Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Acta toxicológica argentina
On-line version ISSN 1851-3743
Acta toxicol. argent. vol.26 no.3 Ciudad Autónoma de Buenos Aires Dec. 2018
ARTÍCULOS ORIGINALES
Analysis of histopathological abnormalities in the gills of Astyanax jacuhiensis (Characidae) for assessment of water quality in the Ijuí River, southern Brazil
Análisis de anormalidades histopatológicas en las branquias de Astyanax jacuhiensis (Characidae) para la evaluación de la calidad del agua del Río Ijuí, sur de Brasil
Rodrigues Batista, Jeferson1; Zimmermann Prado Rodrigues, Gabriela1; da Rosa Neto, Emitério2; Gehlen, Günther1,2; Basso da Silva, Luciano1,2*
1Grupo de Pesquisa em Indicadores de Qualidade Ambiental, Universidade Feevale, RS 239, 2755. CEP 93525-075, Novo Hamburgo, RS, Brazil.
2Programa de Pós-Graduação em Qualidade Ambiental, Universidade Feevale.
Recibido: 21 de febrero de 2018.
Aceptado: 2 de noviembre de 2018
Abstract
The aim of this study was to assess histopathological alterations in the gills of Astyanax jacuhiensis from different points along the Ijuí River and to determine if these abnormalities can be used as biomarkers in biomonitoring studies. Fish specimens were collected from three sites on the Ijuí River in winter and summer and examined histologically for abnormalities of the secondary lamellae of their gills. For each fish, estimates were made of the frequencies of lamellae with edema, hyperplasia, hypertrophy, epithelium lifting, lamellar fusion or deformation, in addition to the overall frequency of abnormal lamellae and the number of abnormalities per lamella. No differences were observed between sampling points during the winter. In summer the frequency of lamellae with hypertrophy was significantly higher at site 3 (Pirapó) than at site 2 (Santo Ângelo) and the frequency of abnormal lamellae was increased at site 1 (Ijuí) in comparison to site 3. Additionally, all three sample points had a significantly higher value in the winter than in the summer for one of the histological parameters analyzed. These results indicate temporal and spatial variation in the level of contamination of the Ijuí River and also shows that fish gill histopathology can be used for in situ biomonitoring studies.
Keywords: Environmental quality; Biomarker; Gills alterations; Histopathology.
Resumen
El agua de la cuenca del río Ijuí, en el sur de Brasil, se utiliza principalmente para irrigar los cultivos y para el abastecimiento público de agua. El objetivo del presente estudio fue evaluar alteraciones histopatológicas en las branquias de Astyanax jacuhiensis en diferentes puntos del Río Ijuí y determinar si tales anormalidades pueden ser usadas como biomarcadores en estudios de biomonitoramiento. Los especímenes fueron recolectados en tres puntos del río durante el invierno y el verano y luego las lamelas secundarias de las branquias fueron examinadas histológicamente para la presencia de anormalidades. Para cada pez se estimaron las frecuencias de lamelas con edema, hiperplasia, hipertrofia, desprendimiento del epitelio, fusión y deformación lamelar. Además, se obtuvieron las frecuencias de lamelas alteradas, así como el número medio de cambios por lamela. No se observaron diferencias entre los puntos de recolección durante el invierno. En el verano, la frecuencia de lamelas con hipertrofia fue significativamente mayor en el punto 3 (Pirapó) que en el punto 2 (Santo Angelo), y el punto 1 (Ijuí) presentó mayor frecuencia de lamelas alteradas que el punto 3. Además, en los tres puntos muestreados, el período de invierno presentó valores superiores para uno de los parámetros histológicos analizados. Estos resultados indican variación espacial y temporal en los niveles de contaminación del Río Ijuí y demuestran que el análisis histológico de las branquias de peces puede ser utilizado en estudios de monitoreo in situ.
Palabras clave: Calidad ambiental; Biomarcadores; Alteraciones branquiales; Histopatología.
Introduction
Pollution of freshwater ecosystems has become a matter of great concern because of the threat to public water supplies and to the aquatic biota (Jordão et al. 2002). Contaminated freshwaters induce changes to structural and physiological aspects of the biota (Vutukuru et al. 2007). In this context, fish have been employed as sentinel organisms in biomonitoring studies, because of the key position they occupy in the trophic chain (Viarengo et al. 2007).
A biomarker has been defined as a biological response to a chemical or complex mixtures that gives a measure of exposure, and sometimes also of toxic effects (Peakall and Walker 1994). Biomarker responses exhibited by fish to pollutants can be used as early warning signs of putative stressors and can often be used to identify the type of stressor (Vutukuru et al. 2007). It is generally accepted that histopathological biomarkers reflect the effects of exposure to a variety of aquatic contaminants (Mallatt 1985; Nascimento et al. 2012).
