Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) v.73 Vicente López ene./dic. 2004
ARTÍCULOS ORIGINALES
Promotion of growth and control of damping-off (Rhizoctonia solani) of greenhouse tomatoes amended with vermicompost
Rivera MC* ,ER Wright, MV López, D Garda, MY Barragué
Faculty of Agronomy. University of Buenos Aires, Av. San Martín 4453 (1417). Buenos Aires. Argentina.
*Corresponding author: Marta C. Rivera, Cátedra de Fitopatología. Facultad de Agronomía. Universidad de Buenos Aires. Av. San Martín 4453 (1417). Buenos Aires. Argentina. Tel (005411)4524-8063; fax (005411)4514-8737/39. E-mail address: mrivera@agro.uba.ar
Thanks are due to Mario Cuniolo, who provided the vermicompost and Dr. Laura Gasoni (IMYZA-INTA) from whom we obtained the isolate of R. solani
Received 12.II.2004: accepted 18.III.2004
Abstract. The pathogen Rhizoctonia solani (teleomorph Tanatephorus cucumeris) can affect tomatoes germination and emergence and cause basal rot of seedlings. It is generally accepted that composts suppress plant diseases, improve soil nutrient availability and stimulate plant growth. However, no reports have been found on the simultaneous evaluation of vermicompost as plant growth promoter and suppressive to damping-off caused by R. solani on tomatoes. This research evaluated the suppressive effects of different concentrations of vermicompost against R. solani and the ability of vermicompost to promote tomato seedlings growth. The microbial composition of the substratum was explored. Thirty six microorganisms were isolated, 13 of which were antagonic to R.solani in vitro. The addition of 25 to 100% of vermicompost promoted seedlings growth and prevented damping-off caused by R. solani.
Additional key words: antagonism, bacteria, biological control, disease incidence, fungi
Tomatoes (Lycopersicon esculentum Mill.) are propagated by seeds. Six-week seedlings with 4 expanded leaves are transplanted into soil. This crop is susceptible to damping-off caused by Rhizoctonia solani Kühn [teleomorph Tanatephorus cucumeris (Frank) Donk] (8). This fungus can affect seed germination or seedling emergence and cause basal rot of seedlings. If it is not prevented, the disease causes economic losses due to plant death, soil infestation with pathogens and delays in crop production. The usual tools for the management of this pathogen include soil chemical disinfection or sterilization and seed treatments. Chemicals registered for the control of soilborne plant pathogens are toxic to humans and are environmental contaminants. Among these, methyl bromide was defined under The Montreal Protocol of 1991 as a chemical that contributes to depletion of the Earth's ozone layer. Accordingly, its manufacture and importation will be phased out completely in 2005 in developed countries and in 2015 in developing countries (26).
In a search for alternatives to soil chemical treatments, biological control may be a useful tool (5). Composts have the potential to control plant diseases biologically (11), as one of their beneficial propeties is the microbiologically induced suppression of soilborne pathogens (2, 6, 9, 10, 16, 27). Another important charactheristic is their role in increasing soil nutrient availability and in plant growth stimulation (4, 12, 13). Vermicomposts, that are produced through the action of earthworms on organic matter, also have a great potential as plant growing media (3). Their physical and chemical properties have been described (1, 19). Their biological properties include suppression of soilborne pathogens (23, 24).
No reports were found on the simultaneous evaluation of vermicompost as plant growth promoter and suppressive to damping-off caused by R. solani in nurseries of tomato. In Argentina, one vermicompost's ability to control damping-off caused by R. solani was confirmed on autumn squash (28), white pumpkin (22) and eggplant (21). The aims of this work were to evaluate the suppressive effect of different concentrations of vermicompost on tomato seedlings growth and health. The microbial composition of the substratum was explored.
