Introduction
Plant extracts are sources of phytochemical substances (Ramos 2008). The Annona muricata tree is commonly known as soursop, being widely used in folk medicine and some studies report that extracts from seeds, branches, roots, stems and fruits have several biological activities (Heinrich et al. 1992; Vieira et al. 2010). Extracts from different parts of the soursop tree were studied and 212 phytochemical compo-nents were identified, mainly acetogenins, al-kaloids, flavonoids, sterols and others (Rady et al. 2018). In 2015, George et al. suggest that the components of the A. murlcata extract have powerful antioxidant properties, being capable of scavenging significant free radi-cals, with a strong correlation with the pheno-lic components. The extract from the leaves of A. murlcata has antioxidant, anti-inflammatory, analgesic, apoptosis-inducing and cytotoxic activity properties (Roslida et al. 2010; Abdul-lah et al. 2017).
In 2018, Rady et al. reported that there is no correlation between the consumption of sour-sop and/or infusions of some parts of the soursop with neurotoxic effects. Arthur et al. (2011) reported that doses higher than 5 g/kg of aqueous extract may be related to toxicity in laboratory animals’ organs. Polycyclic Aro-matic Hydrocarbons (PAH) are environmental pollutants, and 7, 12 - dimethylbenzanthracene (DMBA) is a PAH that undergoes metabolic ac-tivation, producing free radicals and reactive oxygen species capable of inducing deleteri-ous effects and causing oxidative damage in various organs and tissue, being widely used in experimental models using laboratory animals (Macejova and Brtko 2001; Choi 2008).
The objective of this work was to identify the histopathological alterations in organs of Wlstar rats to evaluate the toxic effects of the use of Raw Extract of Annona murlcata Leaves alone or in association with DMBA.
Methods
- Obtaining the Raw Extract of Annonamurlcata Leaves (AMRLE).
The leaves of the soursop tree were collected in Maringá-PR (Brazil) and botanical identifica-tion was performed by Herbarium of Maringá State University (HMSU) with the exsiccate deposited under number 31314. The AMRLE was obtained as described by Barbosa and Mello (2004), where about 30 kg of A. murlcata leaves were crushed and submitted to extrac-tion by turbolysis in Ultra-Turrax® (UTC-115KT) for 15 minutes, with an interval of 15 minutes repeated 3 times. 50% ethanol was used as extracting liquid and the material obtained dur-ing the extraction process was subjected to fil-tration and lyophilization for 24 hours, placed in a plastic bottle and stored in a freezer (-20oC).
- Quantification of total phenols.
The content of total phenols present in AMRLE was determined using the phosphomolybdotungstic reagent by the Folin-Ciocalteu method. The samples and the previously validated standard curve were read in a spectrophotometer (X = 760 nm) (BRASIL Anvisa, 2010).
- Animals and experimental design.
Sixty female Wlstar rats aged 55 days were used, provided by the Maringá State Universitys Central Bioterium and kept in an experimental bioterium. The animals were weighed and randomly divided into 6 groups of 10 animals: Group 1 (G1) - Experimental group treated with 50 mg/kg of AMRLE + DMBA (65 mg/kg)
Group 2 (G2) - Experimental group treated with 100 mg/kg of AMRLE + DMBA (65 mg/kg) Group 3 (G3) - Experimental group treated with 200 mg/kg of AMRLE + DMBA (65 mg/kg) Group 4 (G4) - Group treated only with 200 mg/ kg ofAMRLE
Group 5 (G5) - DMBA treated group (65 mg/kg) Group 6 (G6) - Negative control group (without AMRLE/DMBA)
The animals were housed in polypropylene boxes under standardized conditions of temperature of 22 ± 1°C; 50 ± 10% relative humidity and 12h day-night cycle with free access to feed and water. On the first day of the experiment, animals from G1; G2; G3 and G5 groups received 65 mg/kg of DMBA (Sigma-Aldrich, Saint Louis, Missouri/USA) diluted in 1 mL of corn oil orogastrically (gavage) in a single dose. For 20 weeks, all animals in groups G1; G2; G3 and G4 received 1mL of AMRLE at concentrations of 50 or 100 or 200 mg/kg which was also administered by gavage. While animals in groups G5 and G6 were administered 1 mL of sterile water. At the end of the 20th week, all animals were euthanized using a thiopental solution at dose of 30 mg/kg, administered intravenously. After euthanasia, part of the breast tissue and lung, liver and kidney fragments were surgically removed and fixed in 10% formalin solution and sent for histological processing. Part of the large intestine was also removed and stored in 70% ethanol until the quantification of aberrant crypts (AC) and Aberrant Crypt Focus (ACF).

Table 1: Demonstration of the weight evolution of Wistar rats belonging to experimental groups G1 to G6 during a period of 20 weeks.
