Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) v.76 Vicente López ene./dic. 2007
ARTÍCULOS ORIGINALES
Germination of grasses and shrubs under various water stress and temperature conditions (With 4 Figures & 2 Tables)
Germinación de gramíneas y arbustos bajo varias condiciones de estrés hídrico y temperatura (Con 4 Figuras y 2 Tablas)
1 INTA E.E.A. Bariloche, C.C. 277, 8400 - San Carlos de Bariloche, Río Negro, Argentina.
2 Departamento de Agronomía y CERZOS (CONICET), Altos del Palihue, 8000 - Bahía Blanca, Buenos Aires, Argentina.
Address Correspondence to: Carlos A. Busso, Departamento de Agronomía-CERZOS (CONICET), Altos del Palihue, 8000 - Bahía Blanca, Buenos Aires, Argentina, e-mail: cebusso@criba.edu.ar.
Recibido/Received 20.07.2007. Aceptado/Accepted 10.08.2007.
Abstract. The effects of various temperature combinations and water potentials were determined on the germination of Atriplex lampa Gill. ex Moquin, Larrea divaricata Cav., Leymus erianthus (Phil.) Dubcovsky, Stipa neaei Nees ex Steudel and Poa ligularis Nees ap. Steudel under controlled conditions. The tested hypothesis was that seed germination increases with increasing temperatures and water potentials in A. lampa, L. divaricata, L. erianthus, S. neaei and P. ligularis, and that time to reach 50% of total germination is greater at lower than higher water potentials. PEG 2000 was used to impose water stress conditions. In general, obtained results conducted to accept the posted hyphotesis.
Key words: Water stress; Temperature; Germination index; Perennial grasses and shrubs; Arid Argentina.
Resumen. Los efectos de varias combinaciones de potencial hídrico y temperatura se determinaron en la germinación de Atriplex lampa Gill. ex Moquin, Larrea divaricata Cav., Leymus erianthus (Phil.) Dubcovsky, Stipa neaei Nees ex Steudel and Poa ligularis Nees ap. Steudel bajo condiciones controladas. La hipótesis puesta a prueba fue que la germinación de las semillas se incrementa a mayores temperaturas y potenciales hídricos en A. lampa, L. divaricata, L. erianthus, S. neaei and P. ligularis, y que el tiempo para alcanzar el 50% de la germinación total es mayor a menores que mayores potenciales hídricos. PEG 2000 se utilizó para imponer las condiciones de estrés hídrico. En general, los resultados obtenidos condujeron a aceptar la hipótesis propuesta.
Palabras clave: Estrés hídrico; Temperatura; Indice de germinación; Gramíneas perennes y arbustos; Argentina árida.
INTRODUCTION
Limiting soil water content and extreme temperatures are the major stresses which constrain seed germination in arid and semiarid regions (Sharma, 1976). Time to germination can be the plant life period which requires the highest water potential (Mac Mahon & Schimpf, 1981). Temperature also appears to determine the optimal and minimum water potentials for germination of several species (Sharma, 1976).
Temperatures in the upper limit of the favourable range may shorten time to germination, and it will occur under momentarily favourable hydric conditions (McDonough & Harniss, 1974). Optimum humidity and temperature for seed germination may vary among seasons and years (Romo & Eddleman, 1988). The greater the water stress conditions, the lower will be the total germination and time to germination (Brown, 1995). Seeds will not germinate if they are either under or above specific humidity and temperature conditions for the study species (Raven et al., 1986). Some seeds will not germinate even under favourable conditions because they are dormant; need of a post-maturation period contributes to avoid seed germination under unfavourable conditions.
A linear relationship between seed germination and either humidity or temperature should not necessarily be expected. Sometimes, waterproof seed coats or dormancy caused by chemical substances must be removed from the seed to allow germination. In Oryzopsis hymenoides (Roem. et Schult) Ricker, seed maturation conditions (light quantity and quality, temperature, soil moisture) and genotype play an important role in regulating seed dormancy (Jones & Nielson, 1994). The substratum physical nature can also influence seed germination (Blank & Young, 1992). Germination may be influenced negatively under excess soil moisture: in the gramineae, there may be a space between the lemma and palea which when filled with water constrains oxygen diffusion to the embryo (Black & Young, 1992). The low germination of Oryzopsis holciformis (M. Bieb.) Hack. was related with excessive moisture contents (Dasberg & Mendel, 1971).
