Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) v.73 Vicente López ene./dic. 2004
ARTÍCULOS ORIGINALES
Agrobacterium-mediated transformation of white poplar (Populus alba L.) (with 3 figures & 1 table)
Sánchez N1, JA Manzanera2, MA Bueno1*
1Instituto Nacional de Investigación y Tecnología Agraria y Alimentaría (INIA), Carretera de La Coruña, Km 7, 28040 Madrid, Spain. Tel.: 34-913476863, Fax: 34-913572293.
2Universidad Politecnica de Madrid, E.T.S.I. Montes-Ciudad Universitaria s.n. 28040 Madrid, Spain
* corresponding author, E-mail: bueno@inia.es
Received 27.V.2003: accepted 30.VI.2003
This work has been supported by the Spanish Institute of Agronomic Research (INIA), project SC 989-08. Sanchez was recipient of a INIA post-doctoral scholarship.
Abstract. A simple method is presented for the genetic transformation of white poplar (Populus alba L.). Root suckers and buds from branches of adult trees were established in vitro. Callus was induced on injured leaves cultured on medium supplemented with 0.45 µM thidiazuron and 0.1 µM a-naphthalene acetic acid. Non-transformed leaves and calli did not survive concentrations higher than 85 µM kanamycin or 18 mM hygromycin. The leaf explants were inoculated with the disarmed Agrobacterium tumefaciens LBA4404/p35S GUS INT/pCAMBIA 2301 strain. Twenty % of the cultured callus explants was kanamycin-resistant two months after the co-inoculation experiment. The rate of adventitious bud induction on resistant calli was 17 %. The analysis of b-glucuronidase expression two months after the co-cultivation experiment and the DNA amplification of a 2500 bp band with 35S sense primer and NOS antisense primer confirmed the transformation of 100 % kanamycin-resistant calli and 4 % adventitious shoots. This method forms a basis for genetic transformation of white poplar.
Abbreviations: BAP, benzil aminopurine; TDZ, Thidiazuron; IBA, indole butyric acid; NAA, naphthalene acetic acid; KAN, kanamycin
Genetic transformation of trees has been developed in the genus Populus (5, 7). In the case of white poplar (P. alba L.), this goal has been approached in a few interspecific hybrids and cultivars [Confalonieri et al (2), Delledonne et al (3)]. White poplar is a fast growing species, and high wood producer has many other applications, such as riverbank protection in semi-arid and arid climates. The development of a transformation technique for the introduction of genes conferring resistance against high salt concentrations and other extreme soil conditions would be desirable for this species.
The present work is focused on the introduction of marker genes by Agrobacterium tumefaciens-mediated transformation of white poplar as an easy and efficient technique for the introduction of the desired genes. Preparatory experiments have been conducted: the use of plant growth regulators for the control of plantlet regeneration from callus and the selection of virulent Agrobacterium strains, as well as the determination of the threshold concentration of antibiotics for the survival of untransformed white poplar callus and plantlets. Once these conditions were fulfilled, a reporter gene transfer was performed.
MATERIAL & METHODS
Root suckers from white poplar clone TO were collected in March and April. Shoots from clones M16, AL30 and MN15 of the tissue culture germplasm collection of the Spanish Institute of Agronomic Research (INIA) were also collected for explant preparation. Root suckers and shoots were collected from adult trees and sterilised in ethanol 70 % for 30 s and in 1.2 % NaOCl with a few drops of Tween 20 for 15 min followed by three rinses in distilled sterile water, 10 min each. Leaves of the shoots were discarded before sterilisation and the shoots were cut in pieces and established in culture medium.
Two macronutrient formulas were used: Murashige & Skoog (MS, 1962) [11] and Woody Plant Medium (WPM; Lloyd & McCown, 1980). The culture medium contained the micronutrients of MS, 90 mM sucrose, 8 g L-1 agar (PanreacÒ) and plant growth regulators, BAP, TDZ, IBA and NAA. For in vitro establishment of the shoot pieces from root suckers, 0.4 µM BAP and 0.01 µM NAA were used. The medium was adjusted to pH 5.6 and sterilised in autoclave at one atmosphere (115 ºC) for 20 min. Then the medium was dispensed into sterile tubes or petri dishes.
