Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Phyton (Buenos Aires)
On-line version ISSN 1851-5657
Phyton (B. Aires) vol.73 Vicente López Jan./Dec. 2004
ARTÍCULOS ORIGINALES
Botanical study, phytochemistry and antimicrobial activity of Tabebuia aurea (with 1 table & 1 figure)
Barbosa-Filho José Maria1, Cláudia Sampaio A Lima2, Elba Lúcia Camorim3, Kêsia Xisto FR de Sena4, Jackson Roberto GS Almeida1, Emídio Vasconcelos L da-Cunha1, 5, Marcelo S Silva1, Maria de Fátima Agra1, Raimundo Braz-Filho6
1Universidade Federal da Paraíba, Laboratório de Tecnologia Farmacêutica, 58051-970, João Pessoa, PB, Brazil; E-mail: jbarbosa@ltf.ufpb.br
2Universidade Federal de Pernambuco, Departamento de Biofísica e Radiobiologia, 50740-521, Recife, PE, Brazil
3Universidade Federal de Pernambuco, Departamento de Ciências Farmacêuticas, 50740-521, Recife, PE, Brazil
4Universidade Federal de Pernambuco, Departamento de Antibióticos, 50740-521, Recife, PE, Brazil
5Universidade Estadual da Paraíba, Departamento de Farmácia e Biologia, 58000-100, Campina Grande, PB, Brazil
6Universidade Estadual do Norte Fluminense, Setor de Química de Produtos Naturais, 28015-620, Campos, RJ, Brasil
The authors are grateful to CAPES, CNPq, IMSEAR and FAPERJ (Brazil) for grants and fellowships and express their thanks to the College of Pharmacy of the University of Illinois, Chicago, USA, for granting access to the NAPRALERT database.
Received 30.V:2003; accepted 23.VI.2003
Abstract. We report morphodiagnosis, and phytochemical and antimicrobial investigations of Tabebuia aurea. It was noted that the leaves are compostas with anomocytic stomata only on the lower side; the vascular system is concentric andthe mesophyll arrangement is dorsiventral. From the stem bark eight compounds were isolated, including: lapachol, 3,4´,5-trihydroxy-7-methoxyflavone, b-sitosterol, betulinic acid, p-anisic acid, veratric acid, methyl cinnamate and ethyl p-hydroxy-cinnamate. Antimicrobial assays showed growth inhibition the of Gram positive and Gram negative bacteria, alcohol-acid bacterium and fungi.
Key words:Tabebuia aurea; Bignoniaceae, Pharmacobotany, Lapachol, Chemical constituents, Antimicrobial activity
Tabebuia, established by De Candolle in 1838, is one of the largest genera of the Bignoniaceae, with more than 100 species typical of tropical and subtropical areas. Its timber is used in naval and building construction, mainly because of the hardness and resistance of its wood (15). Several plants of this genus are found in Central and South America and are generally used in traditional medicine as astringents and against skin diseases (13). Many of their chemical constituents have had their pharmacological properties proven, e.g. lapacho discovered in the last century and used in clinical studies (17) as adjuvant in cancer therapy (14, 19).
Tabebuia aurea (Manso) S. Moore (sin. Bignonia aurea Manso, Tabebuia argentea Bureau & K.Schum., Tabebuia caraiba (Mart.) Bureau, Tecoma argentea Bureau & K.Schum. and Tecoma caraiba Mart.) is restricted to South America with wide distribution ranging from Venezuela to Argentina. In Northeastern Brazil it is known by the trivial names "craibeira", "paratudo" and "ipê-amarelo" and is used in folk medicine as anti-inflammatory and against influenza (1). According to Bandoni et al., (2) the stem bark is used against cancer. Previous phytochemical studies with this species reported the isolation of flavonoids (18, 9) and terpenoids (9). The present paper reports the morphodiagnosis, the chemical investigation and anti microbial activity of Tabebuia aurea (Manso) S. Moore, a very common tree in the State of Paraíba, Brazil.
MATERIALS & METHODS
Plant.Tabebuia aurea (Manso) S. Moore was collected near the city of São João do Cariri, State of Paraíba, Brazil in March 2002. A voucher specimen, Agra 2337, is deposited at the Herbarium Prof. Lauro Pires Xavier (JPB), Universidade Federal da Paraíba. Part of the collected plant material was fixed in 70º ethanol for anatomical studies. The taxonomical identifications were carried out by M. F. Agra supported by analytical keys, diagnosis and descriptions found in specialized literature (4, 16, 6, 7). The macroscopical and microscopical morphodiagnosis were based on Agra (1) and Cabral and Agra (5).