Higher temperatures not always determine greater germination percentages. For example, when Elymus cinereus cv. Magnar (Scribn. and Merr.) was exposed to different temperatures profiles from 0 to 40 oC, it showed greater germination percentages when exposed to alternating temperatures between 15 and 25 oC (Evans & Young, 1983). A similar response was obtained with Achnatherum robustum (Vasey) Barkworth which optimum germination occurred with 20 oC during 8 h and 15 oC during 16 h (Young et al., 2003). Leymus cinereus showed the greatest mean germination percentages at alternating temperatures of 15/25 oC, and then at 10/20, 20/30 and 5/15 oC in decreasing order of magnitude (Meyer et al., 1995). Knowledge of the simultaneous effects of water and temperature on species germination percentages would increase our understanding of plant population dynamics (Sharma, 1976).
Atriplex lampa Gill. ex Moquin, Larrea divaricata Cav., Leymus erianthus (Phil.) Dubcovsky (syn.: Elymus erianthus Philippi: Duvcovsky (1997)), Stipa neaei Nees ex Steudel and Poa ligularis Nees ap. Steudel are abundant species in the Monte Austral of the Neuquén Province where the shrub steppe of L. divaricata and A. lampa ocuppies an approximate surface of 1,080,000 ha.
In view of the literature review, we hyphotetized that (1) seed germination increases with increasing temperatures and water potential in A. lampa, L. divaricata, L. erianthus, S. neaei and P. ligularis. Time to reach 50% of total germination is greater at lower than higher water potentials. The objective of this study was to determine the most adequate ranges of temperature and water potential for germination of these species under controlled conditions.
MATERIALS AND METHODS
Seeds were harvested at the field in Picún Leufú (39o30'S, 69o09'W, 383 m.a.s.l.; Province of Neuquén, Argentina) during summer 2000 and fall 2001. Thereafter, they were kept in the laboratory to ambient temperature during 6 to 10 months previously to the study. Before seeding, awns were separated from the anthecia of S. neaei and seeds of L. erianthus were separated from its spikelets. Poa ligularis seeds were used as collected. Seeds of A. lampa and L. divaricata were treated before seeding to break its dormancy. Bracts, which possess chemical inhibitors (Giusti & Grau, 1983), were removed in A. lampa. Seeds of L. divaricata were separated from its hairy involucres, and were then mechanically scarified using fine sand paper following Peretti (1994).
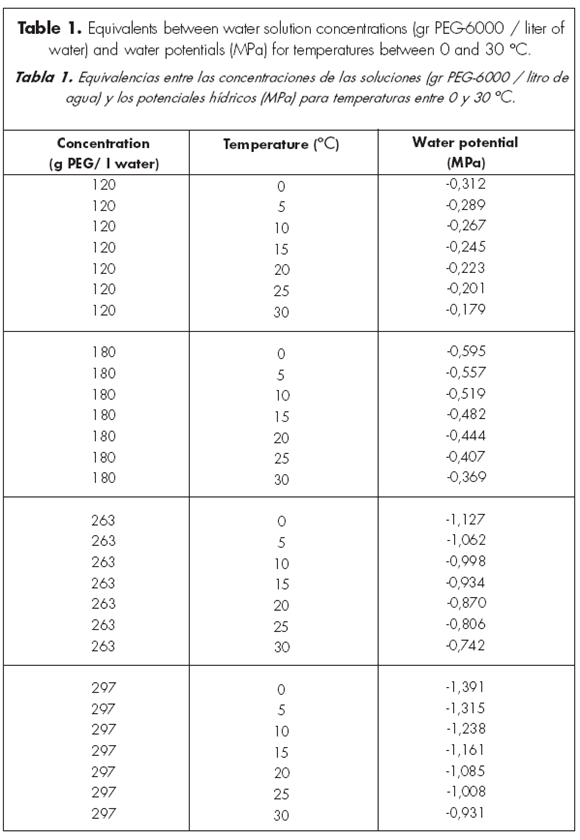
Solutions using PEG-6000 were used to obtain different water potentials at various temperatures. These solutions contained 0, 120, 180, 263 and 297 g PEG-6000/l water. Equivalences between PEG concentrations and leaf water potentials at different temperatures were calculated following Michel & Kaufmann (1973) (Table 1). Temperature combinations used in the growth chamber treated to reproduce those at the field study site from early to late fall. Such temperature combinations were 15-30 oC (end of March), 10-15 oC (late April/early May), 5-10 oC (late May) and 0-5 oC (early June) (Bonvissuto, personal observation). Within each temperature range, minimum temperatures were applied during 12 h of darkness, and maximum temperatures during 12 h of light, which was provided by Osram L 30 watts/41-827 