Introduction
Typically, the fungi that produce mycotoxins as secondary metabolite develop during storage when maize stored in silos presents high moisture content (Márcia and Lazarri 1998). The diseases caused by these toxins are called mycotoxicosis and they mainly lead to lesions in organs such as liver and kidneys, as well as lesions in the epithelial tissue. They can also act on the immune system, either as immunostimulants or immunosuppressives, depending on the type of mycotoxin (Pestka 1990; Diaz 2005; Oliveira and Corassin 2014).
There are over 20 known aflatoxins, but the most important ones in this group are AFB1, AFB2, AFG1, and AFG2. AFB1 stands out among the main AFs given its carcinogenic activity in animals (Zain 2011).
Cyclopiazonic acid is known to be a secondary metabolite of several Penicillium species, but the Aspergillus species, known for aflatoxin synthesis, have CPA as one of their major secondary metabo-lites. The toxicity of CPA in many animal species has been studied: it causes weight loss, diarrhea, degeneration, and necrosis of the muscles and viscera, as well as seizure and death in rodents, birds, dogs and swine (Hayashi and Yoshizawa 2005; Moldes-Anaya et al. 2009; Heperkan etal. 2012).
The Food and Agriculture Organization of the United Nations found that sixty one countries have regulated limits for the presence of aflatoxins, while there are no laws that regulate CPA values (FAO 2004).
In monogastric animals such as broilers, myco-toxicosis is considered a major problem. This is due to the economic impact linked not only to mortality, but also to worsening feed conversion, increased incidence of other diseases, damage to vital organs, and interference with reproductive capacity. The consequences of mycotoxicosis lead to negative effects on public health, espe-cially because birds are used as food, favoring the spread of mycotoxins (Akande et al. 2006; Oliveira and Corassin 2014).
Contamination of animals by more than one my-cotoxin is possible due to the co-occurrence of these toxins in food, proven by co-exposure monitoring research (Alassane-Kpembi et al. 2017). This simultaneous exposure may have antagonistic, additive, or synergistic effects. Thus, this study aims to highlight, through ISI, the damage caused by aflatoxin B1 and cyclo-piazonic acid isolatedly and simultaneously in the histology of the small intestine, which is the site of absorption of mycotoxins, and in the liver, a vital organ in broiler chickens, and also the ef-fect on weight gain.
Materials and Methods
Ethics Committee
This trial was conducted at the Microbiology and Avian Pathology Laboratory, at Federal University of Paraná, Curitiba, State of Paraná, Brazil. This study was approved by the Animal Use Ethics Committee - CEUA of the Federal University of Paraná and registered under the number 23075.139283 / 2016-86.
Experimental design, animals and housingThe experiment was developed considering the importance of the internationally recognized pro-gram 3R, which aims to reduce the number of animals in experiments, refine research to lower animal discomfort and pain, and replace in vivo tests when possible (Cazarin et al. 2004).
A total of 20 Cobb 500 broiler chickens were housed from 1-28 days of age in previously disinfected negative pressure rooms and randomly distributed in cages containing sterilized shackle at 121°C for 15 minutes. Temperature and light were controlled for a comfortable environment according to the age of the bird and its lineage. Water and food were provided ad libitum throughout the period. The diet was a commercial one according to the bird’s necessities, without any anti-coccidial or growth-promoting antibiotic.
The birds were divided into 4 treatments, with 5 birds in each cage that were divided as it follows: group 1) negative control (NC), without challenge; group 2) group challenged with cyclopiazonic acid (CPA); group 3) group challenged with aflatoxin B1 (AFB1); and group 4) group challenged with cyclopiazonic acid and aflatoxin B1 (CPA + AFB1).
Preparation of SolutionsThe standards used in this study, cyclopiazonic acid (batch 0449585-4, Cayman Chemical, Michigan, USA) and aflatoxin B1 (batch 0460083-28, Cayman Chemical, Michigan, USA). The tested mycotoxins were solubilized in a 0.5% methanol solution, which was also the solution administered to the negative control group. The AFB1 and CPA concentrations were 20 pg ml-1 and 120 pg ml-1, respectively.