The fish gill is a multifunctional organ involved not only in respiration but also in osmoregulation, metabolism of circulating hormones, nitrogen excretion and acid base balance (Olson 1991). Gills have large surface areas in direct contact with potential irritants, and so histopathological examination of these organs is critical to assessment of general fish health, and enables their use as biomarkers of water pollution (Olojo et al. 2005; Farombi et al. 2007; Flores-Lopes and Thomaz, 2011; Van Dyk et al. 2012). A variety of factors including environmental pollutants and many parasites can induce morphological anomalies in fish gills. This organ typically responds rapidly to various irritants and the gill lesions that result have a negative effect on the performance of the fish and increase its susceptibility to secondary diseases, potentially causing fish mortality (Flores-Lopes and Thomaz 2011).
In the present study, a fish species known in Brazil as lambarí (Astyanax jacuhiensis [Cope 1894] Characiformes: Characidae) was used to assess the water quality of a river, which is subject to the influence of agriculture. The hydrographic basin of the Ijuí River is located in Brazil's southernmost state, Rio Grande do Sul, and has a total area of 10,779 km2 and a population of 508,336 inhabitants. Water is primarily used to irrigate crops and for the public water supply. The most significant environmental problems in the region of the Ijuí River hydrographic basin are discharge of untreated sewage into water bodies, farming methods that do not include soil conservation practices, indiscriminate use of pesticides, and processes of erosion and silting of springs and other water sources (Fepam 2010). This study was conducted with the objective of conducting a water quality assessment at three different points along the Ijuí River, employing abnormalities observed in the gills of Astyanax jacuhiensis as biomarkers.
Materials and methods
Specimens of A. jacuhiensis were collected with a landing net at three points along the Ijuí River. Collections were conducted during the winter (July) of 2012 and the summer (January) of 2013, exerting sufficient sampling effort to capture five fish at each point. Sampling site 1 (28° 17' 05" S and 053° 48' 32,6" W) is located upstream of Ijuí municipality (78.915 inhabitants); sampling site 2 (28° 19' 23,5" S and 054° 17' 37,4" W) is located downstream site 1 and upstream Santo Ângelo municipality (78.836 inhabitants), and sampling site 3 (28° 03' 03,2" S and 055° 10' 49,2" W) is located downstream site 2 and upstream Pirapó municipality (2.738 inhabitants), around 30 km from the Ijuí River mouth, which flows into the Uruguay River. The distance between sites 1 and 2 is around 40 km and the distance between sites 2 and 3 is approximately 110 km. In the area of the three sampling sites there is intensive use of the land for agriculture. In the present study, ethical guidelines for the protection of animal handling and welfare were followed.
After capture, fish were killed by decapitation and the first arch of the right gill was removed from each fish and fixed in Bouin solution for 16h, washed in running water and then stored in alcohol at 70 %. The samples were later dehydrated in a graded series of alcohols and then set in paraffin. A rotary microtome (Leica, model RM 2125) was used to prepare 7 μm sections which were mounted on gelatin-coated slides preparatory to staining with hematoxylin/ eosin (H.E).
The slides were examined under an optical microscope (Olympus CX 41) and images were digitized using a CCD camera (Micrometrics DSC 3000). For the histological study, from six to ten images were analyzed per fish. Eight secondary lamellae were viewed per image and any occurrences of the following histological abnormalities were noted: hyperplasia of the gill filament epithelium, lamellar hypertrophy, edema, lamellar fusion and deformation and epithelial lifting of gill filament. For each fish, the frequency of lamellae with each type of abnormality was estimated, as was the overall frequency of abnormal lamellae. Additionally, the total number of abnormalities was used to calculate the mean number of abnormalities per lamella.
Data were expressed as mean ± standard deviation. Differences between sampling sites were evaluated using one-way analysis of variance (ANOVA) followed by the Tukey test and different sampling periods at the same site were compared using the T-test. The level of significance was set at p≤0.05. All analyses were conducted using the statistical package for social sciences (SPSS) for Windows, version 18.0.
Results
The results of fish collected during the winter revealed all seven types of gill abnormalities; only lamellar deformation was not observed at site 3 and hyperplasia of the gill filament epithelium was observed at site 1 only. Figure 1 shows images of normal secondary lamella, hyperplasia and epithelial lifting of gill filament.
