTT and A. salina, the following treatments were established: pure flavoring (without dilution) and flavoring diluted in aqueous solution of synthetic sea salt (30 g/L) at concentrations of 0.78; 1.56; 3.12; 6.25; 12.5; 25 and 50%.
Cytotoxicity and genotoxicity test in root meristem cells of Allium cepa L
For the evaluation of the flavorings in root meristems, initially, onion bulbs were placed in aerated flasks with distilled water to obtain 2.0 cm long roots. For the analysis of the all treatment, an experimental group with five onion bulbs was established. Before placing the roots in contact with their respective treatments, some roots were collected and fixed to serve as control of the bulb itself, which was identified as analysis time 0 hour (0 h).Then, the remaining roots were placed in their respective treatments for 24 and 48 hours, procedures called exposure times 24 and 48 h, where root collection was performed every 24 hours. A negative control was prepared only with distilled water, in which roots were also collected at 0, 24 and 48 h. All roots collected during the experiment were fixed in Carnoy 3:1 (ethanol:acetic acid) for up to 24 hours.
Slides were mounted according to the protocol proposed by Guerra and Souza (2002), and analyzed under an optical microscope with a 40x objective. For each bulb, 1,000 cells were analyzed, totaling 5,000 cells for each control group (0 h), 24 h exposure time group and 48 h exposure time group. Cells were counted in interphase and during cell division, and the mitotic index was calculated, thus determining the cytotoxic potential. Genotoxicity was evaluated through micronuclei frequency, and aneugenic or mitotic spindle alterations were evaluated through the frequency of colchicine metaphases, anaphase and telophase bridges, gene amplifications, cells with adhesion, nuclear buds and multipolar anaphases. The results of cytotoxicity and genotoxicity were analyzed by the Chi-square test (?2) at 5%.
Toxicity test in nauplii of Artemia salina Leach
The toxicity test of aroma and flavor additives against A. salina was carried out according to the protocol proposed by Meyer et al. (1982) and Paredes et al. (2016). A. salina eggs were incubated in solution of synthetic sea salt (30 g/L) in a glass vessel equipped with a dark compartment and another with artificial light. The water was maintained at room temperature under constant stirring and aeration for 48 hours until hatching of the larvae.
With the aid of a Pasteur pipette, the larvae or micro crustacean nauplii (n = 10) were transferred to test tubes containing 3 mL of each treatment. Flavorings concentrations were prepared in saline water ranging from 1000 to 7.81 µg/mL. The control was prepared only with solution of synthetic sea salt (30 g/L). All treatments were analyzed in triplicate, and the number of dead larvae was counted after 24 hours of exposure.
Results and discussion
Based on the results in Table 1, the mitotic indices obtained for the root meristem cells of A. cepa exposed to cherry, banana and hazelnut flavorings, in pure form and in the three concentrations analyzed, at 24 and 48 h exposure, were significantly lower than the observed cell division indices for their respective 0 h exposure time. Furthermore, the cell division indices for the 24 and 48 h exposure times of all treatments also demonstrated significant inhibition of cell division to the meristematic tissue when compared to the mitotic index obtained for the negative distilled water related to the same exposure times. The concentrations evaluated were not genotoxic in the A. cepa.
Table 1. Mitotic indices (IM%) observed in root meristem tissue of Allium cepa exposed at 0, 24 and 48 hours exposed to distilled water (control negative) and to different treatments or concentrations of cherry, banana and hazelnut flavorings for evaluation of cytotoxicity.