MATHERIALS & METHODS
Inocula. Isolate R81 (R. solani AG-4) was mantained on 2% potato dextrose agar (PDA). To test its pathogenicity, it was inoculated by sowing tomato cv. UC 82 B (Neuman Seed Co., 92% germination) in soil artificially infested with the pathogen. Plants were kept in humidity chambers at 22 + 2º C, under 12-h periods offluorescent light. The pathogen was reisolated from symptomathic seedlings by superficial disinfection with 2% NaOCl and plating on PDA. Inocula for the pathogenicity test and for the bioassay was obtained by growing on PDA and multiplied on sterilized oat (Avena sativa L.) grains. The concentration of pathogen in the soil was estimated by the method of Ko & Hora (15), as number of colony forming units per gram of soil (cfu/g). For each of 5 replicates, 50 beet (Beta vulgaris L.) glomerules were distributed on the surface of 32 g of soil and covered with an additional 32 g of soil, in 10 cm Ø Petri dishes. Beet glomerules act as baits for the R. solani propagules. After 48 h incubation at 26 ºC, beet glomerules were recovered, washed for 5 min in tap water and placed in Petri dishes containing PDA pH: 4. After other 24 h incubation at 26 ºC, Petri plates were scanned under a dissecting microscope (40 x). The number of glomerules colonized by R. solani were counted.
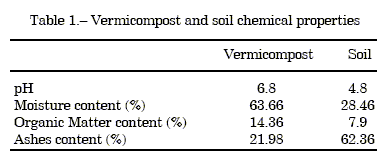
Substrates. A one year old vermicompost produced from cow and horse manure and button mushroom [Agaricus bisporus Lange (Imbach)] crop substrate, was used. Treatments were 100 to 0% of mineral soil artificially infested with R. solani R81 with the amendment of 0 to 100% of vermicompost (by volume), respectively; 100% sterilized and 100% non sterilized soil. Compost and soil chemical properties are summarized in Table 1. The assay was designed in completely randomized blocks, with 7 replicates. Autoclavated soil was inoculated with grains colonized by R. solani (0.1% volume) and incubated in humidity chambers at 21-24 ºC in darkness per ten days. After filling plastic trays (15 ´ 11 ´ 7 cm) with substrate mixtures, they were kept at 25 ºC, in humidity chambers, for 10 days before and 10 days after sowing. Fifty tomato seeds were sown per tray. Disease evaluations were done 11, 14 and 39 days after sowing. Seedlings with damping-off or incipient crown rot, as well as those that did no emerge as expected from the germination control, were considered to be diseased. Data on seedling fresh and dry weight were obtained using Sartorius scales (precision: 0.1 mg).
Statistical analysis. An analysis of variance (a: 5%) was used to compare means. A test described by Di Rienzo et al. (7) was employed as a multiple comparison procedure. The numbers of healthy seedlings were analysed following a repeated measures model. The assumption of sphericity of the covariance matrix was tested by the Maucly sphericity test. Univariate analysis was used when the interaction treatment-observation data were significant (7).
Isolation of compost fungi and bacteria. Samples of vermicompost (3.5 cc) placed in Erlenmeyer flasks containing 250 ml of destilled sterile water were shaked in a shaker at 70 r.p.m. per 1 h. Dilutions of 0.5 ml of 10-1, 10-2, 10-3, 10-4 and 10-5 were incubated on plates with 10 ml of PDA, amended with 100 ppm streptomycin sulfate and nutrient agar (NA Difco) amended with 100 ppm cycloheximide (20). The bacteria developed on NA were counted 3 days after incubation at 28 ºC and the fungi were counted after 7 days incubation at 25 ºC.
Antagonism of fungi and bacteria. Interrelations between each isolated microorganism and R. solani were observed in cultures (18). Circles of 1.1 cm Ø of fungal growth on PDA were placed simultaneously at opposite sites of 10 ml PDA plates. Stripes of 3 cm of bacteria and yeasts were made near the plates edges and a 1.1 cm circle of the pathogen's growth was placed in the plates center after 24 h. Cultures were incubated per 48 h at 25 ºC, and relationships between colonies were observed.
RESULTS
Pathogenicity and density of inoculum. Isolate R81 was pathogenic on tomato, causing damping-off 14-16 days after sowing. The pathogen was succesfully recovered from diseased plant tissues. Numbers of healthy seedlings and fresh and dry weights are given in Tables 2 and 3. Pathogen's population was estimated as 1 cfu g-1 soil.