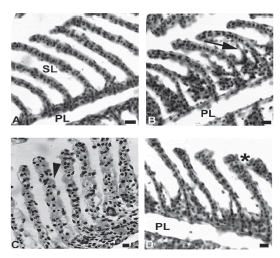
Figure 1. Histological sections of gills of A. jacuhiensis stained with HE. (A) General view of gills, presenting primary (PL) and secondary (SL) lamellae with no alterations from fishes captured in the summer at the site 3. (B) Epithelial lifting and hyperplasia of gill filament (arrow) observed in the animals from site 1 in the winter. (C) Secondary lamella exhibiting hypertrophic cells (arrowhead) in the gills of fishes from site 2, collected in the winter. (D) Secondary filaments from site 3 from fishes obtained in the winter showing less hyperplasia (asterisk). Bars = 10 μm.
There were no significant differences between the three sites in samples collected during the winter considering the frequencies of the individual types of lamellar abnormalities, as well as the overall frequency of abnormal lamellae and the mean number of abnormalities per lamella (Table 1). At all three sites, more than 65 % of lamellae exhibited some type of abnormality, with around 1.2 abnormalities per lamella.
Table 1. Histopathological abnormalities in gills of A. jacuhiensis collected at three sampling sites along the Ijuí River, RS, Brazil.

In summer, fish exhibiting lamellar deformation were not observed (Table 1). In this sampling period significant differences between sampling sites were observed for the frequency of lamellae with hypertrophy (p =0,01) and for the overall frequency of abnormal lamellae (p = 0.046). The frequency of lamellae with hypertrophy at site 3 was significantly higher than at site 2, while site 1 did not differ from sites 2 and 3. The overall frequency of abnormal lamellae differed significantly between sites 1 and 3 (69% vs. 41%), but neither of these two were different from site 2 (61% of abnormal lamellae).
Comparison of results for fish captured in winter with those captured in the summer at the same sampling sites revealed significant differences for all three sites (Table 1). In all cases, higher frequencies of abnormalities were observed in winter samples than in summer samples. At site 1, there was a difference in the frequency of lamellar deformation (p = 0.038), at site 2 the frequencies of lamellar hypertrophy differed (p = 0.013) and at site 3 the number of abnormalities per lamella was different (p = 0.025).
Discussion
Pollution is defined as undesirable alterations in the environment that can have harmful consequences for organisms. Water resources have been impacted by discharge of high levels of pollutants resulting from anthropogenic activities. In this context, biological responses to stress induced by pollutants can be used as early warnings signs of toxic effects (Flores- Lopes and Thomaz 2011). Gills are vital structures for fish health and consequently abnormalities to these structures can damage the animal and can be used as indicators of environmental contamination.
In the present study, abnormalities observed in the gills of A. jacuhiensis were used as potential biomarkers of water quality in the Ijuí River. This species feeds on insects, zooplankton, algae, plants and the eggs of other fish and can tolerate physical and chemical variations to water (Bemvenuti and Moresco 2005). Therefore, the species is a good choice for assessment of the water column and suspended or dissolved materials. Nascimento et al. (2012) observed that the water column dweller Oligosarcus hepsetus (Cuvier 1829) had gills most susceptible to changes, making the species more suitable for assessing environmental quality in rivers.
The results observed for this species demonstrated that about 60% of secondary lamellae presented alterations and the most frequent changes in the gills were hyperplasia of epithelium, followed by lamellar hypertrophy, edema, lamellar fusion and epithelial lifting of gill filament. Most gill damage is either direct, as a result of the action of pollutants on the cells, or indirect, caused by defense or compensatory mechanisms (Wendelaar-Bonga 1997).
The frequencies of abnormalities observed in gills from A. jacuhiensis did not exhibit differences between sample points at either of the two sample times, suggesting that, in terms of their effects on gills, the environmental conditions in the Ijuí River were similar at all three sites. Edema and epithelial lifting were observed at all three sites and are the first signs of serious abnormalities in gills (Tophon et al. 2003). Henares et al. (2008) observed lamellar fusion, edema and epithelial lifting of gill filaments in gills of Oreochromis niloticus after exposure to the herbicide Diquat. Considering that the primary economic activity in the Ijuí River basin is agriculture, it is possible that some of the abnormalities observed in gills of A. jacuhiensis are the result of exposure to pesticides. However, different irritants may cause almost identical lesions and gill structural damage may merely reflect a generalized stress response rather than toxicant- or parasite-specific responses (Mallatt 1985; Haaparanta et al. 1997). At all three sites, samples collected during the winter exhibited significantly higher values for one of the histological parameters analyzed, which indicates temporal variation in xenobiotic concentration and/or composition along the entire extent of the Ijuí River, with winter exhibiting more stressful conditions. The data reported here are similar to findings from another study that also observed more severe damage to fish gills during the winter (Camargo and Martinez 2007).
