Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American journal of sedimentology and basin analysis
versión On-line ISSN 1851-4979
Lat. Am. j. sedimentol. basin anal. v.14 n.2 La Plata ago./dic. 2007
ARTICLES
Identification of microbially induced sedimentary structures over a tidal flat
Diana G. Cuadrado 1,2 and Natalia V. Pizani 1,3
1Instituto Argentino de Oceanografía. CONICET. CC 804. B8000FWB Bahía Blanca. Argentina.
2Depto. Geología. Universidad Nacional del Sur. San Juan 670. B-8000ICN Bahía Blanca. Argentina. Email: cuadrado@criba.edu.ar
3Depto. Biología, Bioquímica y Farmacia. Universidad Nacional del Sur. San Juan 670. B-8000ICN Bahía Blanca. Argentina. Email: npizani@criba.edu.ar
Received: April 15, 2007 - Accepted: December 19, 2007
Abstract. The influence of microbial activity in carbonatic environments leading to stromatolite build-ups is widely known. In siliciclastic environments, however, this influence has been far less studied. The present study was carried out in this type of environment and determined the importance of cyanobacterias in the preservation of sedimentary structures. Similar sedimentary structures were also recognized in the rock record by other studies. Therefore, if we know the environment of formation and conservation of such structures, then the paleoenvironment of the rock could be inferred.
In the tidal flats of the Bahía Blanca Estuary, microbial mats were identified. Their interaction with sediments, lead to a stabilized flat by shielding from erosion the existing sedimentary structures. Since it is the first time these structures are mentioned in a present-day environment in Argentina, a detailed description of the bio-sedimentological interaction is presented and our results compared with others from different climatic zones, namely, temperate humid and subtropical arid. Finally the occurrence of zeolites, an authigenic mineral, indicated that the sediment would be in the early stages of diagenesis.
Resumen. La influencia de la actividad microbiana en ambientes carbonáticos en la construcción de estromatolitos es ampliamente conocida. En ambientes silicoclásticos, sin embargo, dicha influencia ha sido menos estudiada. El presente estudio fue realizado en este tipo de ambiente y ha identificado la importancia de las cianobacterias en la preservación de estructuras sedimentarias. Estructuras similares a las actuales han sido reconocidas en el registro rocoso por otros estudios. Por consiguiente, conociendo con detalle el ambiente de formación y preservación de dichas estructuras se puede inferir el paleoambiente de la roca que las contiene.
En las planicies intermareales del estuario de Bahía Blanca se han identificado matas microbianas que interaccionan con los sedimentos produciendo la estabilización de la superficie de la planicie, resguardando de la erosión las estructuras sedimentarias que en ella se generan. Debido a que es la primera vez que se mencionan estas estructuras en Argentina en un ambiente actual, se presenta una detallada descripción de la interacción bio-sedimentológica encontrada. Asimismo se comparan los resultados del presente trabajo con los obtenidos en ambientes climáticos diferentes; templado húmedo y árido subtropical. En este ambiente y bajo determinadas condiciones se reconoce la formación de zeolitas, minerales autigénicos que son indicadores de una diagénesis temprana.
Keyword: Biostabilization; Microbial activity; Cyanobacteria; Zeolites; Bahía Blanca Estuary.
Palabras clave: Bioestabilización; Actividad microbiana; Cianobacterias; Zeolitas; Estuario de Bahía Blanca.
INTRODUCTION
Over the past few years, the significance of biota for the interpretation of geological processes is increasingly becoming the focus of study for geologists and microbiologists. The interaction of biology with geological parameters has been examined under an interdisciplinary approach, which is reflected by the modern and evolving field of "geobiology" (Noffke and Knoll, 2001). Increasing emphasis on interactions across traditional discipline boundaries rather than on the core disciplines induced Naylor (2005) to point out that geobiology is primarily concerned with exploring the interface and complex interactions between the biosphere and geosphere.
Indeed, many studies have demonstrated the role of organism activity from microbial mats as a key factor for stromatolite formation (Krumbein, 1983; Walter, 1976). Many studies show that metabolic activity of cyanobacteria and heterotrophic bacteria in carbonate marine environments induce the precipitation of carbonates which in turn forms a microbial build-up named stromatolite. Chemical sediments are formed by salt-rich water precipitating into minerals also increasing the preservation of fossils. Microbes found in chemical sedimentary systems are easily visible and well preserved, as is the case with stromatolites, and thus has most geologists focus on carbonate marine rocks where bacterial structures are abundantly preserved.
