Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) v.73 Vicente López ene./dic. 2004
ARTÍCULOS ORIGINALES
Similitude Pattern and Botanical Origin of the Chilean Propolis [with 2 figures & 4 tables]
Montenegro G1, AM Mujica1, RC Peña1, M Gómez1, I Serey2, BN Timmermann3
1Correspondent author: Departamento de Ciencias Vegetales, Pontificia Universidad Católica de Chile, P.O. Box 306 Santiago-22, Chile (Prof. Gloria Montenegro). Email gmonten@puc.cl
2 Depto. Ecología, Facultad de Ciencias, Universidad de Chile, Las Palmeras 3425, Santiago.
3College of Pharmacy, University of Arizona, Tucson USA.
Received 06.V.2003: accepted 18.VI.2003
The authors thank financial supports from FIA C01-1-G-002 (To Gloria Montenegro) and to NIH-NSF 2UO1 00316-08 NIH (To Bárbara N. Timmermann).
Abstract. Correspondence analysis allows identifying various kinds of propolis due to botanical source. The first site, Colliguay is identified by a higher content of pollen of Escallonia pulverulenta, and Nothofagus dombeyi. Two other very close sites are La Vacada and Quilaco, where pollen of Mentha pulegium, besides Echium, Cichorium, and Luma are frequently identified. Other groups including various contents of pollen of Eucalyptus, Salix or Prunus and other native resources such as Peumus, Cryptocarya and Quillaja are not well defined.
Key words: Propolis, botanical source, Mediterranean type climate, and analysis of correspondence
Propolis is a resinous compound, processed by the honey bee (Apis mellifera) from plant exudates, mixed with salivary substances to avoid bacterial contamination, and that is used in sealing and protecting the beehive. The chemical composition of the propolis is complex and includes aldehydes, acid and aliphatic esters, amino acids and aromatic esters, flavanones, ketones and glycosides, among others (1, 3, 6). Those compounds can confer biological properties to the propolis that have not been understood completely. Cinnamic acid, crisine and cinnamic acid ethyl ester, are feature components of the propolis. Inhibitory properties against bacteria that cause dental decays have been reported (2, 4, 14). The clinical effectiveness of an ointment from propolis, in genital herpes, was superior to the commercial standard (aciclovir).
World propolis production is increasing substantially. Major producers include China, Brazil, US, Australia and Uruguay. At least 38 flavonoids have been found in propolis and Brazilian propolis has some of the highest amounts of these essential compounds, including galangin, kaempferol, quercetin, pinocembrin, pinostrobin, and pinobanksin (1).
Chile can become an important producer of propolis and other beehive products, but it needs to fulfill the international standards of quality. Date of collection and identification of beehive are the only standards proposed. Notwithstanding, the complex chemical composition of propolis, many compounds screened in secured samples, i.e. whose beehive is well identified; are susceptible to be traced to source plants. For example, viscidone found in propolis of Cuncumen putatively has been traced to the same compound in Baccharis, a genus found in the surrounding of beehive (11). Flavonoids are difficult to be used with the same purpose, considering its ubiquitous appearance in plants. In the same vein Bankova et al (1), stated that the knowledge of plant sources of propolis is not only of academic interest. It could be useful as a basis for chemical standardization of propolis. It could be easily characterized using its plant source, which might be established by simple chromatographic methods. In addition, knowledge of propolis plant sources is important for beekeepers to be sure that they have the proper plants in their flight range. It is known that colonies suffer when they cannot obtain propolis, bees are even said to use "propolis substitutes" like paints, asphalt and mineral oil which could severely threaten uses of propolis (5).
MATERIAL & METHODS
Propolis material from 14 sites of the Mediterranean region of Central Chile was treated as previously indicated (7). The presence of morphologic rests is analyzed (especially pollen grains) analyzed by means of photon microscopy, documents the findings photographically. Besides samples are conserved in microscope slides by the Prof. Gloria Montenegro in the palinoteque of the Department of Plant Sciences. The samples were collected scraping the marks of the honeycombs to remove the propolis stuck to them, or placing plastic traps with cracks on the marks. The beehives from which the samples have been taken are located between regions V and VIII. Santa Amalia (33º31'S -71º08'W), Cuncumén (33º40' S -71º30'W), Quillota (32º50'S-71º10'W) Sta. Cruz (34º24'S-71º28'W) and, Tanguao (35º30'S-72º05'W) are in slopes of the coastal Mountain range, whereas the others (Loncolemu (33º30'S and 71º58'W), Corintos (35º25'S -71º40'W), San Carlos (36º30'S -71º58'W) and, Salto de Agua (32º50'S -71º10'W) the piedmont and ravines of the Mountain range of the Andes are located on. Colliguay, an off road location (33º12' S 71º05' W). Relatedness was determined by using correspondence analysis aided by Statistica package version 6 Stat Soft®.
RESULTS
The vegetation of the Mediterranean type climate consists of Nothofagus communities ("robledales") or forests of litre and boldo, and lower vegetation sometimes with Baccharis or Rhamnaceae. In all the sites there appear eucalyptuses or Chilean willow (9), that are the sources most visited by the honeybee for the preparation of propolis. The beehives are located between regions V and VIII. All beehives have eucalyptus pollen grains; thus this indicator does not allow to distinguish subtle differences among three types of propolis considering the pattern of pollen types.
Correspondence analysis allows to identify various kinds of propolis according to botanical source. First, Colliguay site is identified by a higher content of pollen of Escallonia pulverulenta, and Nothofagus dombeyi. Two other very close are La Vacada and Quilaco, where pollen of Mentha pulegium, besides Echium, Cichorium, and Luma are frequently identified. Other groups including various contents of pollen of Eucalyptus, Salix or Prunus and other native resources such as Peumus, Cryptocarya and Quillaja are not well defined.