Leaves from in vitro clones TO, M16 and MN15 were injured by cutting the epidermis with a blade and cultured on MS or WPM supplemented with TDZ at various concentrations: 0.45, 2 or 4.5 µM and 0.01 µM NAA for callus induction. One hundred and four leaf explants were cultured per treatment by establishing eight petri dishes with 13 explants per dish in each treatment. One month later, the explants were subcultured on fresh medium supplemented with 0.4 µM BAP and 0.01 µM NAA for bud induction. Bud proliferation was stimulated in MS medium supplemented with 0.4 µM BAP and 0.1 µM NAA. Shoot elongation was induced on MS medium with 0.1 µM BAP and 0.1 µM NAA. Elongated shoots were rooted in WPM with 1 mM IBA [Bueno et al. (1)].
To test the effect of antibiotics on the survival and growth of white poplar cultures, leaves from clones TO and MN15 were injured with a blade and cultured on MS with 0.45 µM TDZ and 0.1 µM NAA and different antibiotic treatments: 40, 85, 170, 340 and 850 µM kanamycin (KAN), or 4, 9, 13, 18, 28, 47, 94, and 188 µM hygromycin (HYG). Sixteen explantswere cultured by establishing four petri dishes with four explants per dish in each treatment.
Three Agrobacterium tumefaciens wild type strains, A281, Ach5 and C58, and two disarmed strains, EHA105/p35S GUS INT and pMp90/p35S GUS INT were kindly provided by Dr. L. Peña from the Valencian Institute of Agronomic Research (IVIA, Valencia, Spain). The disarmed strain EHA105/p35S GUS INT derived from the wild strain A281 and the pMp90/p35S GUS INT derived from C58. The disarmed strain LBA4404/p35S GUS INT/pCAMBIA 2301, which derives from the Ach5 wild type strain was kindly provided by Dr. JM Martinez-Zapater (INIA, Madrid, Spain).
Inoculum was prepared by growing the bacteria for two days on agar-solidified Luria Bertani (LB) medium plus 85 µM KAN and 60 µM rifampicine at 28 ºC in darkness. A sterile stick was placed in the petri dish with bacteria and dipped into a test tube containing 15 ml LB medium plus 85 mM KAN and 60 mM rifampicine. The test tube was gently shaken overnight at 28 ºC and diluted with LB at an O.D. reading of 0.25 at 600 nm. Twenty ml bacterial suspension was dispensed in a sterile petri dish for co-cultivation.
A previous evaluation of the efficiency of the A. tumefaciens strains in the plant tissue infection was performed. The bacterial strains were inoculated twice with a 48 h interval in control plants grown in the greenhouse, in the five internodes closest to the apex, with an A. tumefaciens wild type strain grown in petri dishes. Two months later, the virulence was estimated by the size of the tumour induced.
For the transformation with A. tumefaciens, the protocol of Tzifira et al. (13) was followed. One hundred and four leaves of clone TO were co-cultured with A. tumefaciens disarmed strain LBA4404 pcambia 2301, which contains genes nptII, conferring kan resistance, andgusA gene coding for b-glucuronidase (GUS, Roberts et al.) [12].
The A. tumefaciens cells were eliminated after co-cultivation by the addition of a solution of 628 µM cefotaxime (Duchefa), followed by three rinses in distilled sterile water, and transfer to MS medium with 0.45 µM TDZ, 0.01 mM NAA, 628 µM cefotaxime and 85 µM KAN. Fifteen control explants were transferred to the same induction medium without antibiotics (positive control) and 15 control explants were transferred to the induction medium with628 µM cefotaxime and 85 µM KAN (negative controls). One month later, the explants were transferred to fresh medium with 0.4 µM BAP and 0.01 µM NAA.
GUS expression was histochemically evaluated in 55 putatively transformed explants by the b-glucuronidase activity test, using 5-bromo-4-chloro-3-indolyl-b-D-glucuronide (X-GlcA) sodium salt (Duchefa) as the substrate (Jefferson et al. 1987).