On the other hand, microbial structures in siliciclastic depositional regimes have been far much less investigated. Recent studies show that many sedimentary surface structures found in modern tidal flats are not formed by physical processes alone, but are also the result of biological activities (of which bacteria are an important component) which influence erosional and depositional dynamics (Noffke, et al., 2001). They thus named the siliciclastic counterparts of stromatolites "Microbially Induced Sedimentary Structures" (MISS). Due to their specific microbial-physical mode of formation, their appearance is significantly different from stromatolites and, therefore, they have been classified as a separate category within primary sedimentary structures. The classification is based on the ability of the cyanobacteria to interact with physical processes and sedimentary dynamics. In contrast to carbonate (chemical) sediments which are formed by the precipitation of minerals in marine areas, siliciclastic deposits are allochthonous and are controlled by physical processes of erosion and deposition alone. The studies mentioned above demonstrate the influence of benthic microbial mats on sedimentary dynamics in physical sedimentary systems and thus have given geologists a new perspective on siliciclastic deposit sedimentology.
Microbial mats are typically formed by filamentous, entangled organisms that produce a macroscopic "matlike" structure. Stal (2000) found that the reason why cyanobacteria are the most successful organisms at matbuilding results from a combination of characteristics unique to this group. Cyanobacteria are the only known oxygenic phototropic prokaryotes. They display a great resilience to changes and fluctuations of environmental conditions. As their predominant metabolism is oxygenic photosynthesis, cyanobacteria use light as an energy source, water as an electron donor and CO2 as a carbon source. Where these requirements are met abundant microbial mats will form.
Coastal tidal sand flats often are an ideal environment for microbial mats to develop (Stal, 2000), particularly where they extend over a large area and display a low slope. The near absence of grazing organisms allows mat-building organism such as cyanobacteria to thrive. Most resist long periods of drought, tolerating large fluctuations of salinity and temperature. These coastal microbial mats are typically composed of filamentous cyanobacteria that form a dense entangled mass which traps and binds sediment particles.
Cyanobacteria within sediments secrete complex organic material, often termed extracellular polymeric substances (EPS) which are composed of proteins, carbohydrates and lipids. EPS secretions perform a variety of functions within marine sediment systems including protection from abrasion and desiccation and also act as a food source (Decho, 1990). As studies have demonstrated that the erosion threshold and erosion rate of sediment may be modified by the presence of microbial assemblages, interest in EPS has grown because of its influence on physical properties of sediments and its general biological engineering significance (Paterson, 1994).
Benthic photosynthetic microbes are typically abundant in the upper intertidal and lower supratidal zones (Gerdes et al., 2000). Cyanobacteria establish layered accretions of biomass, thereby making an important contribution to the sediments and sedimentary structure. Bacteria interact with erosion and deposition, and as such can produce structures in the sediment which after lithification are incorporated in rocks. As cyanobacteria colonized the Earth 3 billion years ago, microbially generated structures can be found through most of the geological record. Noffke et al. (2006a, b) discovered sandstones in South Africa and France (Noffke, 2000) whose structures are characteristic signatures of seafloor colonizing bacteria thus indicating that the area formed in an ancient ocean.
The Puerto Rosales tidal flats located in the Bahía Blanca Estuary (Argentina) (Fig. 1), display a homogenous microbial mat veneer over the sediment surface and thus stabilizing it. Because the site is relatively isolated and human use of the area is low, these microbial mats can grow freely without being disturbed and thus turning them into an extraordinary natural laboratory. This paper presents a preliminary study of the influence of benthic cyanobacteria on sedimentary processes in a siliciclastic tidal flat of the Bahía Blanca Estuary. It is worthy to note that this is the first time these structures are mentioned in Argentina, so detailed characteristics are given about the formational environment.
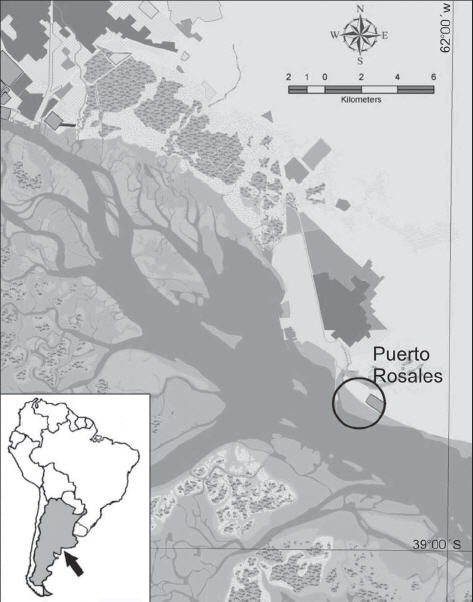
Figure 1. Study area over broad tidal plains in Puerto Rosales, Bahía Blanca Estuary.
Figura 1. Area de estudio en amplias planicies de marea en Puerto Rosales, estuario de Bahía Blanca.