ChallengeFrom the 1st to the 28th day, the birds received a daily gavage dose according to their treatments: group 1) 0,5 ml of 0,5% methanol; group 2) 0,5 ml of CPA 120 pg ml-1; group 3) 0,5 ml of AFB1 20 pg ml-1; and group 4) 0,5 ml of 120 pg ml-1 CPA and 0,5 ml of 20 pg ml-1 AFB1.
Weight
For the analysis of weight gain or loss the birds were weighed at days 1,7, 14, 21 and 28 of the experiment. Values were expressed by group of birds and not individually
Sample Collection and Histological Analysis
At 28 days of age, five birds per treatment were euthanized by cervical dislocation. Duodenum, jejunum, ileum, and liver samples were collected for histológica! evaluation. For each intestinal frag-ment collected, five histological sections were performed; for each liver fragment collected, three histological sections were performed for larger organ sampling.
All samples were collected and fixed in Davidson (100 ml glacial acetic acid, 300 ml 95% ethanol, 200 ml 10% formalin neutral buffer, and 300 ml distilled water) for at least 24 h. All samples were dehydrated, infiltrated and embedded in paraffin, following the common histological routine. Blocks were cut into 5gm sections and stained with hema-toxylin and eosin associated with Alcian Blue for goblet cell staining (Rapp and Wurster 1978). For intestinal and hepatic morphology, an adaptation of the methodology described by Kraieski et al. (2017) and Belote et al. (2018) was performed. For intestinal analyses, 10 villi per cut were analyzed, totaling 50 villi per bird. The villi were observed at 100x magnification (using 400x magnification to confirm changes). For liver morphology, five fields of three cuts were analyzed, totaling 15 fields for each bird. Fields were observed at 100x magnification (using 400x magnification to confirm changes). Both evaluations were performed under light microscopy (Eclipse E200, Nikon).
The ISI methodology in process of patent (INPI BR 1020150036019) is based on a numeric score of alteration. In this methodology, an impact factor (IF) is defined for each alteration in macroscopic and microscopic analysis, according to the re-duction of organ functional capacity, based on previous knowledge from the field literature and background research (necrosis has the highest IF because the functional capacity of affected cells is completely lost). The IF ranges from 1 to 3, with 3 meaning the biggest impact on organ function. In addition, the extent of each lesion (intensity) or the observed frequency compared to a non-affected organ is evaluated in each or-gan/tissue with score (S) ranging from 0 to 3: score 0 (absence of lesion or frequency), score 1 (alteration of up to 25% of the area or observed frequency), score 2 (alteration ranges from 25 to 50% of the area or observed frequency), and score 3 (alteration extends to more than 50% of the area or observed frequency). In order to obtain the final value of the ISI index, the IF of each alteration is multiplied by the respective score number, as shown in Table 1.
1Maximum score represents the sum of all alterations according to the formula ISI = I(IF*S), IF = impact factor (previous fixed) and S = Score (observed), considering the maximum observed S. For example, the lamina propria thickness has IF = 2; this number will be multiplied by observed score (range from 1 to 3); if in a villus, a score S = 3. Source: Belote et al. (2018).
Statistical AnalysisThe experimental unit for each organ was the ana-lyzed section. Initially, data normality was verified using the Shapiro-Wilk Test. Data were compared using one-way analysis of variance (ANOVA) fo-llowed by Tukey test (p <0,05) for parametric data. All analyses were performed by Past for Windows.
ResultsIn this study, birds were challenged with CPA and AFB1 for 28 days. After the treatment period, it was possible to evaluate the damage caused by the presence of mycotoxins in the three evaluated intestinal fragments and in the liver.
Analysis of gain weightThe weights obtained during the 28 days of the experiment are shown in Table 2. In the first days of life of the chickens it was possible to notice that the group contaminated with CPA showed a significantly lower weight gain than the group CN. When weighing the birds on day 28, it was possible to perceive through the coefficient of varia-tion (CV) and the standard deviation (SD) (Table 3) that within each contaminated group there is a great variation between the weights of the birds while the negative control group presents a lesser variation. Thus, it appears that the mycotoxins studied show interference in the weight of the animals, decreasing the uniformity in weight gain.