tubes. International rules for seed testing (Servicio Nacional de Semillas, Comité Federal de Agricultura y Ganadería, República Argentina) say nothing regarding application of a given photosynthetically active radiation (PAR) intensity. They only say if light is either provided or not. Because of this, the following criterion was followed: PAR intensity within the growth chamber was double for temperature combinations during early fall (4 lighting tubes) than for temperature combinations during late fall (2 lighting tubes). Several authors report that there exists a 2:1 relationship between the radiation during early to late fall, respectively (Grossi Gallegos 1998a,b; Kania & Giacomelli, 2005).
This 2:1 relationship was achieved applying the following light intensities (mean±1 S.E., n=6, ìmol photon/m2/s):
• 63.92±10.25 (for 15-30 oC) with 4 lighting tubes (3.82 % of PAR in Picún Leufú on 21 March 2006 between noon and 1:30 P.M.; measurements were effected with a Licor Quantum sensor which precision was ±2.8 µmol photon/ m2/s).
• 46.61±7.59 (for 10-15 oC) with 3 lighting tubes (2.78 % PAR).
• 28.82±4.77 (for 5-10 and 0-5 oC) with 2 lighting tubes (1.72 % PAR).
A total of 1600 seeds were used for each of the 5 species (20 seeds/replicate x 4 replicates x 5 PEG-6000 concentrations x 4 temperature combinations). These seeds, which grew within Petri dishes in the growth chamber, were observed either every 1 to 2 days (when germination rate was faster) or every 6 days (when germination rate was slower) during a 21-day-period. Seeds were considered germinated when the radicle was visible. Observations did not go beyond 21 days because the growth chamber was needed by other researchers.
RESULTS
Stipa neaei and L. erianthus needed a greater amount of days to germination than A. lampa and L. divaricata in all water potentials and temperature ranges (Table 2). Days to reach 50% of total germination tend to increase as solution water potentials decreased in most temperature ranges in S. neaei, L. erianthus and A. lampa (Table 2).
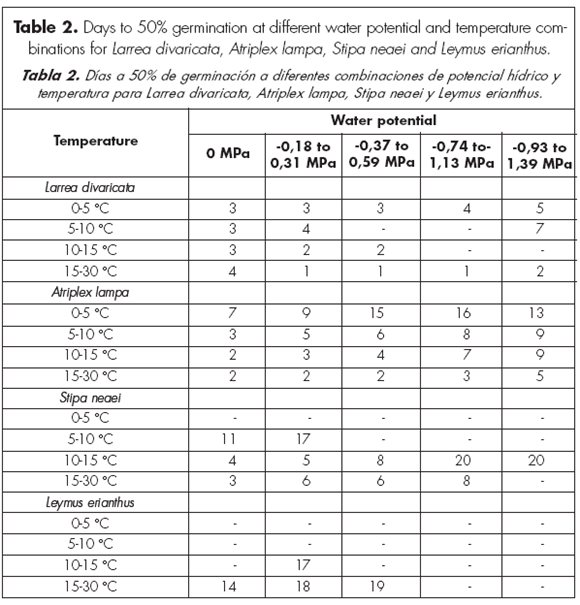
By the third week of study, the lower the solution water potentials, the lower the germination percentages for all species and most of the temperature ranges (Figs. 1 to 4). Days to 50% germination were the lowest at 0 MPa for most species and all temperature ranges. Stipa neaei and L. erianthus needed high solution water potentials to germinate, with almost no germination below -0.59 MPa. Seeds of A. lampa and L. divaricata, however, germinated at water potentials as low as -1.35 MPa (mean of -1.391 and -1.315 MPa) at the 0-5 ºC temperature range. After 21 days, A. lampa showed greater than 80% germination at 0 MPa in all temperature ranges. However, germination percentages in this species at all study temperatures were about 50% at the most, when solution water potentials were between -0.93 and -1.39 MPa. Maximum germination in L. divaricata was 17.6% at 0 MPa and 0-5 ºC temperature range. Percentage germination of S. neaei was greater than 90% at 0 MPa and temperatures over 5 ºC; when solution water potentials were lower than -0.37 MPa, germination percentages were close to 0. Except when temperatures were between 15 and 30 ºC, and water potentials were between 0 and -0.59 MPa, germination of L. erianthus was 0 in most cases.
