Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) v.75 Vicente López ene./dic. 2006
ARTÍCULOS ORIGINALES
Variability in accumulation of free proline on in vitrocalli of four bean ( Phaseolus vulgaris L.) varieties exposed to salinity and induced moisture stress
(With 2 Tables & 4 Figures)
Variabilidad en la acumulación de prolina libre en callos in vitro de cuatro variedades de soja expuestas a salinidad y estrés hídrico inducido
(Con 2 Tablas y 4 Figuras)
Cárdenas-Avila1* ML, J Verde-Star1, RK Maiti 2, R Foroughbakhch-P1, H Gámez-González1, S Martínez-Lozano1, MA Núñez-González1, G García Díaz1, JL Hernández-Piñero1, MR Morales-Vallarta1
1Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León. Apartado Postal 88, Suc. Ciudad Universitaria, C.P. 66450, San Nicolás de los Garza, Nuevo León, México.(*Correspondence address).
2 Universidad de las Américas, Dpto. de Química y Biología, Santa Catarina Martir, C.P. 72820, Puebla, México.
3 This work has been supported by PAICYT CN-150-99
Abstract. This paper reports the genotypic variability in the accumulation of proline on the in vitro calli of bean cultivars exposed to induced water and salinity stress. Remarkable variations in the proline content were found among bean cultivars exposed to both stress factors.
Key words: Proline; In vitro calli; Phaseolus vulgaris; Bean.
Resumen. Este trabajo informa la variabilidad genotípica en la acumulación de prolina en callos in vitro de frijol expuestos a estrés hídrico y salino. Se encontraron variaciones notables en el contenido de prolina de los cultivos sometidos a ambos tipos de estrés.
Palabras clave: Prolina; Callo in vitro; Phaseolus vulgaris; Frijol.
INTRODUCTION
Bean (Phaseolus vulgaris L.) is an important high-protein food crop in Mexico and many Latin American countries of the world. Because of an increasing population, there is a great demand for this food crop, but the production of it is affected by several biotic and abiotic stress factors prevailing in semiarid regions of the world. (Maiti, 1997; Moreno Limon, 1998). The selection of bean cultivars adapted to salinity, drought and other abiotic stresses is a main goal of the breeders. Therefore, several investigations have been directed to study the effect of these stress factors on bean growth. This will contribute to understand the resistance mechanisms involved in different bean cultivars with special reference to drought, salinity, high temperature and low nutrients (Cramer et al., 1988; Mishra et al., 1994; Quintero et al., 1999; Moreno Limón et al., 2000; Nuñez-González et al., 2001).
Proline accumulation is a plant resistance mechanism to various stress factors, such as drought (Naidu et al., 1992; Stewart & Lee, 1974; Singh et al., 1972); salinity (Treichel, 1975), low temperature (Benko, 1986; Chu et al., 1974 and high temperature (Oshanina, 1972). In tolerant genotypes, a high accumulation of proline and little anomaly on chloroplast ultrastructure were observed (Nuñez-Gonzalez et al., 2001). Therefore, the accumulation of proline is considered an indicator for selection of plants tolerant to various stress factors (Singh et al., 1972; Van Rensburg & Kruger, 1994; Flores, 1997). Techniques of in vitro tissue culture have been utilized as a valuable tool in the selection of crop cultivars for tolerance to salinity, and for studying the tolerance mechanisms to this stress factor (Revilla & Cañal, 1999; Kim & Song, 1984; Lupotto et al., 1988; Mongodi et al., 1988; Huang-Peiming & Ge-Koulin, 1989; Mahammed et al., 1992). Assays with this technique have demonstrated that increased salinity raised the accumulation of free proline in calli of bean. At the same time, proline content diminished with a decrease in salinity (Broetto et al., 1995; Sawires et al., 1997 cited by Maiti et al., 1997). The objective of the present study was to study the effect of water and salinity stresses on the proline accumulation in cultivars of bean, and to establish its relation to the resistance mechanism of this species to these stress factors.
MATERIALS AND METHODS
Seeds of four varieties of Phaseolus cultivars, (Pinto Americano, Pastilla, Flor de Mayo and Flor de Junio) were disinfected in 70% ethanol and sodium hypochlorite (15% v/v) for 10 min. and immediately sown under asceptic conditions on sterilized 0.7% agar under a laminar flow chamber. The explants of 1 cm2 cotyledonary leaves were obtained from in vitro seedlings. They were sown asceptically in glass vials of 120 mL with approx. 30 mL of MS (Murashige- Skoog, 1962) medium with vitamins, 100 mg.L of mio-inositol, 1.5, 2.0, 3.0, 5.0 and 10 mg.L of 2,4-D (for induction of callus in vitro) and agar at 0.7%. The pH was adjusted to 5.7 with NaOH 0.1 N or HCl 0.1 N before addition to agar which was sterilized in a pressure chamber at 121ºC and 15 pound pressure for 15 minutes. The in vitro culture was maintained in a photoperiod of 16 h to a temp. of 26±1ºC.