Field studies using biomarkers offer the advantage of only quantifying pollutants that are biologically available and can integrate the effects of multiple stressors (Adams 1990). Morphological parameters are higher-level responses following chemical and cellular interaction and are generally indicative of irreversible damage. Histopathological alterations in the gills of A. jacuhiensis proved to be a very suitable biomarker for in situ studies, since seasonal or temporal variation was detected at all three sampling sites. However, caution should be taken in interpreting these results because the number of samples by region and season are small to establish definitive conclusions.
References
1. Adams S.M., Shugart L.R., Southworth G.R., Hinton D.E. Application of bioindicators in assessing the health of fish populations experiencing contaminant stress. In McCarthy J.F., Shugart L.R., editors. Biomarkers of environmental contamination. Boca Raton: Lewis Publishers; 1990. P. 333-353. [ Links ]
2. Bemvenuti M.A., Moresco A. Peixes: áreas de banhados e lagoas costeiras do extremo sul do Brasil. Porto Alegre: ABRH; 2005, P. 63. [ Links ]
3. Camargo M.M.P., Martinez C.B.R. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop Ichthyol. 2007;5(3):327-336. [ Links ]
4. Farombi E.O., Adelowo O.A., Ajimoko Y.R. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in african catfish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health. 2007;4(2):158-165. [ Links ]
5. Flores-Lopes F., Thomaz A.T. Histopathologic alterations observed in fish gills as a tool in environmental monitoring. Braz J Biol. 2011; 71(1):198-188. [ Links ]
6. Fundação Estadual de Proteção Ambiental (FEPAM) Henrique Luiz Roessler. 2010. Qualidade das águas da Bacia Hidrográfica do Rio dos Sinos. [online]. Available from: http://www.fepam.rs.gov.br/qualidade/bacia_uru_ijui.asp (Accessed: December 12, 2017). [ Links ]
7. Haaparanta A., Valtonen E.T., Hoffmann R.W. Gill anomalies of perch and roach from four lakes differing in water quality. J Fish Biol. 1997;50(3):575-591. [ Links ]
8. Henares M.N., Cruz C., Gomes G.R., Pitelli R.A., Machado M.R.F. Toxidade aguda e efeitos histopatológicos do herbicida Diquat® na brânquia e no fígado de tilápia nilótica (Oreochromis niloticus). Acta Sci Biol Sci. 2008;30(1):77-82. [ Links ]
9. Jordão C.P., Pereira M.G., Bellato C.R., Pereira J.L., Matos A.T. Assessment of water systems for contaminants from domestic and industrial sewages. Environ Monit Assess. 2002;79(1):75-100. [ Links ]
10. Mallatt J. Fish Gill structural changes induced by toxicants and other irritants: A statistical review. Can J Fish Aquat Sci. 1985;42(4):630-648. [ Links ]
11. Nascimento A.A., Araújo F.G., Gomes I.D., Mendes R.M.M., Sales A. Fish gills alterations as potential biomarkers of environmental quality in a eutrophized tropical river in South-Eastern Brazil. Anat Histol Embryol. 2012;41(3):209-216. [ Links ]
12. Olojo E.A.A., Olurin .KB., Mbaka G., Oluwemimo A.D. Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol. 2005;4:117-122. [ Links ]
13. Olson K.R. Vasculature of the fish gill: anatomical correlates of physiological functions. J Electron Microsc Tech. 1991;19(4):389-405. [ Links ]
14. Peakall D.B., Walker C.H. The role of biomarkers in environmental assessment (3). Vertebrates. Ecotoxicology. 1994;3(3):173-179. [ Links ]
15. Thophon S.M., Kruatrachue M., Upatham E.S., Pokethitiyook P., Sahaphong S., Jaritkhuan S. Histopathological alterations of white seabass, Lates calcarifer in acute and subchronic cadmium exposure. Environ Pollut. 2003;121(3):307-320. [ Links ]
16. Van Dyk J.C., Cochrane M.J., Wagenaar G.M. Liver histopathology of the sharptooth catfish Clarias gariepinus as a biomarker of aquatic pollution. Chemosphere. 2012;87(4):301-311. [ Links ]
17. Viarengo A., Lowe D., Bolognesi C., Fabbri E., Koehler A. The use of biomarkers in biomonitoring: A 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146(3):281-300. [ Links ]
18. Vutukuru S.S., Pauleena J.S., Rao J.V., Anjaneyulu Y. Architectural changes in the gill morphology of the freshwater fish, Esomus danricus as potential biomarkers of copper toxicity using automated video tracking system. Environl Bioindic. 2007;2(1):3-14. [ Links ]
19. Wendelaar-Bonga S.E. The stress response in fish. Physiol Rev. 1997;77(3):591-625. [ Links ]