Study Site and Physical Environment
Broad intertidal sand and mudflats are found bordering many of the world's estuaries. These are depositional areas of low current velocities. The physical environment and characteristics of intertidal sediment deposits differ markedly from their terrestrial and shallow counterparts due to regular emersion which leaves the surficial sediment layers exposed to wind and rain erosion, subsequent drying, compaction and exposure to extreme temperatures.
Puerto Rosales has a dry temperate climate with low precipitation and high evaporation rates. The annual mean temperature is 15.6ºC, while summer (January) temperatures average 22.7ºC and 8.1ºC in winter (July). The mean precipitation in Puerto Rosales is 460.5 mm (Piccolo and Diez, 2004).
The Bahía Blanca Estuary uncovers large tidal flats at low tide. In Puerto Rosales, these extend nearly 1000 m between the high and low tide line, exhibit a low slope and isolated patches of vegetation. Semi-diurnal tides predominate and therefore, large areas are covered by water for only short periods at high tide and often left exposed for several days at neap tide. The sediment often experiences large fluctuations in water content, salinity and temperature resulting in extreme conditions that limit the range of organisms able to inhabit this environment. Lower areas are submerged for longer periods of time.
FIELD AND LABORATORY METHODS
Field work was conducted over a period of twelve months at monthly intervals, from May 2006 to May 2007. During high-water spring tide, water depth was measured using as a reference a graduated wood stake (1 m high) buried in the flat and current velocities were determined by measuring the duration of transport of a swimming mark along a distance of 1 m. The wave height was also measured by referencing it to the stake and the time for 11 successive crests to pass over the same point used to determine the wave period.
Sedimentary surface structures of Puerto Rosales tidal flats were characterized by photography and microscopic studies. Fresh samples of microbial mats were prepared (dried and gold coated) for analysis under scanning electron microscopy (SEM JEOL35 CF 8 Tokio) and energy dispersive X-ray microanalysis system (EDAX). Undisturbed mat and sediment samples were collected in plastic Petri-dishes to observe the composition and structure under the optical microscope Nikon SMZ 1500. Sediment samples were obtained with tube corers (10 cm long, 3 cm in diameter) separating the surficial mat from the rest and the latter into 1-cm thick layers. They were stood in hydrogen peroxide for three days and then sediment grain size was measured using a Malvern Mastersizer 2000 laser particle analyzer.
We compared the structures found in Argentina based on a catalogue of microbial signatures (Gerdes, et al., 2000) of sedimentary structures from two different study sites in Europe (North Sea) and Africa (Tunisian sabkha coast).
RESULTS
Typical conditions at the site are reflected by data collected on May 11th, 2007. The intertidal study area was flooded by seawater 12-15 cm deep during high tide. Current speed reached up to 40 cm s-1 and waves reached 5 cm in height with periods of 2 s. Southwest wind speed was 5-6 m s-1 during the measurement, although previously they reached up to 10-11 m s-1. Another event where strong onshore winds and higher wave heights was also examined (Cuadrado and Gómez, 2007) is discussed below.
The granulometry of the sediments from the tidal flat was measured in 1 cm thick-layers and displayed varying grain size distribution with depth. The upper layer which consisted of microbial mats had a bimodal grain distribution reflecting the presence of two different populations (Fig. 2). There was a peak at 0.350 mm (medium to fine sand) and another at 0.020 mm (fine silt), indicating mixed sediments. Similar characteristics were observed in underlying layers down to 2 cm depth. Between 2 and 4 cm depth, sand was absent and fine sediment including silt 0.040 mm and clay 0.004 mm approximately in size was observed.

Figure 2. Grain size distribution at different layers from the tidal plain surface. a) surface layer. b) 0-1 cm. c) 1-2 cm. d) 2-3 cm. e) 3-4 cm.
Figura 2. Distribución del tamaño de grano en diferentes capas desde la superficie de la planicie de marea. a) capa superficial. b) 0-1 cm. c) 1-2 cm. d) 2-3 cm. e) 3-4 cm.
The mineralogical composition of the sediments was primarily feldspar and quartz (Fig. 3). There were abundant alterites displaying inclusions and fluids, and a minor proportion of heavy minerals (density > 2.83 g cm-3 at 20ºC) mostly pyroxenes (augite and hypersthene) and amphiboles. The mineralogical composition of tidal flat sediments was comparable to the results obtained by Gelós and Spagnuolo (1982) studying the Canal Principal of Bahía Blanca Estuary whom determined the volcanic origin of its sediments.

Figure 3. Vertical section cut through the tidal plain. a) The arrow indicates the presence of a layer immediately beneath the microbial mat where the sediments are glued by microorganisms activity. b) A close-up view showing extra cellular polymeric substance (EPS) binding sediment grains. Single mineral particles "float" in the mat matrix without any grain-to-grain contact.