Extraction and isolation. Air dried ground bark of T. aurea (5 kg) was exhaustively extracted with 95% ethanol. The solvent was evaporated to yield a dark syrup residue (167 g, 3.3%), which was partitioned with water and successively treated with hexane, chloroform, ethyl acetate and n-butanol yielding 8.5 g, 0,2%, 4.1 g, 0,08%, 6.2 g, 0.13% and 74 g (1.5%) respectively. The hexane residue was fractionated by column chromatography over Merck silica gel 70-230 mesh, yielding compound 1 (0.525 g, 0.011%). The chloroform residue was also subjected to column chromatography over silica gel, and eluted with chloroform-hexane gradient. 75 fractions of 100 ml were collected. After analysis by TLC silica gel-60 F254 developed with either I2 or Neu/Peg reagent, some fractions were combined yielding 27 fractions. The compounds were obtained in the following order: fraction 3 afforded compound 2 (0.065 g, 0.0013%), compound 3 (0,032 g, 0.0006%) was obtained as orange needles from fractions 9-11 after recrystallization in methanol-ether, fractions 26-28 afforded compound 4 (0.086 g, 0.0017%), combined fractions 41-50 were rechro-matographed on CC column silica gel (chloroform-methanol gradient) to afford 5 (0,015 g, 0.0003%) and 6(0.126 mg, 0.0025%). The AcOEt residue was fractionated on a silica gel column. Elution was started with CHCl3, and then continued with a mixture of CHCl3-MeOH containing increasing proportions of MeOH (up to 10%). Twenty fractions of 100 ml were collected. Compounds 7 (0.095 g, 0.0019%) and 8 (0.009 g, 0.0002%) were purified from fraction 9 by a combined use of CC and PTLC on silica gel.
Microorganisms used. A modified diffusion test (3) was used to determine the antimicrobial activity. The compounds were dissolved in DMSO and tested against Bacillus subtilis DAUFPE 16, Candida albicans DAUFPE 1007, Enterococcus faecalis DAUFPE 138, Escherichia coli DAUFPE 224, Micrococcus luteus DAUFPE 06, Monilia sitophila DAUFPE 2083, Mycobacterium smegmatis DAUFPE 71, Serratia marcensis DAUFPE 398 and Staphylococcus aureus DAUFPE 01. All these microorganisms were obtained from the Culture Collection of the Departamento de Antibióticos of the Universidade Federal de Pernambuco (10). From fresh cultures of microorganisms, standardized suspensions were prepared in physiological solutions for comparison with 0.5 of the MacFarland scale, equivalent to 107 UFC (11, 20). Antimicrobial activities were evaluated by the diffusion test on paper discs over Müller Hinton agar, glucose-yeast extract agar and Sabouraud-dextrose media. The suspensions were spread on the surface of the medium in Petri dishes with Drigalsky's loop (100 µL per dish). Whatman nº 2 paper Discs (6,0 mm diameter) were moistened with 20 µL of the compounds at the concentration of 30 mg/mL, which is equivalent to 600 µg/disc. After incubation at 30 or 35º C for 24 or 48 h, the inhibition zones around the discs were measured (3). The tests were performed in triplicate and the results were expressed in mm as the arithmetic mean of diameters of the inhibition zones. The blank control was performed with DMSO. Standard solutions of kanamicin and ketoconazol were used as positive controls for bacteria and fungi, respectively.
RESULTS & ANALYSIS
Botanical description. Tree 10-20 m high; branches subteretes to subtetragonous, glabrous to lepidote in young plants and rugose in old plants. Opposite and composed leaves; 5-leaflets digitate; petiole 2.5-8 cm long; petiolules 0.2-3.5 cm long; canaleted, lepidote; blade 2.0-21 cm long and 0.4-2.5 cm wide, oblong-lanceolate, entire, leathery and smooth; rounded to subcordate and asymmetric at the base; acute, rounded to obtuse at the apex; both surfaces lepidote with peltate trichomes. Inflorescences in dense terminal panicles. Flowers pentamers, hermaphrodite; pedicel terete, 1.0-1.5 cm long, glabrous. Calyx 1.0-1.7 cm long, campanulate; 2-3-sepals free on 1/3 apical, irregularly labiated, and lepidote. Corolla tubular-campanulate, yellow; tube 0.5-8.0 cm long, pubescent at the insertion of the stamens with simple trichomes. Androecia with4-stamens inserted, didinamous; anthers divaricated, 0.3-0.4 cm long. Ovary 0.2-0.3 cm long, linear-oblong, bilocular, pluriovular; ovules bisseriate in each locule; hypogenous disc, ca 1.0 mm, densely lepidote. Capsula linear oblong, subpyramidata, 20-35 cm long and 1.5-2.0 cm wide, polyspermic; epicarp lepidote, greywish. Seeds flattened, 1.0-1.5 cm long, wings fine with hyaline margin, body ovoid, differentiated from wings, embryo sub-reniform and compressed.