DNA of the putatively transformed tissues was extracted according to the protocol of Doyle and Doyle (1990). Also, DNA of the A. tumefaciens wild strains and tissue cultured white poplar shoots were analysed as negative controls, and DNA of the disarmed A. tumefaciens pCAMBIA 2301 strain was analysed as positive control. Primers 35S-1, 35S-2, NOS-1 and NOS-2 were used (Lipp et al. 1999). The amplification reaction was performed in a 25 µl volume containing 67 mM Tris-ClH pH 8.8, 16.6 mM (NH4)2SO4, 0.1% Tween-20, 3 mM MgCl2, 0.25 mM dNTPs, 0.3 mM both sense and antisense primers (Progenetic S.L., Pacisa Giralt), 0.5 units. Taq DNA-polymerase (Ecogen S.R.L.) and 10 to 50 ng/mL DNA. Amplification in a Perkin Elmer 9600 termocycler was programmed with a previous denaturation at 95 ºC for 3 min, and 40 cyclesof 36 s at 95 ºC, 72 s at 54 ºC and 84 s at 72 ºC. The final extension lasted 10 min at 72 ºC. Amplified fragments were separated in a 3% agarose (SeaKem, FMC BioProducts) electrophoresis gel, with Tris-Acetic acid-EDTA buffer, at 80 v for 2 h. A ladder of l PstI digest was used as size marker. Bands were visualised in a UV transilluminator by staining with 1 mg L-1ethidium bromide. The analysis was repeated twice.
For statistical calculations, a log-linear model was fitted to all experiments, using a Chi-square test. Asymptotic standard errors of the parameter estimates were computed by the Delta method (Lee, 1977). The ratio of the log-linear parameter estimate to its standard error was used to obtain the frequency significance level.
RESULTS & DISCUSSION
Clone MN15 showed good growth and healthy aspect in both MS and WPM media. Calli appeared on leaf explants one month after the beginning of the induction treatment. Clone TO grew better in MS, with green and healthy leaves, while in WPM, symptoms of chlorosis and poor growth were obvious. Clones M16 and AL30 had a poor and chlorotic growth in both media and were discarded for further experiments. Adventitious buds appeared on the calli after one and a half month in culture. Significant differences were observed between clones, MN15 producing buds more frequently than clone TO (Fig. 1). Also, the effect of TDZ concentration was statistically significant on adventitious bud induction, the highest rate being obtained with 0.45 mM TDZ in both TO and MN15 clones (Fig. 1). Similar results were obtained in both MS and WPM media.

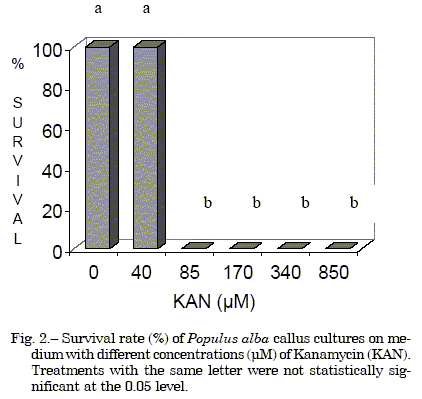
Both antibiotics assayed were lethal at high concentrations. Injured leaf explants did not produce callus or survive on medium with KAN at concentrations higher than 85 µM (Fig. 2) nor in medium with HYG at concentrations higher than 18 µM (Fig. 3).

Bacterial strain A281 was not pathogenic for white poplar plants. Therefore the respective disarmed strain was not used in further transformation experiments. Strains Ach5 and C58 showed pathogenicity with a moderate virulence. The disarmed strain LBA4404 pCAMBIA 2301, which derives from the Ach5 wild type strain, was used for the transformation of white poplar leaves. After co-cultivation, the bacteria were eliminated by subculturing on callus induction medium supplemented with628 µM cefotaxime and 85 µM KAN. A 20% of 55 callus explants was resistant two months after the co-cultivation experiment. The rate of adventitious bud induction on these kan-resistant calli was 17%.