Figura 3. Corte vertical de la planicie de marea. a) La flecha indica la presencia de una capa por debajo de la cubierta microbiana donde los sedimentos están pegados por la actividad de microorganismos. b) Vista cercana mostrando la sustancia polimérica extracelular (SPE) uniendo los granos de sedimento. Partículas individuales de sedimento "flotan" en la matriz orgánica careciendo de contacto grano a grano.
Main occurrences of microbial films and mats found in tidal flats of Bahía Blanca Estuary are in the intertidal and lower supratidal range. The areas closely related to the tidal cycle are covered by layers of mats and biofilms at an early stage of development. In some cases, mats can even be peeled off from the sediment as a large coherent piece (Fig. 4). In autumn, the microbial mat begins to colonize the sediment of the higher intertidal flat. Different stages of development can be found in the late winter probably because of the pattern of successive colonization in the area, a feature recorded in the changing thickness of the microbial mat layer.

Figure 4. Piece of coherent biofilm acting as a protective layer on the tidal flat.
Figura 4. Trozo de biofilm que actúa como capa de protección de la planicie de marea.
The microbial mat protects the sedimentary structures developed over the tidal flat from wave and wind erosion. The structural characteristics are those of non cohesive sand layers where upper bedding planes with high contents of clastic minerals were binded by microbial films mainly made of filamentous cyanobacteria (Fig. 5) that builds a fibrillar meshwork. Furthermore, such mats are visible on tidal flats of Bahía Blanca Estuary to the naked eye as extensive layers that appear to resist erosion very well.

Figure 5. Cyanobacteria culture present in the study area.
Figura 5. Cultivo de cianobacterias presentes en el área de estudio.
To study the microbial influence on grain sediments, undisturbed samples including surficial mat covered sediments were collected perpendicularly to the sediment surface for optical microscope analysis (see Fig. 3a). These samples showed that individual sedimentary grains are binded by microbial extra cellular polymeric substances (EPS) immediately below the tidal flat surface. This adhesive substance (EPS) occurs as a matrix between grains in figure 3b. Only a few millimeters below the surface the EPS layer cannot be seen, however, some grains maintain their connection.
Small-sized particles with a flaky appearance can be observed (Fig. 6). A crystal is also noticeable from the upper middle part of the image, which the X-ray spectrum identifies as authigenic zeolite with characteristic elements of chabazite: Ca, Na, Si and Al. Identification of these crystals as chabazite is based on correlation of the rhombic morphology and EDX spectrum (Welton, 1984). Chabazite also occurs as rosette-shaped crystals as can be seen in figure 7a. Another spectrum analysis indicates the presence of a different zeolite: clinoptilolite (Fig. 7b).
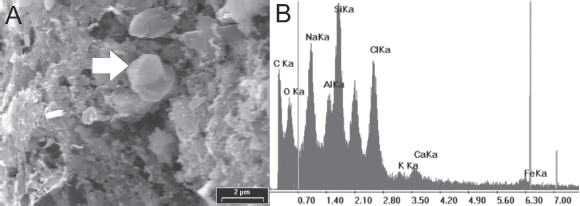
Figure 6. a) Photo taken by SEM showing a crystal of zeolite recognized by the X-ray spectrum ( b).
Figura 6. a) Foto tomada con el microscopio electrónico mostrando un cristal de ceolita, reconocido por el espectro de Rayos X (b).

Figure 7. a) Rosette-shaped chabazite crystals. b) clinoptilolite crystal.
Figura 7. a) Cristal de chabazita en forma de roseta. b) cristal de clinoptilolita.
DISCUSSION
EPS was found in the samples collected from the Puerto Rosales tidal flat as binded sediment grains located in the top surface layer of the tidal flat due to the biological activity (Fig. 3). The process that glues particles to the mat surface was described by Shin (1983) as "trapping". The mat gradually incorporates allochthonous grains as it grows upwards, a feature called "binding" (Dunham, 1962). Mineral grains are then incorporated into the organic matrix by the trapping and binding process mentioned above (Fig. 3b). These processes could explain the occurrence of two different populations in the upper layer's sediment distribution. Since the study area is located in the lower supratidal zone, it is probable that some sand grains might have been transported to it by wind and trapped by the microbial mat and then added to the sand transported as bedload before high tide. During ebb tide, water had a clearer appearance probably due to the trapping and binding action of the microbial mat.
Microbial influences, in addition to biostabilisation, trapping and binding, also control the protective property of the organic matrix. Microbial mats thus increase the critical shear stress value required to resuspend deposited grains. In addition, microbes stabilize the sediment and thus protect their surroundings against destruction by water generated turbulences. The preservation of footprints on the flat over several months seemed to corroborate this, even if the tidal flat was flooded twice a day and was affected by currents and waves, including strong storm waves (Fig. 8).