Anatomy. The leaflets are hypostomatyc with anomocytic stomata.The epidermal walls of the adaxial and abaxial surfaces are straight with a thick cuticle with peltate trichomes present on both surfaces. The mesophyll is dorsiventral with the palisade tissue regularly stratified. The petiole is glabrous and canalated with concentric vascular structure constituted by 10 vascular nodes fused into an arc. The epidermis of the corolla at the insertion point of the stamens is pilose with simple unbranched, pluricellular, unisseriate trichomes.
Phytochemical analysis. A sample of each compound isolated was submitted to NMR spectroscopic analysis, using a 200 MHz Varian model Mercury NMR spectrometer. The results are the following:
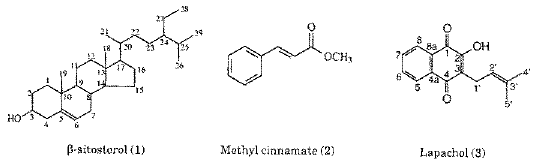
b-Sitosterol (1). 1H NMR (200 MHz, CDCl3): 5.32 (1H, m, H-6), 3.49 (1H, m, H-3), 2.24 (2H, m, H-4), 2.20-0.9 (40 H, m); 13C NMR (50 MHz, CDCl3): 140.73 (C-5), 121.72, 71.79 (C-3), 56.75 (C-14), 50.11 (C-9), 56.04 (C-17), 45.81 (C-24), 42.30 (C-13), 42.25 (C-4), 39.76 (C-12), 37.23 (C-1), 36.48 (C-10), 36.13 (C-20), 33.92 (C-22), 31.90 (C-7), 31.88 (C-8), 31.62 (C-2), 29.13 (C-25), 28.23 (C-16), 26.06 (C-23), 24.29 (C-15), 23.05 (C-27), 21.06 (C-11), 19.80 (C-29), 19.37 (C-19), 19.02 (C-26), 18.76 (C-21), 11.96 (C-28), 11.84 (C-18).
Methyl cinnamate (2): 1H NMR (200 MHz, CDCl3): 3.78 (3H, s, H-10); 6.42 (1H, d, J 16.2 Hz, H-8), 7.36 (3H, m, H-3, H-4, and H-5), 7.50 (2H, m, H-2 and H-6), 7.68 (1H, d, J 16.2 Hz, H-7); 13C NMR (50 MHz, CDCl3): 167.29 (C-9), 144.75 (C-7), 134.26 (C-1), 130.19 (C-4), 128.77 (C-3 and C-5), 127.96 (C-2 and C-6), 117.69 (C-8), 51.55 (C-10).
Lapachol (3). 1H NMR (200 MHz, CDCl3): 1.66 (3H, s, H-5'), 1.77 (3H, s, H-4'), 3.28 (2H, d, J 7.2 Hz, H-1'), 5.19 (1H, m, H-2'), 7.40 (1H, br s, OH), 7.67 (1H, td, J 7.5, 1.5 Hz, H-6), 7.75 (1H, td, J 7.5, 1.5 Hz, H-7), 8.05 (1H, ddd, J 7.5, 1.5, 0.5 Hz, H-8), 8.10 (1H, ddd, J 7.5, 1.5, 0.5 Hz, H-5); 13C-NMR (50 MHz, CDCl3): 184.36 (C-4), 181.52 (C-1), 152.61 (C-2)134.69 (C-7), 133.66 (C-C-3'), 132.79 (C-4a), 132.71 (C-6), 129.34 (C-8a), 126.64 (C-5), 125.92 (C-8), 123.40 (C-3), 119.59 (C-2'), 25.72 (C-4'), 22.60 (C-1')17.87 (C-5').
