Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) v.73 Vicente López ene./dic. 2004
ARTÍCULOS ORIGINALES
Evaluation of almond shell as a culture substratefor ornamental plants. II. Ficus benjamina (with 3 tables & 2 figures)
Lao MT1, S Jiménez2
1Dpto. Producción Vegetal. Escuela Politécnica Superior. Universidad de Almeria. Almeria (España). Corresponding author e-mail: mtlao@ual.es
2Centro de Investigación y Formación Agrícola. Departamento de Horticultura. Apdo. 91. El Ejido. Almeria (España)
Received 26.V.2003; accepted 30.VI.2003
Abstract. Some technical and economic problems currently limit the use of substrates. The main problems include the lack of reciprocal adaptation of the cultivation technics and the substrate,the possible presence of pathogens, and the cost involved. To these we must add the ecological problems of the extraction areas, since there are no short-term renewable resources, especially in peat, the classic substrate. This has motivated the search for substitutes, especially amongst indigenous materials and those easily obtainable locally, such straws cereals, rice husk and cork residuous.
The use of these substrates should be evaluated agronomically for: physical, chemical and cultural properties. The characterisation and use of almond shell (Prunus dulcis) as a horticultural substrate substitute for growing ornamental plants were studied.
The study involved 4 almond shell and peat mixtures (20:80,40:60, 60:40 and 80:20 in almond shell and peat volume respectively), as well as those of a control mixture consisting of peat and expanded clay (33.3: 66.6 in volume of expanded clay and peat respectively). The evaluated plant was Ficus benjamina. The plants crop in mixture 20:80 present hight height, dry and fresh weight aerial and root zone and nitrogen foliar level.
Key words: Substrate, mixture, peat, expanded clay
Soilless culture can be divided into hydroponic culture and substrate culture (1). The term substrate is applied in horticulture to all solid material different fromnatural soil in situ, composed of synthesis or residual, mineral or organic substances that is placed in a container, in pure form or in a mixture, and which allows the anchorage of the root system, and is able to intervene (chemically active material) or not (inert) in the complex process of the plant's mineral nutrition(2, 3, 4).
Some technical and economic problems currently limit the use of substrates (5, 6). The main problems include the lack of reciprocal adaptation of the cultivation techniques and the substrate,the possible presence of pathogens, and the cost involved. To these we must add the ecological problems of the extraction areas, since there are no short-term renewable resources, especially of peat, the classic substrate. This has motivated the search for substitutes, especially among indigenous materials and those easily obtained locally, such as cereal's straw, rice husk and cork residual (2).
The almond tree, originally from Asia Minor has existed in the Mediterranean basin since before the birth of Christ.At the present time, Spain is the second largest producer (20.33% in the world production) after the USA (32.39%) (7).
The current uses of almond shell are for furfural production, fertilisers made from the residue of previous production,coal and fuels, xylose and xylitol, some bioproducts andmulching in gardens and growth substrates.
MATERIALS & METHODS
Five different mixtures of substrate were prepared, four of them composed of almond shell and peat, in different volumetric ratios, and one formed by expanded clay and peat:
T1: 20% shell + 80% peat; T2: 40% shell + 60% peat; T3: 60% shell + 40% peat; T4: 80% shell + 20% peat; Control: 33,3% expanded clay + 66,6% peat.
Cracked almond shells varying in size from 0.5 to 2 cm were obtained from one of the local companies, and the kernel was cleaned out. The peat used in all the mixtures was of Danish origin and its characteristics guaranteed by the manufacturing firm were: Screening: sifter of 20 mm, pH6.0 (in water), elements added by m3: nitrate-N, 63 g; ammonium-N, 11 g; P205, 121 g; K2O, 142 g; MgO, 35 g; CaO, 300g; and micronutrients, 200 g; FTE 3600 (slow liberation).
The expanded clay used had sizes between 3 and 5 mm.
Growth trial: The experimental design was unifactorial (substrate mixture) with 40 repetitions for each treatment (T1,T2,T3,T4 and control). The trialperiod in the field was from April 18/ 1997to June 27/ 1998. Plants of Ficus benjamina sp were cultivated in pots of 1.5 L in volume, in a rigid badge multitunnel greenhouse with a transmissibility of 10% and without heating.
Fertirrigation was applied, starting from May 19 with a balance of 2:1:2 N: P2O5: K2O
(1 g L-1) and micronutrients. From June 6 the balance was modified by 3:1:2 N: P2O5:K2O (1 g L-1).
The measured parameters for the plants were height (data were taken weekly) and biomass (at the end of the trial from a sample of 4 plants per treatment). The fresh weight of the aerialandroot zone were also determined.
Foliar analysis was carried out on the samples for each treatments according to standard procedures (8). Data were analysed having the analysis variance and means were separated using the least significant difference (LSD) at p<0.05.
RESULTS & DISCUSSION
Measure of the height: Table 1 shows the average values and the results of least significant difference test (LSD) at p<0.05 of the height, the figure 1 shows the height evolution, both throughout the trial.