Figure 8. Footprints on the tidal flat a) in July b) and in October, 2007.
Figura 8. Pisadas sobre la planicie de marea a) en Julio, b) y en Octubre de 2007.
Impacts of physical disturbances were analyzed. The physical processes that control growth responses of biofilms and microbial mats include sedimentation, erosion and fracturing of surface layers (Gerdes et al., 2000). The sedimentation in the study area is almost null (Cuadrado and Gómez, 2006). This fact is demonstrated by the results obtained after sprinkling finely ground brick powder (<63 mm) over a test area. After one month, the allochthonous material could still be seen over the flat and two months later there was scarcely any left. This point to a very low sedimentation rate which also demonstrates the trapping and binding processes mentioned above.
In some areas, ripple marks occur as "frozen" ripples due to a biological binding of the physically generated morphology. The ripples were formed by tidal flushing, followed by a microbial recolonization of the surface, preferentially after the water drains out. The following tide may then reinitiate sediment transport; however, surface patches successfully stabilized by the cohesive matrix may withstand the shear stress. This also generates "erosional remnants" and "pockets" (Fig. 9a), the resulting surface topography from both biostabilization and erosion interaction was also found by Noffke (1999) on the North Sea coast. The erosional remnants are a topographic feature a few centimetres high which are residual biolaminated build-ups left over from the destruction of the former biostabilized surface layer. On the other hand, erosional pockets are round depressions with soft margins caused by partial erosion of cohesive sand layers as a result of tide and wave controlled hydrodynamics. This morphology changed after a winter storm where the wave height was three times over normal values (Cuadrado and Gómez, 2007). Following that event, erosional remnants were covered by microbial mats protruding several centimetres above the surrounding sediment, whereas erosional pockets had developed sharp margins (Fig. 9b). The resulting morphology is the typical signature of an erosional stress that was applied to the biostabilised sediments. The distribution of both erosional structures suggests different degrees of biostabilisation resulting from the interaction of currents and waves as well as stabilizing microbial communities composition and maturity stage.

Figure 9. a) Erosional remnants and pockets over Puerto Rosales tidal flat. b) The tidal flat after a storm.
Figura 9. a) Remanentes erosivos y depresiones en la planicie de marea de Puerto Rosales. b) La planicie de marea luego de una tormenta.
In addition, microbial mats are commonly fractured as a result of desiccation as found on the Tunisian coast (Gerdes, et al., 2000). Shrinkage of microbial mats is evidenced by polygonally organized cracks (Fig. 10a). Fracturing enables the mat-forming microbes to overgrow these margins resulting in pillow-like structures.
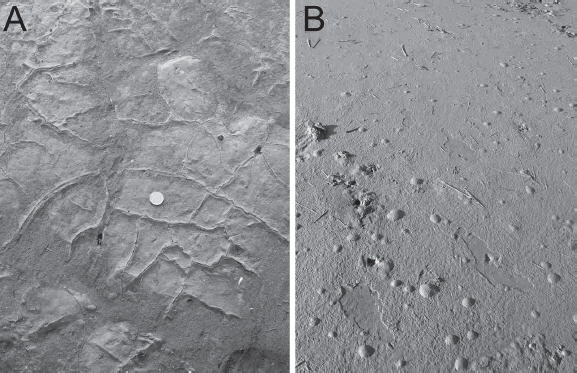
Figure 10. a) Polygonally fractured microbial mats. Coin is 2 cm in diameter. b) Surface deformation through bubbling caused by gas formation in the substrate. Note that microbial mat formation is weaker only in isolated spots where the erosion may tear off pieces of the organic layer. Consequently, the sandy surface in those exposed areas can be eroded and depressions may form.
Figura 10. a) Fracturas poligonales en coberturas microbianas. La moneda tiene 2 cm de diámetro. b) Deformación de la superficie por burbujas debido a la formación de gas en el sustrato. Nótese que la cobertura microbiana es más débil en algunos sectores aislados donde la erosión puede romper trozos de la capa orgánica. Consecuentemente, la superficie arenosa expuesta puede ser erosionada y se pueden formar depresiones.
Another mechanical deformation of biostabilized sediments is their levering by gas pressure, a surface deformation caused by gas accumulation in the substrate. Fig. 10b shows the results: domes and folds with a hollow centre and a typically circular or elongated base. The gas migrates from deeper buried organic deposits towards the surface and the cohesive microbial films inhibit escape of the gas into the air or water. When accumulating beneath the surface mat, gas pressure leads to doming the mat and extreme tension may cause rupturing.