Ethyl p-hydroxycinnamate (4). 1H NMR (200 MHz, CDCl3): 1.15 (3H, t, J 7.1 Hz, H-11), 4.20 (2H, q, J 7.1 Hz, H-10), 6.53 (1H, d, J 15.9 Hz, H-8), 7.11 (2H, d, J 8.6 Hz, H-3 and H-5), 7.55 (2H, d, J 8.6 Hz, H-2 and H-6), 7.89 (1H, d, J 15.9 Hz, H-7). 13C-NMR (50 MHz, CDCl3): 167.14 (C-9), 161.26 (C-4), 144.87 (C-7), 130.43 (C-2 and C-6), 125.94 (C-1), 116.66 (C-3 and C-5), 115.09 (C-8), 60.01 (C-10), 14.29 (C-11).
Betulinic acid (5). 1H NMR (200 MHz, CDCl3): 0.77 (3H, s, H-25), 0.97 (3H, s, H-24), 0.99 (3H, s, H-26), 1.01 (3H, s, H-27), 1.17 (3H, s, H-23), 1.70 (3H, s, H-30), 0.8-2.50 (22H), 2.69 (1H, tl, J 11.4 Hz, H-13), 3.49 (1H, H-19), 3.40 (1H, t, J 8.2 Hz, H-3), 4.71 (1H, br s, H-29a), 4.88 (1H, d, J 2.0 Hz, H-29b); 13C-NMR (50 MHz, CDCl3): 180.0 (C-28), 151.19 (C-20), 109.68 (C-29), 77.96 (C-3), 56.48 (C-17), 55.74 (C-5), 50.79 (C-9), 49.60 (C-18), 47.58 (C-19), 42.67 (C-14), 40.94 (C-8), 39.32 (C-4), 39.10 (C-1), 38.42 (C-13), 37.42 (C-22), 37.34 (C-10), 34.65 (C-7), 32.73 (C-16), 31.05 (C-21), 30.09 (C-15), 28.46 (C-23), 28.09 (C-2), 25.94 (C-12), 21.02 (C-11), 19.29 (C-30), 18.59 (C-6), 16.24 (C-26), 16.22 (C-25), 16.13 (C-24), 14.71 (C-27).
3,4´,5-Trihydroxy-7-methoxyflavone (6). 1H NMR (200 MHz, pyridine-d5): 3.77 (3H, s, OMe-7), 6.58 (1H, d,J 2.2 Hz, H-6), 6.70 (1H, d, J 2.2 Hz, H-8), 7.35 (2H, d, J 8.8 Hz, H-3' and H-5'), 8.54 (2H, d, J 8.8 Hz, H-2' and H-6'), 13.16 (1H, br s, OH-5); 13C NMR (50 MHz, pyridine-d5): 177.25 (C-4), 165.50 (C-7), 161.79 (C-5), 160.78 (C-4'), 156.95 (C-9), 147.89 (C-2), 137.99 (C-3), 130.50 (C-2' and C-6'), 123.06 (C-1'), 116.32 (C-3' and C-5'), 105.28 (C-10), 97.94 (C-6), 92.02 (C-8), 55.80 (7-OMe).
Veratric acid (7). 1H NMR (200 MHz, pyridine-d5): 3.60 (3H, s, 4-OMe), 3.63 (3H, s, 3-OMe), 6.74 (1H, d, J 8.0 Hz, H-5), 8.03 (1H, br s H-2), 8.10 (1H, br d, J 8.0 Hz, H-6); 13C-NMR (50 MHz, pyridine-d5): 174.17 (C-7), 158.45 (C-4), 148.96 (C-3), 127.89 (C-1), 124.19 (C-6), 114.03 (C-2), 110.97 (C-5), 55.66 (3-OMe), 55.62 (4-OMe).
p-Anisic acid (8). 1H NMR (200 MHz, CDCl3): 3.54 (3H, s, 4-OMe), 6.81 (2H, d, J 8.2 Hz, H-3 and H-5), 8.40 (2H, d, J 8.2 Hz, H-2 and H-6); 13C-NMR (50 MHz, CDCl3): 173.21 (C-7), 162.64 (C-4), 132.69 (C-2 and C-6), 113.43 (C-3 and C-5), 55.11 (4-OMe).
Antimicrobial activity.Microbiological assays with the compounds isolated in galore showed a wide spectrum of activity against Gram positive and Gram negative bacteria and also against alcohol-acid bacterium and fungi (see Table 1).


DISCUSSION
The macroscopical and microscopical morphodiagnosis constitute an important set of diagnostic characters to identify and separate T. aurea from other species of the Tabebuia. The substances isolated from T. aurea (Figure 1) had their structures elucidated by the analysis of the their 1H and 13C NMR spectroscopic data. The bibliographic survey of the genus showed that b-sitosterol [1], lapachol [3] and veratric acid [7] were previously isolated from species of Tabebuia (9, 12). Although, methyl cinnamate [2], ethyl p-hydroxycinnamate [4], betulinic acid [5], 3,4´,5-trihydroxy-7-metoxyflavone [6] and p-anisic acid [8] are known, they are reported here for the first time as isolated from the genus Tabebuia.