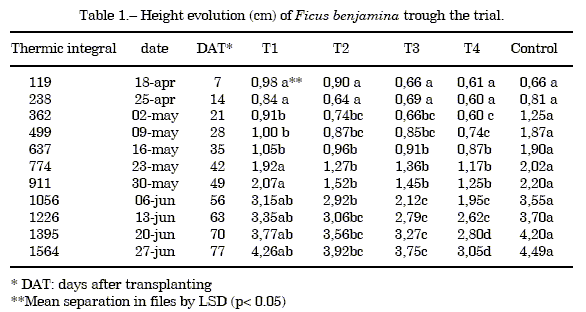

A saw tooth shaped (9) growth tendency was observed, with a precocious increase of the height for the control that generates significant differences in this parameter during 3 weeks with the T1 treatment. However, starting from this moment both treatments present a similar growth. The substrates T2, T3 and T4 produced plants of smaller height, diminishing significantly as the volumetric ratio of almond shell in the mixture increased.
Biomass study: The data for fresh and dry weights, aerial and root weights are presented in table 2, The T1 treatment was similar to the control. However, the other treatments had lower biomass. Figure 2 shows the fresh and dry weights of the aerial and the root zones.
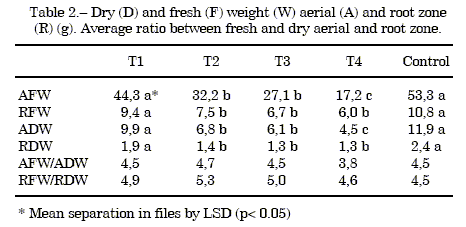

Table 2 also shows the ratio between fresh weight and dry weight, in the aerial and root zone, which lower in the aerial fraction. This could be due to water stress because of the great porosity of the substrate in treatments 3 and 4, undersimilar watering management with alltreatments.
Foliar analysis: The following elements were analysed from a representative sample of each treatment and the control: nitrogen, phosphorus, potassium, calcium and magnesium (table 3).
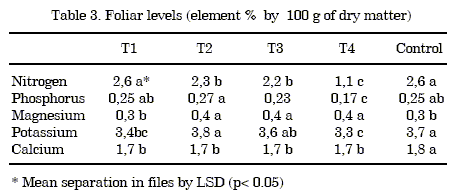
The foliar nitrogen levels in the treatment 1 and the control are within the levels (2.5-4%) considered adequate by Jiménez et al. (10). However the level decreased when the shell percentage in the mixture was increased. This result can be due to the "nitrogen hunger" generated by the substrate, and it is related to the C/N ratio, the effect of which was not corrected by basic fertilizers.
The P levels were low in all the treatments and the control compared with the sufficiency range of 0.6-1% (10). This effect may be due to the pH levels of bothtreatments andcontrol, which may have reduced availability.
The K and Mg levels in all treatments were within the recommended range for cultivation (2.5-5%) and (0.25-0.5%) as well as the levels of Ca.
CONCLUSIONS
The contribution of almond shellin the substrate was to improve its physical and chemical properties and, therefore, this can be the starting point for the design of the substrate´s composition. The Ficus benjamina crops with the mixture 20:80 is similar to the culture with the control. The shell of almond is a good substrate component to culture for ornamental plants.
REFERENCES
1.Abad M, P Noguera, In Fertirrigación: cultivos hortícolas y ornamentales; Cadahía C, Ed; Mundiprensa: Madrid (1998) 289 [ Links ]
2.Abad M, P Noguera, V Noguera, V. Turbas para semilleros. II Jorn sobre semillas y semilleros hortícolas. Congresos y Jornadas, 35/96. Jta de Andalucía, Consej Agricult Pesca: Sevilla (1996) 79 [ Links ]
3.Abad, M. In Los sustratos hortícolas: características y manejo. Actas del II Congr Nacional de Fertirrigación. Almería sep 18-20; Brader, L Eds; FIAPA Almería (1991) 1 [ Links ]
4.Abad M, PF Martínez, Sustratos Hortícolas y cultivos sin suelo. Horticultura 11 (1995) 32 [ Links ]
5.Abad M, Sustratos para el cultivo sin suelo: Inventario y Características. In cultivos sin suelo; Canovas F,Díaz J, Eds FIAPA: Almería, Spain (1993) 47 [ Links ]
6.Martínez PF, Acta Horticulturae 323 (1992) 129 [ Links ]
7.Baudoin WO, GW Winsor, M Schwarz, Soiless culture for horticultural crop production, FAO Plant Production & Protection Paper. 20101. FAO: Rome, 1990 [ Links ]
8.Métodos Oficiales de Análisis, Secret Gen Técn Ed. Minist Agricultura Pesca Aliment: Madrid, Tomo II (1986) [ Links ]
9.Baille M, Actas de horticultura S.E.C.H. 2º Jornadas de sustratos. Valencia. (1994) 1 [ Links ]
10.Jiménez R, M Caballero, In El cultivo industrial de plantas en maceta. Ed Hortic Reus, Spain (1990) [ Links ]