The amplification of DNA from leaf and callus explants co-cultivated with A. tumefaciens produced bands of 189 bp for NOS terminator primers, 195 bp for 35S promoter primers, and of 2500 bp for 35S sense primer and NOS antisense primer (Table 1). The use of 35S promoter primers did not allow the detection of transgenic explants, as the negative controls showed a 195 bp amplification band. The use of NOS terminator primers produced amplification of DNA samples from co-cultivated leaves that did not form resistant calli and from brown non-resistant calli co-cultivated with the bacteria probably due to the presence of A. tumefaciens DNA traces. The amplification of DNA of putatively transformed callus with 35S sense primer and NOS antisense primer confirmed this hypothesis, while no amplification was observed in the negative controls.

The analysis of GUS expression two months after the co-cultivation experiment confirmed that 100% kan-resistant calli were GUS positive, and that 4% adventitious shoots induced after co-cultivation with A. tumefaciens disarmed strain LBA4404 pCAMBIA 2301 were GUS positive.
These results show a simple method for checking the genetic transformation of white poplar.
1.Bueno MA, R Astorga, JA Manzanera, Micropropagación de Populus alba "Siberiaextremeña" a partir de amentos. Investigación Agraria Sist Recur For 1(1992) 163 [ Links ]
2.Confalonieri M, M Belenghi, A Balestrazzi, S Negri, G Facciotto, G Schenone, M Delledonne, Plant Cell Rep 19 (2000) 978 [ Links ]
3.Delledonne M, G Allegro, B Belenghi, A Balestrazzi, F Picco, A Levine, S Zelasco, P Calligari, M Confalonieri, Molecular Breeding 7 (2001) 35 [ Links ]
4.Doyle J, J Doyle, Focus 12 (1990) 13 [ Links ]
5.Fillatti JJ, J Sellmer, B McCown, B Haissig, L Comai, Mol Gen Genet 206 (1987) 192 [ Links ]
6.Jefferson RA, TA Kavanaugh, MW Bevan, EMBO J6 (1987) 559 [ Links ]
7.Kim MS, NB Klopfenstein, YW Chun, Agrobacterium-mediated transformation of Populus species. pp. 51-59. In Micropropagation, genetic engineering, and molecular biology of Populus. Fort Collins, Colo. (USA). U.S.D.A. Forest. Service Gen. Tech. Rep. RM-GTR. [1997]. [ Links ]
8.Lee SK, On the asymptotic variances of u-terms in log-linear models of multidimensional contingency tables. J Am Statist Assoc 72 (1977) 412 [ Links ]
9.Lipp M, P Brodmann, K Pietsch, J Pauwels, E Auklam,. IUPAC Collaborative Trial Study of a Method to Detect Genetically Modified Soy Beans and Maize in Dried Powder. Journal of AOAC International 82 (1999) 923 [ Links ]
10.Lloyd G, BH McCown, Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by shoot-tip culture. Comb Proc Int Plant Prop Soc 30 (1981) 421 [ Links ]
11.Murashige T, F Skoog, Physiol Plant 15 (1962) 473 [ Links ]
12.Roberts CS, S Rajagopal, W Yang, S Nugroho, L Smiyh, T Nguyen, KS Ravi, L Dransfield, R Harcourt, K Vijayachandra, V Patell, C Sallaud, N Desamero, I Slamet, P Keese, A Kilian, RA Jefferson, A comprehensive new set modular vectors to allow both routine and advanced manipulations and efficient transformation of rice by Agrobacterium and direct gene-transfer methods at the Rockeffeller Foundation Meeting of the International Program of Rice Biotechnology 15-19 Sep. 1997, Malacca, Malaysia. [ Links ]
13.Tzfira T, CS Jensen, W Wang, A Zuker, B Vinocur, A Altman, A Vainstein, Transgenic Populus tremula: a step-by-step protocol for its Agrobacterium-mediated transformation. Plant Molecular Biology Reporter (USA). 15 (1997) 219 [ Links ]