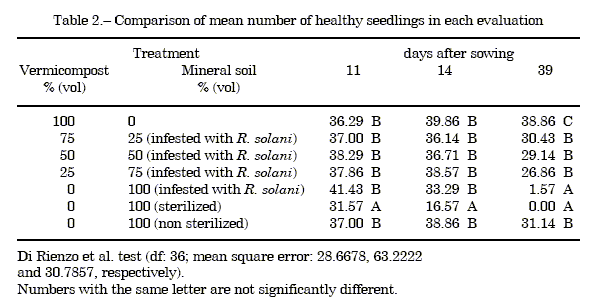

Disease incidence. The numbers of healthy seedlings were not different among treatments 11 and 14 days after sowing, except for sterilized soil, which differed from all the rest. More differences among treatments were detected on day 39. Trays with 25 to 75% of vermicompost with infested soil did not differ from tomatoes sown in non sterilized (and non inoculated) soil. Non sterilized soil provided control equal to 100% vermicompost. Disease incidence did not differ between trays with 100% infested soil and 100% sterile soil. No disease control was observed in both of them (Table 2).
Seedlings growth. Data on seedlings fresh and dry weights are summarized in Table 3. The use of between 25 and 100% of vermicompost improved seedling growth, as demonstrated by mean seedling fresh weights. Minimum fresh weights were obtained by sowing in either sterilized-inoculated or sterilized-non inoculated soil. There were no differences in dry weights among treatments.
Isolation of compost fungi and bacteria. Numbers and types of microorganisms isolated from the vermicompost are summarized in Table 4. There was a high prevalence of fungal population in the compost.
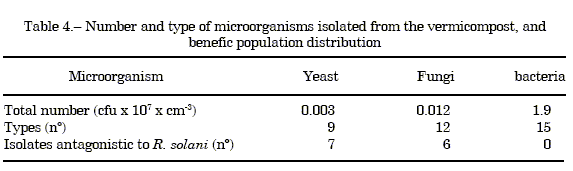
Antagonism of fungi and bacteria. The results of dual cultures (Table 4) show that 77% of the yeasts, 50% of the filamentous fungi and 0% of the bacteria demonstrated in vitro competitive ability against R. solani.
DISCUSSION
The plant growth and health responses to the addition of vermicompost seemed independent of amendment percentages incorporated to the soil. Twenty five percent of vermicompost was enough to promote tomatoes seedling growth (measured as fresh weight) and control the pathogen at transplant time (day 39). However, these results differ from those obtained with identical vermicompost and pathogen on different hosts. The relationships between percentages of vermicompost and healthy seedlings of white pumpkin (Benincasahispida (Thunb.) Cogn.) could be described by a linear function (28). The percentage of healthy seedlings increased significantly with 75% of vermicompost on eggplant (Solanummelongena L.) (21). In experiments on african daisy (Gerbera jamesonii H. Bolus) vermicompost incorporation at 20% rate reduced the incidence of Rhizoctonia root and crown rot. Also, plant length, chlorophyll content, number, lenght and diameter of floral peduncle and number and diameter of inflorescences were higher (23). In this work, with the amendment with 25 to 75% of vermicompost in infested soil, the number of healthy tomato seedlings was equal to the treament with non sterilized soil without the presence of the pathogen.
Plant health and growth did not differ between tomatoes in 100% sterilized soil or soil infested with R. solani. So as to know the origin of the disease occured on sterile soil, seedlings basal tissues were disinfested in 2% NaOCl during 1 min and cultivated on PDA. An isolate of Phytophthora sp. was obtained, and its pathogenicity was confirmed by inoculation on healthy nurseries. Absent soil life due to autoclaving may have let Phytophthora propagules on the seeds or in irrigation water colonize the substratum. In this case, heat sterilization had similar effect to methyl bromide applications, creating a biological vacuum (14).
The higher the level of microbial activity, the more difficult it will be for any given portion of the microbial population to obtain the N and/or C and energy necessary for their growth (5). Isolations provided an interesting number of compost microorganisms, and in vitro tests added evidence for specific forms of pathogen suppression. Our results on number of microorganisms in vermicompost are similar to those obtained by Szczech (24). Whether the effect of vermicompost on seedling weight is due to nutrient content or growth promotion microorganisms needs further studies. Also, mineral soil microbial composition has to be explored.