- Weight development assessment.
All animals were weighed on the first day of the experiment, and later on each day for 20 weeks.
- Histopathological Analysis.
Fragments of breast tissue, lungs, liver and kidneys were dehydrated in alcoholic solutions with increasing degrees, cleared in xylene and embedded in paraffin. The paraffin blocks were sectioned in a semi-automatic microtome, obtaining histological sections of 4 - 5 g, which were used to make permanent slides stained with Hematoxylin-Eosin (HE). The reading of the slides was performed using an optical microscope with a camera (Opticam Microscopy Technology - Lopt 14003) with magnification of 400X. Histopathological changes were identified and noted for the presence or absence of animals belonging to each group.
- Quantification of Aberrant Crypts (ACs) and Aberrant Crypt Focus (ACF).
The colonic mucosa was stained with 1% methylene blue as described by Sousa et al. (2010). The ACs and FCAs were identified and quantified according to the criteria established by Bird (1987). After this procedure, the intestinal tissue was also submitted to histopathological analysis, where ACF were classified as hyperplasia or dysplasia according to Yoshimi et al. (2004).
- Statistical analysis.
Results were expressed as mean ± standard deviation (SD). And the statistical analysis was evaluated by the Fischer and Kruskal Wallis tests. Values of P < 0.05 were considered statistically significant.
- Ethical aspects.
All experiments involving animals were done in conformity with the worldwide accepted ethical guidelines for animal experimentation and approved by the Ethics Committee for Animal Experimentation of Maringá State University (Protocol n° 4816181115).
AMRLE did not influence the development of the treated animals, since weight gain showed a progressive increase in the animals’ body mass, and there was no statistically significant change between the analyzed groups (Table 1).
- Histopathological analysis to assess the toxic effects of DMBA and/or AMRLE.
The histopathological analysis identified some pathological alterations, such as: hepatic steatosis; inflammatory foci in the liver, kidney and lung; pulmonary lymphoid hyperplasia, ectasia and hyperplasia mammary glandular epithelium (Figure 1).
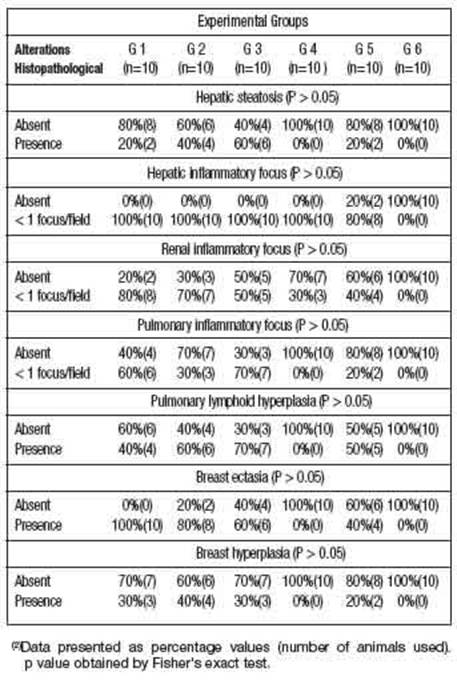
Table 2: shows that many animals in G1, G2, G3 and G5 groups showed histopathological changes in liver, kidney, lung and breast tissue. These histopathological changes may be related to the isolated toxic action of DMBA and/or associated with AMRLE, since these changes were not seen in animals belonging to group 6 (negative control - without DMBA/AMRLE.
Results
- Chemical composition.
Approximately 1 kg of AMRLE was obtained, corresponding to approximately 3% of total weight of the leaves. The number of total phenols was determined to be 274.4 ± 1.62 mg/g of AMRLE.
- Effect of DMBA and/or AMRLE on weight development.
Apparently, administration of 65 mg/kg of DMBA and/or 50, 100 and 200 mg/kg of - Quantification of Aberrant Crypts (CA) and Aberrant Crypt Focus (aCf) and histopathological analysis.
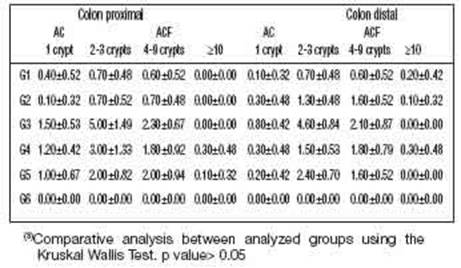
In Figure 2A, ACF present in the large intestine and stained with methylene blue can be identified. The quantification results are shown in Table 3, and in the animals in group G6 (negative control without DMBA and/or AMRLE there was no development of AC or ACF. However, the mean values of the number of AC and ACF present both in the proximal and distal regions of the colon of animals in G1, G2, G4 and G5groups were very similar to each other. Kruskal Wallis test was used for statistical analysis, obtaining a P = 0.4895. The portion of the intestinal colon used for the quantification of ACs and ACF was also submitted to histopathological analysis, showing that in Figures 2B and 2C hyperplasia and dysplasia were observed in all groups, except in groups G1 and G6. While lymphoid nodules (Figure 2D) were observed in animals from groups G2 and G3.