Images obtained by SEM of a biofilm showed a halite crystal (Fig. 11) as found in an extremely hypersaline area of the Tunisian coast and probably induced by bacterial presence (Noffke, et al., 2003). Cornée et al. (1992) stated that halite precipitates from a solution after 90% evaporation of the initial volume of seawater.

Figure 11. Image of a halite crystal.
Figura 11. Imagen de un cristal de sal.
A variety of sedimentary structures and textural features was used by Schieber (1998) who considers them as the result of microbial colonization of sedimentary surface in shales and sandstones from the Mid-Proterozoic period. He identified at least some exceptionally well preserved specimens displaying early diagenetic silicification, where proof of organosedimentary origin was established. This would help establish sedimentological and textural criteria for mat recognition. From his point of view, to have any chance to prove the existence of organosedimentary structures in a rock, one will first have to identify localities where modern counterparts occur. Additionally, sedimentological and textural feature criteria indicating the presence of microbial mat deposits need to be established.
Among structures found in the rocks by Schieber (1998), the "irregularly wrinkled patches" was also recognized in the modern tidal flats of Puerto Rosales (Fig. 12), where transition between rippled surfaces and non rippled surrounding areas are smooth. This author stated that this transition is critical for the interpretation of ripple patches as microbial mat indicators. As long as transitions are smooth it is fair to assume that the rippled and non-rippled areas are penecontemporaneous, and on the contrary, if there is a sharp edge delineating the rippled and non rippled surface, it can be assumed that there are two different sediment layers, an upper non-rippled layer and a lower rippled layer. The ripple patch appearance is produced when weathering tears off portions of the upper layer, thus forming "windows" that reveal the lower rippled layer (Schieber, 1998). Nevertheless, this assumption does not always prove true, since figure 12 shows that the two above mentioned features, a smooth transition and a sharp edge between rippled and non-rippled areas, are simultaneously present in the same sediment layer. The detrital grains of a featureless surface are held together by a binding property that may be weakened in the ripple patches by wave action, e.g. storm surges. It is possible that locally, where the microbial mat structure is weaker, erosion can tear off parts of the organic layer and with the sandy intertidal bottom at those spots then being eroded and creating depressions as "pockets".

Figure 12. The arrow shows the rippled surface passes without break into surrounding smooth surface. Below, a sharp step limits the rippled area with the non-rippled area.
Figura 12. La flecha muestra la superficie ondulada que pasa a una superficie lisa sin interrupción. Por debajo, un agudo escalón limita el área ondulada del área no-ondulada.
Noffke et al. (2003) has detected MISS in siliciclastic deposits from the Paleo-Archean to the Pleistocene, where they found a record of extensive ancient microbial mats covering large areas of the sea floor. This study presents facies and sequence analyses conducted on data sets from sandstone successions of the Paleo- and Meso-Archean of South Africa, the Neo-Proterozoic of Namibia, and the Ordovician of the Montagne Noire, France. The features named "erosional remnants" and "pockets" by Noffke and Krumbein (1999) are also found in sandstone of Paleo-Archean age (Noffke, et al., 2006a). In her study, Noffke demonstrates the microbial origin of the sedimentary structures in sandstones throughout Earth history, making their detection and interpretation a most valuable tool for paleobiologists. The description of MISS in their studies resembles the modern wrinkle structures found in Puerto Rosales tidal flats whose bed surfaces are characterized by regular and irregular ripple patterns (Fig. 13).

Figure 13. a) Wrinkle structures present in the modern tidal flats of Puerto Rosales. b) the same feature called "old elephant skin structures" found in rocks by Noffke et al. (2006a) (with publisher permission).
Figura 13. a) Estructuras onduladas presentes en las planicies de marea actuales de Puerto Rosales. b) el mismo diseño, llamado"estructuras de piel de elefante", encontradas en rocas por Noffke et al. (2006a) (con permiso de publicación).
The link between sedimentary structures found in modern environments and similar structures found in rocks are diagenetic processes. All sediments after their deposition are affected by diagenesis and include a fundamental suite of physical, chemical and biological processes that control the texture, mineralogy and fluidflow properties of sedimentary rocks. Usually the sediments are cemented by minerals that precipitate from a solution. Understanding the processes and products of diagenesis is thus a critical component in the analysis of sedimentary basin evolution. Eogenesis involves the initial interaction of the original sedimentary assemblage from its depositional pore water. Eogenetic processes are influenced strongly by bacterial degradation of organic matter present in finer grained sediments (Kantorowicz, 1985). Under certain conditions, microbial mats induce mineral precipitation such as calcite (Krumbein, 1979), whereas heterotrophic bacteria decompose primary producers and induce in situ formation of minerals like pyrite (Giblin, 1988; Pye et al., 1990). All these results were obtained from carbonatic environments. The present study site was on the other hand located in a siliciclastic environment where other minerals, different kinds of zeolites, were found.