A significant decrease in mitotic index, as observed in Table 1, is indicative of high cytotoxicity of the test substance. As mentioned by Herrero et al (2012) and Türko lu (2007), mitotic indices significantly lower than the control in dices, such as those observed in the present study for cherry, banana and hazelnut, indicate the presence of agents whose toxic action impairs the growth and development of exposed organisms. In addition, these authors state that the inhibition of cell proliferation triggered by cytotoxic compounds in tissues of intense cellular proliferation and normal performance, as used in this research is very harmful to the organism by inhibiting or limiting the replacement of cells, altering the production of proteins and result in dysfunction of the organ where it is located. Such losses, according to Valavanidis et al. (2013) and Zilifdar et al. (2014) can significantly compromise the cellular division of the affected tissue or organ and trigger and/or potentiate cancerous processes.
In relation to the condition of the flavorings have shown cytotoxic but not genotoxic potential to A. cepa, it is important consider to the principle of the cell cycle is the formation of identical cells, the production of new cells with significant changes in structure and/or chromosome It was neither found in the literature and nor on the labels of flavorings, the specific chemical composition of cherry, banana and hazelnut additives. However, the literature contains studies demonstrating the toxicity, at the cellular level, of chemical constituents with diluent and preservative actions (Brasil 2007), according on the basic formulation of flavorings, and corroborating the data obtained for the three flavorings evaluated in this study. Among these compounds, stands out the benzoic acid, responsible for maintaining uniformity and facilitating incorporation and dispersion of the flavor in food products. Analyzing the action at the cellular level of this diluent, Demir et al. (2015) fou nd that the alcohol promoted significant damage to the mitotics pindle and therefore to cell division in human peripheral blood cells. Another diluent found in the flavoring formulation is diacetyl (2, 3-butadione). Whittaker et al. (2008) reported that this comnumber make cell functioning unfeasible and tend to be eliminated from tissues with normal performance, which may lead to a significant antiproliferative effect (Marques et al. 2015; Sales et al. 2017).
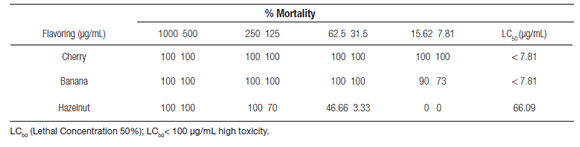
In the evaluation of toxicity of cherry, banana and hazelnut food flavorings against nauplii of A. salina are given in Table 2. The results revealed that all the samples tested showed high toxicity (LC50<100 µg/mL) against A. salina, according with Paredes et al. (2016). Cherry was the most toxic with 100% lethality at all concentrations tested, followed by banana and hazelnut, respectively. Hazelnut and banana still killed the larvae at the lowest dose (7.81 ppm). The toxicity results observed in A. salina (Table 2) corroborate the results of cytotoxicity of these flavorings observed in A. cepa.
Table 2. Toxicity of cherry, banana and hazelnut flavorings to Artemia salina after 24 hours of exposed.
It was neither found in the literature and nor on the labels of flavorings, the specific chemical composition of cherry, banana and hazelnut additives. However, the literature contains studies demonstrating the toxicity, at the cellular level, of chemical constituents with diluent and preservative actions (Brasil 2007), according on the basic formulation of flavorings, and corroborating the data obtained for the three flavorings evaluated in this study.
Among these compounds, stands out the benzoic acid, responsible for maintaining uniformity and facilitating incorporation and dispersion of the flavor in food products. Analyzing the action at the cellular level of this diluent, Demir et al. (2015) fou nd that the alcohol promoted significant damage to the mitotics pindle and therefore to cell division in human peripheral blood cells. Another diluent found in the flavoring formulation is diacetyl (2, 3-butadione). Whittaker et al. (2008) reported that this compound in gene mutation assay in rat lymphoma caused significant damage to the loci of chromosome 11 of these cells, causing loss of expression of genes for thymidine kinase enzyme. Still, More et al. (2012) verified that the diacetyl diluent had the potential to replace thymine with guanine in regions of euchromatin and to cause disruption of hydrogen and disulfide bonds in the tertiary structure of enzymes involved in the process of cell division.