Figure 1 shows species arrangement with three clearly delimited clusters: Escallonia pulverulenta,Nothofagus dombeyi, Echium, Cichorium, Luma, Mentha pulegium. Side by side Eucalyptus and Salix are near the centroid. Figure 2 depicts the fourteen sites clearly delimiting two groups of propolis and a heterogeneous linking the rest of accessions.
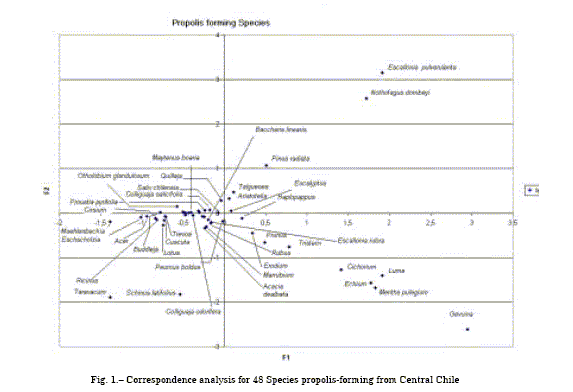
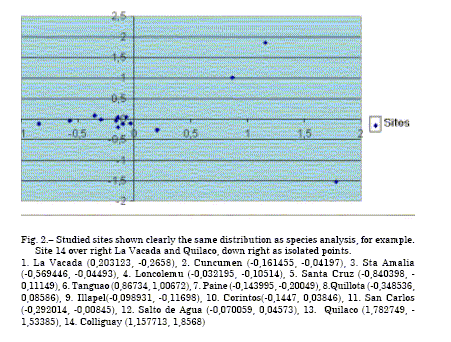
Table 1 shown matrix for frequency of pollen grain of the propolis-forming species. Table 2 Major inertia contributors are Escallonia pulverulenta and Luma, representatives of Colliguay and La Vacada sites. Other species with minor contribution are Populus (seven sites), Buddleja (three sites), Galega (Cuncumen and Santa Amalia), Gevuina (Quilaco) and Otholobium from Quillota.
Table 1. Results of frequency of pollen grain in fourteen studied hives
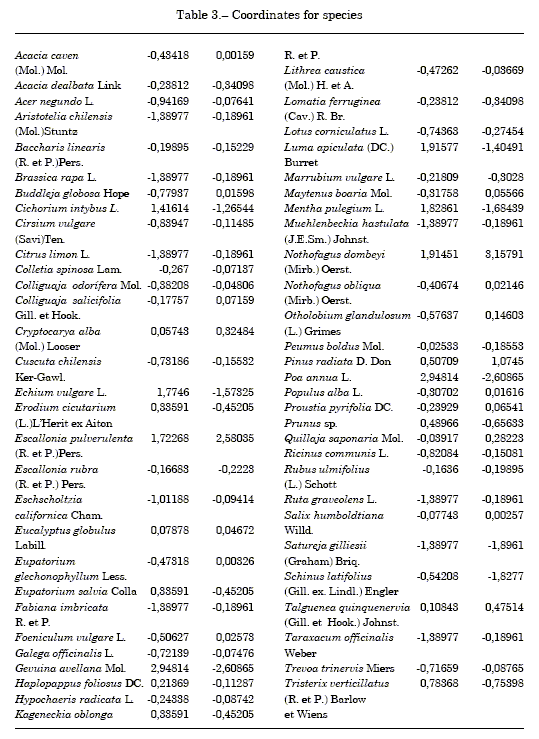
Table 3 shows orthogonal coordinates for studied species, same as shown in figure 1. And, Singular and eigenvalues, and significance c2 are shown in table 4.

1.Bankova VS, S de Castro, MC Marcucci, Apidologie 31 (2000) 3 [ Links ]
2.Bretz WA, DJ Chiego Jr, MC Marcucci. Z Naturforsch 53c (1998) 1045 [ Links ]
3.Burdock GA, Food and Chem Tox 36 (1998) 347 [ Links ]
4.Ikeno T, C Miyazawa, Caries Res 25 (1991) 345 [ Links ]
5.König B, Bee World 66 (1985) 136 [ Links ]
6.Marcucci MC, Apidologie 26 (1995)83 [ Links ]
7.Montenegro G, BN Timmermann, RC Peña, AM Mujica, G Avila, FUTON 66 (2000) 15 [ Links ]
8.Montenegro G, RC Peña, G Avila, BN Timmermann, Bol Bot Univ São Paulo 19 (2001) 1 [ Links ]
9.Montenegro G, RC Peña, AM Mújica, R Pizarro, FUTON 2001 (2001) 191 [ Links ]
10.Muñoz O, RC Peña, E Ureta, G Montenegro, Z Naturforsch 56c (2000) 269 [ Links ]
11.Muñoz O, RC Peña, E Ureta, G Montenegro, C Caldwell, BN Timmermann, Z Naturforsch 56c (2000) 273 [ Links ]
12.Valcic S, G Montenegro, BN Timmermann, J Nat Prod 61 (1998) 771 [ Links ]
13.Valcic S, G Montenegro, AM Mújica, G Avila, S Franzblau, MP Singh, W Maiese, BN Timmermann, Z Naturforsch 54c (1999) 406 [ Links ]
14.Vinograd N, I Vinograd, Z Sosonowski, Phytomedicine 7 (2000) 1 [ Links ]