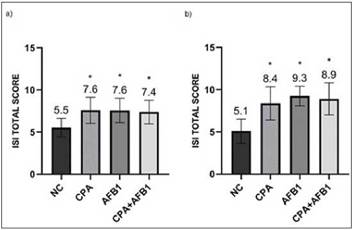
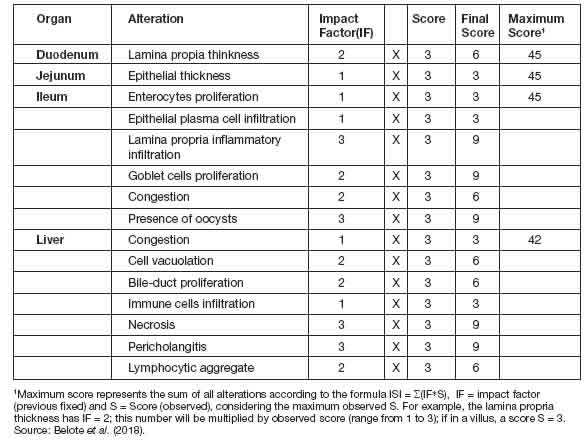
Figure 1: ISI total histological alteration scores in duodenum (a) and ileum (b) in different groups. Error bars represent standard error of the mean. NC: non-challenged group, CPA: cyclopiazonic acid challenge, AFB1: aflatoxin B1 challenge, and CPA+AFB1: cyclopiazonic acid and aflatoxin B1 challenge.
IntestineIn both duodenum and ileum fragments, the challenged groups (CPA, AFB1 and CPA + AFB1) presented significant difference (p<0,05) when compared to the negative control group (NC). However, when compared to another chal-lenged group, the difference is absent (Figure 1). In the jejunum fragments, the CPA treatment presented a higher total number of ISI than all other treatments, while the AFB1 and CPA + AFB1 groups were higher than the NC (Figure 2).

Figure 2: ISI total histological alteration scores in jejunum in different groups. Error bars represent standard error of the mean. NC: non-challenged group, CPA: cyclopiazonic acid challenge, AFB1: aflatoxin B1 challenge and CPA+AFB1: cyclopiazonic acid and aflatoxin B1 challenge.
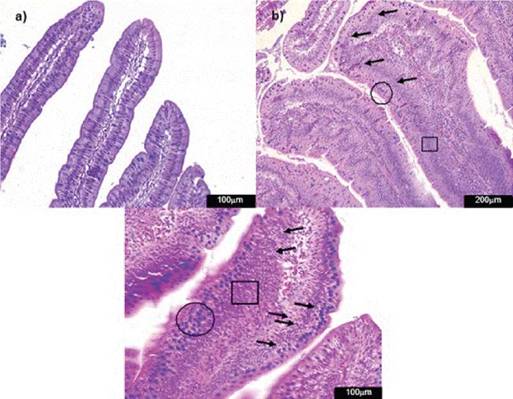
Figure 3: Photomicrographs of hematoxylin and eosin-stained chicken jejunum sections. Alcian Blue was used to stain the goblet cells. a) Normal histological structure of non-challenged group NC (200x); b) Epithelial inflammatory infiltration cells (arrow), goblet cells proliferation (circle) and enterocytes proliferation (square) in the challenged group CPA+AFB1 (200x).
Enterocyte proliferation, inflammatory infiltration into the epithelium and goblet cell proliferation (Figure 3) demonstrate the alterations suffered by the intestinal epithelium of the three fragments with the presence of CPA and AFB1 analyzed (Table 4). In relation to lamina propria, in the duodenum, mycotoxins showed no difference between the challenged groups and the NC. In the jejunum, all challenged groups presented high thickness scores and also inflammatory infiltration in the lamina propria, with the CPA treatment having the highest ISI number for both parameters, differing signifi-cantly from the AFB1 and CPA + AFB1 groups. In the ileum, the challenged groups presented high values for lamina propria thickness, but only the
CPA + AFB1 group showed significant difference for inflammatory infiltration when compared to NC (Figure 4). No oocysts or significant values for con-gestion were found in any treatment.