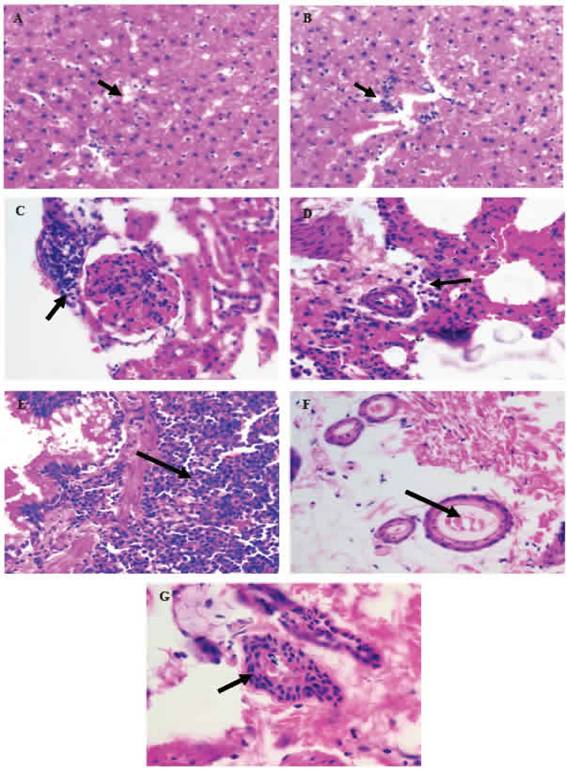
Figure 1: Histopathological alterations in organs or tissues of Wistar rats resulting from the performance of DBMA and/or AMRLE 400X magnificaron. A) hepaticsteatosis; B - D) inflammatory foci in the hepatic, renal and pulmonary parenchyma; E) bronchial lymphoid hyperplasia; F) ectasia ductal; G) mammary cell hyperplasia.
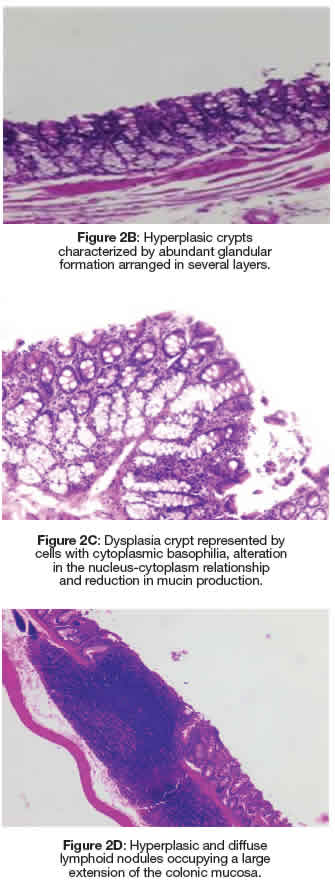
Figure 2: Dysplasia crypt represented by cells with cytoplasmic basophilia, alteration in the nucleus-cytoplasm relationship and reduction in mucin production. Figure 2A: Colonic mucosa stained with methylene blue representing an FCA composed of four CAs with irregular luminal opening and thicker epithelial lining compared to normal adjacent crypts. Figure 2D: Hyperplasic and diffuse lymphoid nodules occupying a large extension of the colonic mucosa.
Discussion
The Brazilian population and also from different regions of the world consume soursop and also parts in the form of teas or infusions due to its antioxidant, anti-inflammatory, analgesic and also cytotoxic properties against different forms of cancer (Baskar et al. 2007; Roslida et al. 2010; Abdullah et al. 2017; Ekaprasasti 2012). The number of total phenol spresent in AMRLE was like that described in other studies (Rady et al. 2018). The phenolic components are responsible for the antioxidant activity present in A. muricata extracts (George et al. 2015; Moghadamtousi et al. 2015; Coria-Téllez et al. 2018; Rady et al. 2018).
Ethanolic extract of A. muricata leaves has low toxicity. Its requiring exaggerated daily con-sumption (Sousa et al. 2010; Abdullah et al. 2017). This low toxicity was also observed dur-ing the treatment of animals with AMRLE that showed a progressive increase in body mass, but there was no statistically significant differ-ence in weight development between the nega-tive control group (G6) and the groups treated with DMBA and/or with AMRLE, even when ad-ministered simultaneously with the environmental pollutant DMBA, which after hepatic metabo-lism gives rise to 7-hydroxymethyl - 12 - methyl-benzanthracene (7-HMBA); 12 - hydroxymethyl
- 7 -methylbenzanthracene (12-HMBA) and 7,12
- dihydroxymethylbenzanthracene (7,12-HMBA) which are active metabolites and responsible for oxidative damage (Ravi et al. 2005).