In the composition of food flavorings, preservative compounds are also found: boric acid, citric acid, potassium citrate and sodium citrate (Brasil 2007), which, according to Türko lu (2007), resulted in a significant reduction in the cell division index of root meristem cells A. cepa, proving to be cytotoxic. In turn, among the chemical constituents responsible for delaying the action of microorganisms, enzymes, and physical agents in flavoring solutions, potassium benzoate, sodium benzoate and potassium nitrate (Brasil 2007) are preservatives that, according to Mpountoukas et al. (2010) and Zeguin et al. (2013), were cytotoxic and genotoxic to normal human peripheral blood cells.
The results obtained in this study and those already available in the scientific literature on the cellular toxicity of some constituents of the chemical composition of flavorings show that -although the use of flavoring additives is allowed by FAO, FEMA and ANVISA -there is an urgent need for clarification, through more detailed studies in the medium and long term, in different test systems, doses and exposure time, as to the toxicity of these substances. It results also indicate the need to set, as by high performance liquid chromatography, the chemical composition of flavorings in general, so as to consistently determine the toxicity of these additives property and ensure the safety of consumers.
References
1. Adami F.S., Conde S.R. Food and nutrition in life cycles. Lageado: Univates, 2016. Brasil. Ministério da Saúde. [ Links ]
2. Agência Nacional de Vigilância Sanitária. Regulamento Técnico sobre Aditivos Aromatizantes / Aroma [on line]. Resolução N. 104 de 14 de maio de 1999. [Accessed on: June 13th, 2018]. Available at: http://www.engetecno.com.br/port/legislacao/norqual_aditivos_aromatizantes.htm. [ Links ]
3. Agência Nacional de Vigilância Sanitária. Ministério da Saúde, Brasil. Resolução da Diretoria Colegiada RDC nº. 05, de 15 de Janeiro de 2007 [on line]. [Accessed on: June 3rd, 2018]. Available at: http://www.anvisa.gov.br/legis/resol/2007/rdc/02_170107rdc.pdf. [ Links ]
4. Carvalho B.D.L., Sales I.M.S., Peron A.P. Cytotoxic, genotoxic and mutagenic potential of UHT whole milk. Food Sci Technol. 2017;37:275-279. [ Links ]
5. Demir E., Kocaoglu S., Kaya, R. Assessment genotoxic effects of benzyl derivatives by comet assay. Food Chem Toxicol. 2015;48:1239-1242. [ Links ]
6. Gomes K.M.S., Oliveira M.V.G.A.D., Carvalho F.R.S., Menezes C.C., Peron A.P. Citotoxicity of food dyes sunset yellow (E-110), bordeax red (E-123), and tatrazine yellow (E-102) on Allium cepa L. root meristematic cells. Food Sci Tecnh. 2013;33:218-223. [ Links ]
7. Guerra M., Souza M.J. How to observe the chromosomes: a guide to techniques in plant, animal and human cytogenetics. Ribeirão Preto: FUNPEC, 2002. [ Links ]
8. Herrero O., MartÍn J.P., Freire O.F., López L.C., Peropadre A., Hazen M.J. Toxicological evaluation of three contaminant of emerging concern by use of Allium cepa test. Mut Res. 2012;743:24-34. [ Links ]
9. Koca N., Erbay Z., Kaymark-Ertekin, F. Effects of spray-driping conditions on the chemical, physical and sensory properties of cheese powder. J Dairy Sci. 2015;98:2934-2943. [ Links ]
10. Marques G.S., Silva S.I.O., Ferreira P.M.P., Peron A.P. Cytotoxic and genotoxic potential of liquid synthetic food flavorings evaluated alone and in combination. Food Sci Techn. (Campinas) 2015;35:183-188. [ Links ]