The use of organic-by products as amendments to reduce soilborne pathogen diseases is gaining the interest of plant pathologists, manufacturing and processing industries, regulators, consumers and growers (17). Our results confirm that vermicomposts can be included in the development of effective alternatives to control tomato damping-off. In addition, it may be a tool to promote seedlings growth.
1.Albanell E, J Plaixats, T Cabrero, Biology and Fertility of Soils 6 (1988) 266 [ Links ]
2.Alvarez MA, S Gagne, H Antuon, Appl Environ Microbiol 61 (1995) 194 [ Links ]
3.Atiyeh RM, S Subler, CA Edwards, J Metzger, Pedobiologia 43 (1999) 724 [ Links ]
4.Chiu AL, JW Huang, Plant Pathology Bulletin 6 (1997) 67 [ Links ]
5.Cook RJ, KF Baker, The nature and practice of biological control of plant pathogens. APS Press, St. Paul (1983) [ Links ]
6.Craft CM, EB Nelson, Applied and Environmental Microbiology 62 (1996) 1550 [ Links ]
7.Di Rienzo J, A Guzmán, F Casanoves, Journal of Agricultural, Biological and Environmental Statistics 7 (2002) 129 [ Links ]
8.Farr DF, GF Bills, GP Chamuris, AY Rossman, Fungi on plants and plant products in the United States. APS Press, St. Paul (1989) [ Links ]
9.Gorodecki B, Y Hadar, Crop Protection 9 (1990) 271 [ Links ]
10.Hoitink HA, PC Fahy, Annual Review of Phytopathology 24 (1986) 93 [ Links ]
11.Hoitink HA, ME Grebus, Compost Science & Utilization Spring (1994) 6 [ Links ]
12.Kale RD, BC Mallesh, K Bano, DJ Bagyaray, Soil Biology and Biochemistry 24 (1992) 1317 [ Links ]
13.Kalembasa D, Zesyty Problemowe Postepow Nauk Rolniczych 437 (1996) 249 (in Polish). [ Links ]
14.Katan J, Journal of Plant Pathology 81 (1999) 153 [ Links ]
15.Ko W, FK Hora, Phytopathology 61 (1971) 707 [ Links ]
16.Kuter GA, EB Nelson, HAJ Hoitink, LV Madden, Phytopathology 73 (1983) 1450 [ Links ]
17.Lazarovits G, M Tenuta, KL Conn, Australasian Plant Pathology 30 (2001) 111 [ Links ]
18.Mariano RLR, Revisâo Anual de Patologia de Plantas 1 (1993) 369 [ Links ]
19.Orozco FH, J Cegarra, LM Trujillo, Roig, Biology and Fertility of Soils 22 (1996) 162 [ Links ]
20.Peng G, JC Sutton, Canadian Journal of Plant Pathology 13 (1991) 247 [ Links ]
21.Rivera MC, ER Wright, MV López, GS Guastella, Biological and Cultural Tests for control of plant diseases (2001) http://www.scisoc.org/online/B&Ctests [ Links ]
22.Rivera MC, ER Wright, MV López, MC Fabrizio, FUTON 54 (2004) 131 [ Links ]
23.Rodríguez-Navarro JA, E Zavaleta-Mejía, P Sánchez-García, H González-Rosas. Fitopatologia 35 (2000) 66 [ Links ]
24.Szczech MM, J Phytopathology 147 (1999) 155 [ Links ]
25.Szczech MW, MW Rondomanski, US Brzeski, JF Kotowski, Biological Agriculture and Horticulture, 10 (1993) 47 [ Links ]
26.USDA ARS Methyl Bromide Research. http://www.ars.usda.gov/is/mb/mebrweb.htm#why 2002. [ Links ]
27.Voland RP, AH Epstein, Plant Disease 78 (1994) 561 [ Links ]
28.Wright ER, MC Rivera, A Cheheid A, MC Fabrizio, J Mosedale, Biological and Cultural Tests for Control of Plant Diseases 14 (1999) 183 [ Links ]