Some of the more common environments for zeolites to occur are saline, alkaline-lake deposits where the first zeolites formed from the dissolution of natural rhyolitic glass are chabazite and/or phillipsite (Tummer and Wirsching, 2000). Therefore, the zeolite found in the sediment of Puerto Rosales, chabazite, may be a product from the dissolution of glass present in the volcanic materials of the tidal flat sediments. These zeolites are silicates of aluminium mostly composed of sodium and calcium which crystallize in a water-rich environment under low pressure. The most important feature is that zeolite precipitation occurs during the fist stage of diagenesis: the eogenesis. Therefore, the results obtained from this study clearly demonstrate the initial process of lithification needed to form a sedimentary rock.
CONCLUSIONS
Sedimentary structures induced by microbial activity were identified for the first time in modern tidal flats of Argentina. The comparison with two other modern siliciclastic environments subjected to different climatic conditions (temperate humid and subtropical arid), reveals that the specific structures analyzed by the current study are similar to both of them. Some features are similar to those found in the arid condition as the cracks formed over the lower supratidal level where minerals such as halite and zeolites have been identified. As well, the structural topography of erosional remnants and pockets was observed in a temperate humid climate environment and under winter conditions.
Microorganisms growing over the sediment stabilize microbial mats by releasing extra cellular polymeric mucilages and adhesive slimes, which bind the sediment together. This increases the resistance of the microbial mats against erosion. An environment with considerable current and wave activity where the sediment surface is stabilized by microbial mats thus may easily be mistaken for a calm one if the mats are not identified.
The formation of authigenic minerals such as zeolite was determined as the influence of microbial action. This highlights the need for further interdisciplinary research in order to gain more insight into this particular diagenetic process. It is important to characterize the variety of geochemical and microbiological factors that can affect the composition and ability to precipitate zeolites. Finally, the petrified structures identified in rocks and their comparison with modern sedimentary counterparts from tidal systems may help with paleoenvironmental and palaeoclimatic reconstructions.
Acknowledgements
The authors gratefully acknowledge funding for their work from the PGI 24/ZH13 (Universidad Nacional del Sur) and PICT´03 N°07-14652 (ANPCyT). We greatly appreciated the help of Mr. Hugo Pellegrini in the field and for laboratory analysis and Dr Jorge Spagnuolo for the mineralogical analysis. The SEM images were taken by Viviana Sorrivas (CCT BB). Useful comments by the reviewers, Dr Thorbjørn J. Andersen and Lic. Fernando Gómez, helped to improve the manuscript.
REFERENCES
1. Cornée, A., M. Dickman and G. Busson, 1992. Laminated cyanobacterial mats in sediments of solar salt works: some sedimentological implications. Sedimentology 39:599-612. [ Links ]
2. Cuadrado, D.G. and E.A. Gómez, 2006. Acumulación en planicies de marea causada por vegetación. Resúmenes IV Congreso Latinoamericano de Sedimentología y XI Reunión Argentina de Sedimentología:80, Bariloche, Argentina. [ Links ]
3. Cuadrado, D.G., E.A. Gómez, N. Pizani y E.R. Parodi, 2006. Bioestabilización de sedimentos en planicies de marea. Resúmenes VI Jornadas Nacionales de Ciencias del Mar y XIV Coloquio de Oceanografía:39, Puerto Madryn, Argentina. [ Links ]
4. Cuadrado, D.G. and E.A. Gómez, 2007. Preservación de estructuras sedimentarias en planicies de marea. Actas XII Colacmar. 3pp. Brasil. [ Links ]
5. Decho A.W., 1990. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanographic and Marine Biology: an Annual Review 28:73-153. [ Links ]
6. Dunham, R.J., 1962. Classification of carbonate rocks according to depositional texture. Memoir American Association of Petroleum Geologists 1:108-121. [ Links ]
7. Gelós, E. and J. Spagnuolo, 1982. Estudio composicional de los sedimentos de fondo de la ria de Bahía Blanca entre Puerto Cuatreros y Puerto Ingeniero White. RAGA 37(1):3-22. [ Links ]