Salt concentrations utilized followed Lupotto et al. (1988) and Moreno Limón (1998). Sections of calli in vitro were subcultured in glass of 120 mL with approximately 30 mL of MS culture. Then, solutions of (0.1 and 0.15 M) were added to the main culture for having salinity stress. Each test was compared against its respective control.
Polyethylene glycol (PEG 6000) was used to induce water stress. Sections of calli in vitro were subcultured in test tubes (18 x 150mm) in MS medium supported with filter paper. A glass of 120 mL with approximately 30 mL of MS culture and two concentrations of polyethylene glycol (PEG) (10 and 15% which are equivalent to -0.6 and -1 Mpa, respectively) was used for inducing water stress. Each test was compared with its respective control.
Determination of proline. For determination of proline in calli exposed to drought and salinity treatments, we utilized the techniques of Zuñiga et al. (1989) and Bates (1973).
Five g samples of calli were homogenized in 10 mL of aqueous solution of sulfosalicylic acid at 3%. Then the solution was filtered rapidly through a buchner funnel using Whatman filter paper Nº 2. A small volume (2 mL) of the filtrate was thereafter taken to a test tube where added 2mL of ninhydric acid and 2 mL of glacial acetic acid were. This was kept in an incubator at 1000C for one hour. Finally the reaction was completed in an ice bath. Thereafter, 4 mL of toluene were added and contents of the tube were inverted during 20 seconds. After this, toluene phase was sucked using a pipette and it was kept at room temperature to stabilize. Finally, absorbance was determined at 520 nm with a visible light spectrophotometer Turner Sequoia 690. We used a tube with toluene as control for calibration of the apparatus.
The concentration of proline was determined from the calibration curve and calcutated by adopting the following equation (ppm of proline from the curve) X volume of aforation / Fresh material, g = ppm proline in tissue.
RESULTS AND DISCUSSION
Determination of free proline on in vitro calli exposed to salinity stress.
The results obtained from the analysis of variance indicated that there were highly significant differences (P< 0.01) in proline contents among varieties and treatments (Table 1).
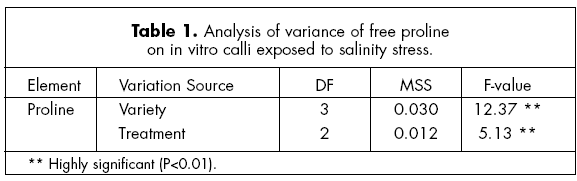
Pinto americano showed higher (p<0.01) free proline contents than the other varieties (Fig. 1). Flor de Mayo and Flor de Junio had the lowest (p<0.01) contents. Free proline content was lowest (p<0.01) at 0.15 M NaCl compared with the control (Fig. 2). This is in contrast to the report by Sawires et al. (1997), cited by Maiti et al. (2000) who reported that free proline in general increased with an increase in salinity. Sivaramkrishnan et al. (1988) and Revilla & Cañal (1999) also reported that salinity stress in calli of olive showed an increase in proline under salinity stress. Our results need to be verified in further studies.


Determination of free proline on in vitrocalli exposed to drought stress with PEG. Free proline contents of all 4 bean varieties were significantly different among each other (p<0.01), (Table 2 and Fig. 3). On the other hand, the lower the water potential (15% PEG), the higher (p<0.01) the free proline content (Fig. 4). This result is coincident with findings of Sivaramkrishnan et al. (1988) who reported that an increase in proline was related with a decrease in leaf water potential.



CONCLUSIONS
A higher free proline content in varieties reported as tolerant (pinto americano and pastilla), compared to the other two varieties indicates that this aminoacid may be highly involved as a plant adaptation mechanism to water and salinity stresses. Proline may act as a osmoregulator compound to equilibrate the osmotic potential in bean cells.