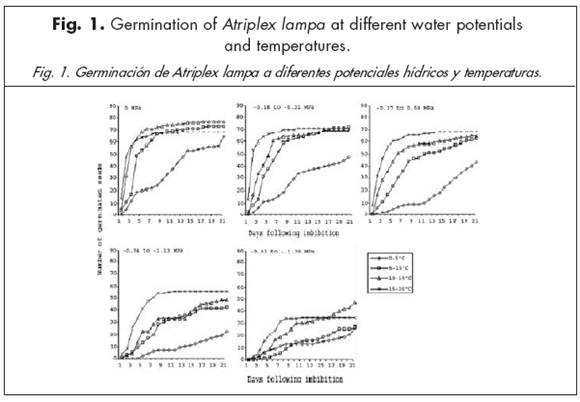
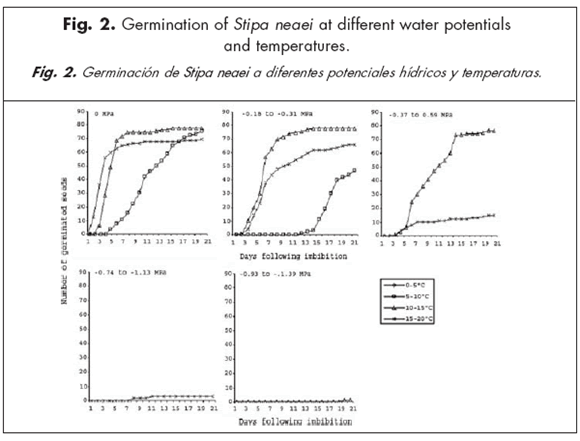


When solution water potentials were 0, -0.18 to -0.31 and -0.74 to -1.13 MPa, germination of A. lampa and S. neaei increased at greater temperature ranges, while it increased at lower temperature ranges in L. divaricata. Atriplex lampa was the only species which germinated under all study solution water potentials and temperature ranges. Poa ligularis did not germinate under any study water potential or temperature range.
DISCUSSION
In our study, seed germination of A. lampa, L. divaricata, L. erianthus and S. neaei increased with increasing temperatures and soil water potentials. These results agree with the posted hypothesis and results of Sharma (1976) and Brown (1995). In addition, days to reach 50% of total germination were greater at lower than higher solution water potentials and at lower than higher temperatures in both grass and shrub species. These results are similar to those reported by McGinnies (1960), Owens & Call (1985) and Brown (1995) in several shrub and grass species. However, this is not always the case. For example, Dasberg & Mendel (1971) and Black & Young (1992) related low germination percentages with high moisture contents. They suggested that there could be mechanisms that have a negative influence under saturated or oversaturated seed germination conditions. Also, a major source of variation in defining the critical water status at which seed germination is inhibited is temperature, which appears to mediate the optimum and minimum water potential for germination of many species (Owens & Call, 1985; Romo et al., 1991). It needs to be taken into account that the optimum water and temperature levels for germination may shift in different seasons and for seeds collected in different years (Romo & Eddleman, 1988), presumably due to environmental conditions during seed development (Brown, 1995).
Regarding temperature, not always there would be greater germination with increasing temperatures. Depending on the species, optimal germination profiles can be obtained at intermediate temperatures (Evans & Young, 1983; Meyer et al., 1995; Young et al., 2003). This was the case for A. lampa and S. neaei, although germination was highest at the lowest temperatures in L. divaricata and at the highest temperatures in L. erianthus.