8. Gerdes, G., Th. Klenke and N. Noffke, 2000. Microbila signatures in peritidal siliciclastic sediments: a catalogue. Sedimentology 47:279-308. [ Links ]
9. Giblin, A., 1988. Pyrite formation in marshes during early diagenesis. Geomicrobiology Journal 6:77-97. [ Links ]
10. Kantorowicz J.D., 1985. The petrology and diagenesis of Middle Jurassic clastic sediments, Ravenscar Group, Yorkshire. Sedimentology 32:833-853. [ Links ]
11. Krumbein, W.E., 1979. Photolithotropic and chemoorganotrophic activity of bacteria and algae as related to beachrock formation and degradation (Gulf of Aqaba, Sinai). Geomicrobiology Journal 1:139-203. [ Links ]
12. Krumbein, W.E., 1983. Stromatolites. The challenge of a term in space and time. Precambrian Research 20:493-531. [ Links ]
13. Naylor, L., 2005. The contributions of biogeomorphology to the emerging fiekd of geobiology. Palaeogeography, Palaeoclimatology, Palaeoecology 219:35-51. [ Links ]
14. Noffke, N., 1999. Erosional remnants and pockets evolving from biotic-physical interactions in a recent lower supratidal environment. Sedimentary Geology 123:175-181. [ Links ]
15. Noffke, N. and W. Krumbein, 1999. A quantitative approach to sedimentary surface structures contoured by the interplay of microbial colonization and physical dynamics. Sedimentology 46:417-426. [ Links ]
16. Noffke, N., 2000. Extensive microbial mats and their influences on the erosional and depositional dynamics of a silisiclastic cold water environment (Lower Arenigian, Montagne Noire, France). Sedimentary Geology 136:207-215. [ Links ]
17. Noffke, N., G. Gerdes, T. Klenke and W. E. Krumbein, 2001. Microbially Induced Sedimentary Structures: A New Category within the Classification of Primary Sedimentary Structures. Journal of Sedimentary Research 71 (5):649-656. [ Links ]
18. Noffke, N. and A.H. Knoll, 2001. Geobiology: Its application to sedimentary geology. Pardee Keynote Symposium, Annual Meeting Geological Society of America, Boston. Geological Society of America, Boulder, Colorado. [ Links ]
19. Noffke, N., G. Gerdes and T. Klenke, 2003. Benthic cyanobacteria and their influence on the sedimentary dynamics of peritidal depositional systems (siliciclastic, evaporitic salty, and evaporitic carbonatic). Earth-Science Reviews 62:163-176. [ Links ]
20. Noffke N., 2005. Geobiology-a holistic scientific discipline. Palaeogeography, Palaeoclimatology, Palaeoecology 219:1-3. [ Links ]
21. Noffke, N., N. Beukes, J. Gutzmer and R. Hazen, 2006a. Spatial and temporal distribution of microbially induced sedimentary structures: A case study from siliciclastic storm deposits of the 2.9 Ga Witwatersrand Supergroup. South Africa. Precambrian Research 146:35-44. [ Links ]
22. Noffke, N., K. Eriksson, R. Hazen and E. Simpson, 2006b. A new window into Early Archena life: Microbial mats in Earth´s oldest siliciclastic tidal deposits (3.2 Ga Moodies Group, South Africa). Geology 34:253-256. [ Links ]
23. Paterson D.M., 1994. Microbiological mediation of sediment structure and behaviour. In Cuamette P., Stal L.J. (eds), Microbial mats. NATO ASI Series:35-97. [ Links ]
24. Piccolo, M.C. and P.G. Diez, 2004. Meteorología del Puerto Coronel Rosales. In M.C. Piccolo y Hoffmeyer M. (ed), Ecosistema del Estuario de Bahía Blanca:87-90. Bahía Blanca, Argentina. [ Links ]
25. Pye, K., A.D. Dickinson, N. Schiavon, M.L. Coleman and M. Cox, 1990. Formation of siderite-Mg-calcite-iron sulfide concretions in intertidal marsh and sandflat sediments, north Norfolk, England. Sedimentology 37:3255-343. [ Links ]
26. Schieber, J., 1998. Possible indicators of microbial mat deposits in shales and sandstones: examples from the Mid-Proterozoic Belt Supergroup, Montana, USA. Sedimentary Geology 120:105-124. [ Links ]
27. Shin, E.A., 1983. Tidal flat environments. American Association of Petroleum Geologists Memoir 33:172-210. [ Links ]
28. Stal, L.J., 2000. Cyanobacterial mats and stromatolites. In Whitton, B.A. and Potts, M. (eds), The ecology of Cyanobacteria. Netherlands: 61-120. [ Links ]
29. Trummer, B. and U. Wirsching, 2000. Formation of zeolites in saline, alkaline-lake deposits: an experimental approach. In C.Colella and F.A. Mumpton (eds.), Natural Zeolites for the Third Millenium:211-225. Italy. [ Links ]
30. Walter, M.R., 1976. (ed). Stromatolites. Elsevier, Amsterdam, 790pp. [ Links ]
31. Welton, J., 1984. SEM Petrology Atlas. American Association of Petroleum Geologists. Tulsa Oklahoma, USA, 237 pp. [ Links ]