All the compounds tested, except betulinic acid [5] inhibited the development of the microorganisms Staphylococcus aureus, and Enterococcus faecalis. Only ethyl p-hydroxycinnamate [4] presented weak activity against Escherichia coli. Compound [4] also presented marked activity against a yeast and a filamentous fungi (Candida albicans and Monilia sitophila, respectively). The antimicrobial activity of lapachol was confirmed (8). These results lend support to some traditional medicinal uses of the stem bark.
1.Agra MF, Plantas da medicina popular dos Cariris Velhos, Paraíba Brasil: espécies mais comuns. Editora União, João Pessoa, Brasil (1996) [ Links ]
2.Bandoni AL, ME Mendiondo, RVD Rondina, JD Coussio, Survey of Argentine medicinal plants. Folklore and phytochemical screening. II. Economic Botany 30 (1976) 161 [ Links ]
3.Bauer AW, WMM Kirby, JC Sherris, M Turc, Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology 45 (1966) 493 [ Links ]
4.Bureau E, C Schumann, Bignoniaceae. In: Martius CFP (Ed.) Fl. Bras. 8 (1897) 1-452 [ Links ]
5.Cabral SCM, MF Agra, Etnomedicina e farmacobotânica das Bignoniaceae medicinais da caatinga paraibana, Brasil. In: Iniciados 4(1999) 27-50, Editora Universitária, João Pessoa [ Links ]
6.Gentry AH, Bignoniaceae Parte II (Tecomeae) Flora Neotropica 25 (1992) 1-204 [ Links ]
7.Gentry AH, A field guide to the families and genera of woody plants of northwest South America (Colombia, Ecuador, Peru) with supplementary notes on herbaceous taxa. Conservation International, Washington, DC. USA (1993) [ Links ]
8.Lima OG, IL D´Albuquerque, MP Machado, Primeiras observações sobre a ação antimicrobiana do lapachol. Anais da Sociedade de Biologia de Pernambuco, 14 (1956) 129 [ Links ]
9.Makboul MA, AM Abdel-Baky, DW Bishay, Pharmacognostic study of Tecoma argentea Bur. & Schum cultivated in Egypt. Bulletin of Pharmaceuutical Sciences of Assiut University, 7 (1984) 190 [ Links ]
10.Méllo BR, Catálogo da coleção de microrganismos. 2a Ed., Editora Universitária, Recife, Brasil, (1988) [ Links ]
11.Murray PR, EJ Baron, MA Pfaller, FC Tenover, RH Yolken, Manual of clinical microbiology, 6a Ed., Washington, DC. USA (1995) [ Links ]
12.Oliveira MF, TLG Lemos, R Braz-Filho Investigação fitoquímica de plantas bioativas: Tabebuia serratifolia e Tabebuia rosea (Bignoniaceae). Revista Brasileira de Farmácia, 80,(1999) 46 [ Links ]
13.Pio Correa M, Dicionário das plantas úteis do Brasil e das exóticas cultivadas. Vol. IV, Ministério da Agricultura, Rio de Janeiro, (1984) [ Links ]
14.Rao KV, TJ Mcbride, JJ Oleson, (1968) Recognition and evaluation of lapachol as an antitumor agent. Cancer Research, 28: 1952 [ Links ]
15.Rizzini CT, WP Mors,(1976) Botanica yureayica brasileira, Edições EDUSP, São Paulo [ Links ]
16.Sandwith NY, DR Hund, (1974) Flora Ilustrada Catarinense. Parte I. Florianópolis [ Links ]
17.Santana CF, AAF Silva, Primeiras observações com o emprego de lapachol em pacientes humanos portadores de neoplasias malignas. Revista do Intituto de Antibióticos, 8 (1981) 89 [ Links ]
18.Swarnalakshmi T, K Gomathi, N Sulochana, Phytochemical studies on Tabebuia argentea. Proceedings - National Academy of Sciences, India, 52 (1982) 340 [ Links ]
19.Tyler VE, The new honest herbal. Philadelphia, (1988) [ Links ]
20.Washington JA, E Warren, AG Karlson, Stability of barium sulfate turbidity standards. Applied Microbiology, 24 (1972) 1013 [ Links ]