REFERENCES
1. Bates L, Rapid determination in free proline of water stress studies. Plant Soil 39 (1973) 205. [ Links ]
2. Benko A, The content of same aminoacids in young apple shots in relation to frost resistance. Biología Plantarum 2 (1986) 334. [ Links ]
3. Cramer G, R Epstein, A Lauchli, Kinetics of root elongation of maize in response to short/term exposure to NaCl and elevated calcium concentration. Journal of Experimental Botany 39 (1988) 1513. [ Links ]
4. Huang-Peiming, Ge-Koulin, Acta-Agriculturac-Shangahi (China), 5 (1989) 31. [ Links ]
5. Chu MT, D Aspinall, GL Paleg, Stress metabolism. VI. Temperature stress and the accumulation of proline in barley and radish. Australian Journal Plant Physiology I (1974) 87. [ Links ]
6. Flores HA, Características Bioquímicas relacionadas con el estrés por calor en nopal (Opuntia spp). Tesis Doctoral, Colegio de Postgraduados. Instit. de Enseñanza e Investigación en Ciencias Agrícolas. Instituto de Recursos Genéticos y Productividad, Montecillo, México (1997). [ Links ]
7. Kim SG, JH Song, Korean Journal of Botany 27(1984) 173. [ Links ]
8. Lupotto E, M Mongodi, MC Lusardi, Salt tolerance in vitro selection with regard to the regenerative potential. Maize Genet. Cooperation Newsletter 62 (1988) 30. [ Links ]
9. Maiti RK, Phaseolus spp. Bean Science. Fst Ed Science Publishers. USA (1997) PP.15. [ Links ]
10. Mishra PK, AS Mehta, AK Srivastava, Effect of salt stress on the physiology of 15-day old seedlings of maize. Neo- Botanica 2 (1994) 1. [ Links ]
11. Mohammed MF, PE Read, DP Coyne, Journal of the American Society for Horticultural Science 117 (1992) 2. [ Links ]
12. Mongodi M, MC Lusardi, WE Lupotto, Regeneration in salt tolerant cultures of maize (Zea mays). Importance of genotype and somaclonal variation in the scheme of selection. Genetica Agraria 42 (1988) 85. [ Links ]
13. Moreno Limón, Respuestas morfofisiológicas, biquímicas y ultraestructurales en frijol (Phaseolus vulgaris L) al estrés de salinidad, altas temperaturas y sequía. Ph.D. Thesis. Facultad de Ciencias Biológicas, División de Estudios de Postgrado, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León. (1998) 99. [ Links ]
14. Moreno-Limón S, RK Maiti, A Núñez Gonzalez, J Verde Star, H Gamez-Gonzalez, Biochemical mechanism in bean cultivars (Phaseolus vulgaris L) for resistance to salinity stress at the germination stress. Research on Crops 1 (2000) 25. [ Links ]
15. Naidu PB, D Aspinall, G L Paleg, Variability in proline accumulating ability of barley (Hordeum vulgare L.) cultivars induced by vapor pressure deficit. Plant Physiology 98 (1992) 716. [ Links ]
16. Murashige T, F Skoog, A Revised Medium For Rapid Growth and Bioassays With Tobacco Tissue Cultures. Physiology Plantarum 15 (1962) 473. [ Links ]
17. Núñez-González MA, Bases bioquímicas y ultraestructurales de respuesta al estrés nutrimental en frijol (Phaseolus vulgaris L.) a nivel de plántula. Ph.D. Thesis. Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México (2001) 107. [ Links ]
18. Oshanina NP, Nitrogen exchange of plants in the south-western kyzilkum. In: Ecophysiological Fundation of ecosystems and productivity in arid zones. Inter. Symp. U.S.S.R. (1972). [ Links ]
19. Quintero FJ, I Mendoza, JM Rodríguez-Galán, A Hernández, MT Ruiz, JM Pardo, Regulación de la homeostasis de Na+ y K+ en plantas y hongos. Instituto de Recursos Naturales y Agrobiología. Consejo Sup. de Investigaciones Científicas, XIII Breunión Nac de la Soc Española de Fisiología Vegetal. VI Congreso Hispano-Luso de Fisiología Vegetal 19-22 Sept. (1999). [ Links ]
20. Revilla MA, MJ Cañal, Respuestas a estrés salino de callos de olivo. Departamento de Biología de Organismos y Sistemas. Fac de Biología Univ. De Oviedo. XIII Reunión Nacional de la Sociedad Española de Fisiología Vegetal. VI Congreso Hispano-Luso de Fisiología Vegetal 19-22 Sept. (1999). [ Links ]
21. Singh T N, D Aspinall, LG Paleg, Proline accumulation and varietal adaptability to drough in barley: A potential metabolic meaure of drough resistance. Nature (London) New Biology 236 (1972) 188. [ Links ]
22. Sivaramakrishnan S, VZ Patell, DJ Flower, JM Peacock, Physiology Plantarum 74 (1988) 418. [ Links ]
23. Stewart CR, JA Lee, The role of proline accumulation in halophytes, Planta 120 (1974) 279. [ Links ]
24. Treichel S, The effect of NaCl on the concentration of proline in different halophytes. Z Pflanzenphysiol 76 (1975) 56. [ Links ]
25. Van Rensburg LV, GHJ Kruger, Aplicability of abscisic acidand (or) proline accumulation as selection criteria for drought tolerance in Nicotiana tabacum, Canadian Journal of Botany 72 (1994) 1535. [ Links ]
26. Zuñiga, G. E. V. H. Argandona, y L. J. Corcuera, Distribution of glycine, betaine and proline in water stress and un stress barley leaves. Phytochemistry 28 (1989) 419. [ Links ]