LiverIn this study, through the ISI methodology, it was observed that all challenged groups presented liver damage, but the treatments containing AFB1 presented greater organ damage than the other treatments (Figure 5).
The groups challenged with aflatoxin B1 presented more harm to the liver due to parameters of bile duct proliferation, inflammatory infíltrate, necrosis and pericholangitis (Figure 6).
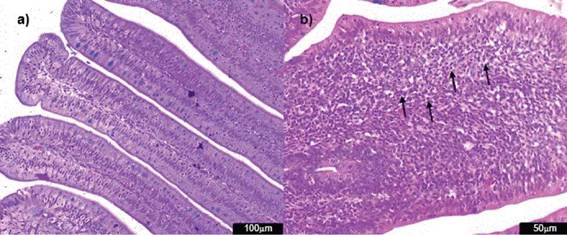
Figure 4: Photomicrographs of hematoxylin and eosin-stained chicken ileum sections. Alcian Blue was used to stain the goblet cells. a) Normal histological structure of non-challenged group NC (200x); b) lamina propria inflammatory infiltration cells (arrow) in the challenged group CPA+AFB1 (400x).
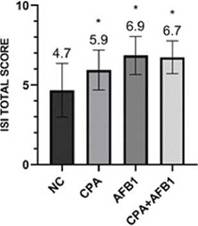
Figure 5: ISI total scores for histological liver changes in different groups. Error bars represent standard error of the mean. NC: non-challenged group, CPA: cyclopiazonic acid challenge, AFB1: aflatoxin B1 challenge and CPA+AFB1: cyclopiazonic acid and aflatoxin B1 challenge.

Figure 6: Photomicrographs of hematoxylin and eosin-stained chicken liver sections. a) Normal liver histological structure of non-challenged group NC (100x); b) necrosis (arrow) in the challenged group AFB1 (400x) c) inflammatory cell infiltration (circle) in the challenged group AFB1 (400x); d) pericholangitis (arrow) in the challenged group AFB1 (100x).
Discussion
The lower weight gain realized in the first days of the broiler’s life corroborate with Hayashi and Yoshizawa (2005), which indicates this effect of CPA in different animal species.
This interference caused by mycotoxins presents damage both to the animal’s health and to its pro-ducers. The broiler chicken is a food consumed and traded worldwide, its unregulated growth can interfere in the schedule of its breeders, generating greater expenses for the development of the bird.
IntestineThe mucosa of the gastrointestinal tract (GIT) is a link between the external environment and the internal environment of birds. Thus, the mucosa acts as a selectively permeable barrier that allows the absorption of important substances - such as nutrients - and exclusion of harmful substances - such as toxins (Perry 2006).
Several studies report effects of mycotoxins on intestinal villus morphology. The villi increase the surface absorption of water and nutrients through the intestinal wall and, because of this, abnor-malities in their morphology may compromise the animal’s health (Grenier and Applegate 2013).
The lesions found on the epithelium are in accor-dance with the study of Akinrinmade et al. (2016) who, when exposing mice to AFB1, found pro-liferation of enterocytes, inflammatory cells and other structural changes in the epithelium. Robert et al. (2017) point out mycotoxins as pathology inducers during their course in the GIT, which may result in disturbance of epithelial function as an intestinal barrier. Epithelial thickness increases as a result of increased goblet cells and proliferation of enterocytes.
Enterocytes and mucus produced by goblet cells are responsible for absorbing water and nutrients (Liew and Mohd-Redzwan 2018), but they also function as a physical barrier to antigens present in the digestive tract. Thus, we can assume that the high ISI values of these parameters indicate a gut defense mechanism against the presence of mycotoxins.