The study of histopathological changes in or-gans or tissues of Wistar rats was also use-ful in the assessment of toxicity resulting from the use of AMRLE alone or in conjunction with DMBA. Hepatic steatosis (Figure 1A and Table 2) is a reversible cell lesion caused by the action of exogenous chemical substances or xenobi-otics (da Silva and Escanhoela 2009). Inflam-matory foci in the hepatic, renal and pulmonary parenchyma (Figure 1B-1D and Table 2) may be due to toxic action of the active metabolites of DMBA (7-HMBA, 12-HMBA and 7,12-HMBA) and/ or AMRLE, but it should be noted that in the composition of AMRLE there are phenolic components with potent antioxidant properties, capable to neutralize the active metabolites (George et al. 2015; Rady et al. 2018).
The harmful effects of 7-HMBA, 12-HMBA and 7, 12-HMBA may have been partially neutral-ized by the phenolic components of AMRLE, reducing formation of cell and tissue lesions, and consequently formation of moderate or intense inflammatory processes. The anti-in-flammatory activity of A. muricata extracts has already been described (Roslida et al. 2010; Sousa et al. 2010).
In Figures 1E-1G, bronchial lymphoid hyperplasia and breast ductal cell ectasia and hyperplasia were observed. These histopathological changes were observed in all animals, except those in groups G4 and G6 without contact with DMBA, which is a polycyclic aromatic hy-drocarbon (PAH), found in tobacco combustion, having a strong association with lung diseases and breast carcinogenesis (Macejová and Brtko 2001).
Ductal cell hyperplasia is not considered a pre-neoplastic lesion, but it presents a very low risk for the development of invasive breast carcinoma. Furthermore, there is also a relationship between smoking and the formation of ductal ectasia (Guray et al. 2006). As DMBA is one of the combustion products of tobacco, there is a possibility that this chemical compound may participate in the formation of these pathological changes in the breast tissue. Bronchial lymphoid hyperplasia and intestinal mucosa can be considered as a defense mechanism of the body against the harmful action of DMBA and/orAMRLE, since the lymphoid system has a large area of interface between the internal and external environment, being prepared to inactivate or neutralize aggressive agents of chemical, physical and biological origin through a structure organized in follicles composed of B lymphocytes and interfollicular zone with T lymphocytes, in addition to dendritic follicular cells that present antigens to B lymphocytes, providing co-stimulatory signals which increase the activation and proliferation of germinal centers, resulting in lymphoid hyperplasia (Halle et al. 2009).
The number of isolated AC and ACF in the distal and proximal portions of the colon was similar and there was no statistical difference between the analysed groups. However, in Table 3, it is observed that groups G1 and G2 (distal and proximal region) presented a decrease in the number of ACF with 02-03 and 04-09 crypts/ focus compared to group G3. Although this decrease has not been confirmed by statistical analysis, the prospect still opens up that the experimental treatment with 50 and 100 mg/ kg of AMRLE may have contributed to the inhibition of ACF development, controlling the multiplicity of crypts/focus probably due to the chemoprotective action of the phenolic components present in AMRLE, whose action may be related to the administered dose.
During the investigation of the antioxidant activity of essential oils, the quantification of ACF was considered useful for studies that assess the antioxidant activity that acts in all stages of the carcinogenesis process, thus promoting a reduction in the potential risk of cancer. Since antioxidant substances can inactivate reactive oxygen and nitrogen species that play an important role in carcinogenesis (Lima et al. 2020). In the histopathological analysis using HE stains, hyperplastic and dysplastic ACF were visualized in all groups, except in groups G1 and G6, suggesting that AMRLE, was effective in controlling the clonal expansion of AC and ACF, although it was not demonstrated statistical significance. Furthermore, it should be noted that no tumour was observed in the experimental groups, which is relevant information, since the development of tumours is related to the large number of ACF (Ansil et al. 2013; Kilari et al. 2016).
Conclusions
Histopathological alterations and the formation of ACs and ACF did not show a statistically significant difference between the groups analysed. However, although AMRLE has antioxidant effects due to the presence of phenolic components, there was still the formation of some pathological processes that may be related to the isolated toxic action of DMBA and/ or associated with other components of AMRLE, since these changes were not seen in the negative control group.
Acknowledgement
Coordenagao de Aperfeigoamento de Pessoal de Nivel Superior -Brasil (CAPES) - (graduate scholarship).












 uBio
uBio