11. Meyer B.N., Ferrign R.N., Putnam J.E., Jacobson L.B., Nicholas D.E., Mclaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31-34. [ Links ]
12. More S.S., Raza, A., Vince, R. The butter flavorant, diacetyl, forms a covalent adduct with 2-deoxyguanosine, uncoils DNA, and leads to cell death. J Agricul Food Chem. 2012;12:3311-3317. [ Links ]
13. Moura A.G., Santana, G.M., Ferreira P.M.P., Sousa J.M.C., Peron A.P. Cytotoxicity of Cheese and Cheddar Cheese food flavorings on Allim cepa L root meristems. Braz J Biol. 2016;76:439-443. [ Links ]
14. Mpountoukas P., Pantazaki A., Kostareli E., Christodoulou P., KarelI D., Poliliou S., Lialiaris T. (2010): Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem- Toxicol. 2010;48:2934-2944. [ Links ]
15. Neves E.S., Ferreira P.M.P., Lima L.H., Peron A.P. Action of aqueous extracts of Phyllanthus niruri L. (Euphorbiaceae) leaves on meristematic root cells of Allium cepa L. Anais Acad Bras Ciênc. 2014;86:1131-1137. [ Links ]
16. Paredes P.F.M., Vasconcelos F.R., Paim R.T.T., Marques M.M.M., Morais, S.M., Lira S.M., Guedes M.I.F. Screening of bioactivities and toxicity of Cnidoscolus quercifolius Pohl. Evid Based Complement Alternat Med. (Print) 2016;2016. [ Links ]
17. Rosa C.S., Veras K.S., Silva P.R., Lopes-Neto J.J., Cardoso H.L.M., Alves L.P.L., Brito M.C.A., Amaral F.M.M., Maia J.G.S., Monteiro O.S., Moraes, D.F.C. Composição quÍmica e toxicidade frente Aedes aegypti L. e Artemia salina Leach do óleo essencial das folhas de Myrcia sylvatica (G. Mey.) DC. Rev Bras de Plantas Med. 2016;18:19-26. [ Links ]
18. Sales I.M.S., Sousa J.B., Sousa F.K.S., Silva F.C.C., Ferreira P.M.P., Sousa J.M.C., Peron, A.P. Acute toxicity of grape, plum and orange synthetic food flavourings evaluated in vivo test systems. Food Techn Biotechn. 2017;55:51-55. [ Links ]
19. Tabrez S., Shakil S., Urooj M., Damanhouri G.A., Abuzenadah A.M., Ahmad, M. Genotoxicity testing and biomarker studies on surface waters: an overview of the techniques and their efficacies. J Environ Sci Health, Part C.2011;9:250-275. [ Links ]
20. Türkoglu S., Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mut Res. 2007;626:04-14. [ Links ]
21. Valavanidis A., Vlaclogranni T., Fiotakis K., Lorida R. S. Pulmonary oxidative stress, inflammation and cancer: Respirable particular matter fibrous duts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanism. Inter J Environ Res Pub Health. 2013;10:3886-3907. [ Links ]
22. Whittaker P., Clarke J.J., San R.H., Begley T.H., Dunkel V.C. Evaluation of the butter flavoring chemical diacetyl and a fluorochemical paper additive for mutagenicity and toxicity using the mammalian cell gene mutation assay in L5178Y mouse lymphoma cells. Food and Chem Toxicol. 2008;46:2928-2933. [ Links ]
23. Xu Z., Gu C., Wang K., Ju J., Wang H., Ruan K., Feng Y. Arctigenic acid, the key substance responsible for the hypoglycemic activity of Fructus arctii. Phytomedicine. 2013;22:128- 137. Doi: 10.1016/j.phymed.2014.11.006 [ Links ]
24. Zilifdar F., Alper-Hayta S., Yilmaz S., KaplanÖzen ç., Foto E., Aydogan Z., Yildiz I., Aki E., Yalçin I., Diril N. Genotoxic potentials and eukaryotic DNA topoisomerase I inhibitory effects of some benzoxazine derivatives. Med Chem Res. 2014;23:480-486. [ Links ]