Müller et al. (2005) may corroborate elevated ISI scores for inflammatory epithelial infiltration showed by challenged groups. According to the authors, the intestine is an organ that has components of acquired immunity called gut-associated lymphoid tissue (GALT). The presence of M cells in the intestinal epithelium carries the antigen present in the lumen to dendritic cells present in the Peyer’s Patch, where the activation of defense cells against this antigen occurs. Following this activation, GALT cells produce plasma and mature T cells that are spread throughout the intestinal mucosa. According to Olkowski etal. (2006) and Belote etal. (2018), as a consequence of bird exposure to antigens, the lamina propria is hyperemic and infiltrated with numerous inflammatory cells. Significant changes are mainly found at the interface of the enterocyte basal domain and lamina propria, which cause damage to the integrity of the intestine. In this study, the fragments behaved differently in relation to the thickness and inflammatory infiltration in the lamina propria due to exposure to mycotoxins. The duodenum did not present difference between challenged groups and NC while in jejunum, all challenged groups presented high thickness scores and also inflammatory infiltration, and in ileum, the challenged groups presented high values for lamina propria thickness, but only the CPA + AFB1 group showed significant difference for inflammatory infiltration when compared to NC.
Akinrinmade et al. (2016) conducted a morpho-logical study of the intestine in the presence of AFB1. The authors not only found alterations in the intestinal epithelium, but also inflammatory infiltration in the lamina propria, a result that agrees with the findings of this study. Aflatoxin B1 is considered the most life-threatening myco-toxin, but its intestinal toxicity can be compared to other mycotoxins (Liew and Mohd-Redzwan 2018). Because of this, the results found in this study showed similar toxicity between CPA and AFB1 in mucosa intestinal.
Liver Mycotoxins have several biological effects that are harmful to animal health, including hepatotoxicity. The effects of bile duct necrosis and proliferation found in this study had been previously identified in the 1960s, when aflatoxins were isolated and characterized (D’Mello and Macdonald 1997). Due to this, many studies related to AFB1 liver damage have been conducted. AFB1 is known to be mu-tagenic and carcinogenic in chronic intoxication and its acute effects were also previously studied (Steyn 1995). Cyclopiazonic acid does not present this variety of studies and its hepatotoxic effects still lack some clarification.
Jaskiewicz et al. (1988) challenged non-human primates with AFB1 and CPA, separately and simultaneously. When analyzing the liver of the animals in the CPA group, no necrosis was found.
However, there was hepatocyte necrosis in the groups containing isolated AFB1 and in associa-tion with the CPA. In this study, this result was repeated. Therefore, since we worked with another animal species, the findings showed that AFB1 is also responsible for cell necrosis in liver tissues of broilers.
Results found for aflatoxin B1 are in agreement with those reported by Ortatatli and Oguz (2001) and Saleemi et al. (2020), in which signs of necrosis and bile duct proliferation are identified. All parameters, when analyzed separately, did not indicate a significant difference between the CPA group and the NC group, but when summed to generate the total number of ISI for the liver, the CPA group was significantly altered (p<0,05).Antony et al. (2003) identified CPA as potentially hepatotoxic by testing it in rats, which corrobo-rates the results found.
According to Oliveira and Corassin (2014), the sensitivity of birds to AFB1 is due to the rapid absorption of mycotoxin by the GIT. Following this absorption, AFB1 binds rapidly to albumin and is distributed to tissues, mainly to the liver. The mycotoxins in question have harmful effects on the small intestine and liver, both alone and together. It was also concluded that mycotoxins behave differently in small intestine fragments. When the parameters of thickness and mixed inflammatory infiltrate of the lamina propria were analyzed, the scores were different between the challenged groups. It is known that there is a great concern regarding the contamination of commodi-ties by AF, but it is valid that this care extends to the CPA, since it also caused damage to the con-taminated birds on the small intestine and liver. The ISI method has proved to be a tool that can show which parameters have suffered the most damage according to the challenges performed. Thus, it demonstrates the ability of the tested mycotoxins to affect different areas of the intestine and liver.
Acknowledgments. Thank are given to Postgraduate Program in Pharmaceutical Sciences (PPGCF) and Postgraduate Program in Veterinary Sciences (PPGCV) for collaboration. We thank Co-ordination for the Improvement of Higher Education Personnel (CAPES) for the financial support.












 uBio
uBio