Larrea divaricata and A. lampa germinated at water potentials between -0.39 and -1.39 MPa at 0-5 ºC. Even more, A. lampa germinated under all temperature combinations at -1.3 MPa. Despite large varitions among species and environmental conditions, the minimum water potentials at which seeds of species from widely different habitats germinate is surprising. For example, Koller & Hadas (1982) and Lindstrom et al. (1976) quote critical soil matric potentials for germination of crop species such as Zea mays L., Sorghum bicolor L. Moench and Triticum aestivum L. of -1.4, -2.0 and -2.0 MPa, respectively. In rangeland species, results showed are similar to those reported for crops. Roundy (1985) found that Agropyron elongatum (Host.) Beauv. and E. cinereus, common grasses of the Great Basin in North America, both germinated at soil matric potentials of about -2.0 MPa, although germination declined rapidly below -0.5 MPa. Sharma (1976) found similar results for one grass (Danthonia caespitose Gaud.) and two bush species (Atriplex nummularia Lindl. and A. vesicaria Heward ex Benth.). All three species displayed some, although different, germination levels at the lowest tested matric potential of -1.5 MPa. Graded osmotic solutions have been used extensively to determine the effects of water potential on seed germination of different range species. Knipe (1968) found that the effect of water status on germination of Sporobolus airoides (Torr.) Torr., Hilaria jamesii (Torr.) Benth. and Bouteloua gracilis (H.B.K.) Lag. ex Steud. not only varied with species, but that germination occurred down to osmotic potentials of -1.6 MPa. Finally, Hardegree & Emmerich (1990) and Emmerich & Hardegree (1991) observed germination of Bouteloua curtipendula (Michx.) Torr., Panicum coloratum L., Cenchrus ciliaris L. and Eragrostis lehmanniana Nees at osmotic potentials as low as -1.2 MPa in various solutions of salts and polyethylene glycol.
We have to recognize, however, that use of osmotic solutions as a medium for definition of critical water potentials may be misleading because seeds tend to germinate more readily in solutions than in soil (matric) environments (Wright, 1975; Koller & Hadas, 1982). In some cases, results may also be difficult to interpret because of changes in solution water potential during the course of germination (Emmerich & Hardegree, 1991). Thereafter, our understanding of the interactions of seed germination and soil water status is not adequate for most rangeland species and more comprehensive research is needed. However, based on what is currently known about the water relations of seed germination, the land manager does have some important options available that may be useful during range improvement and revegetation efforts. For example, during revegetation of arid or semiarid ranges, seeds of adapted species should be planted during the proper season and at specific depths in the soil to ensure optimum germination (Hull, 1974).
All four species can be ordered according to its germination capacity under all temperature and water stress conditions (sensu Sharma, 1976): A. lampa > S. neaei > L. divaricata > L. erianthus. Our results agree with those of Sharma (1976), who reported the capacity of A. lampa for germinating under extreme temperature and water stress conditions. Our results also suggest that this species could thus germinate after rains in any season of the year. This could represent a competitive advantage for A. lampa in an area with unfavourable environmental conditions.
REFERENCES
1. Blank, R.R. & J.A. Young (1992). Influence of matric potential and substrate characteristics on germination of Nezpar Indian ricegrass. Journal of Range Management 45:205-209. [ Links ]
2. Brown, R. (1995). The water relations of range plants: adaptations to water deficits. In: Bedunah, D.J. and Sosebee, R.E. (eds), pp. 291-413. Wildland Plants: Physiological Ecology and Developmental Morphology. Society for Range Management, Denver, Colorado, USA. 710 p. [ Links ]
3. Dasberg, S. & K. Mendel (1971). The effect of soil water and aeration on seed germination. Journal of Experimental Botany 22:992-998. [ Links ]
4. Duvcovsky, J., A.R. Schlatter & M. Echaide (1997). Genome analysis of South American Elymus (Triticeae) and Leymus (Triticeae) species based on variation in repeated nucleotide sequences. Genome 40: 505-520. [ Links ]
5. Emmerich, W.E. & S.P. Hardegree (1991). Seed germination in polyethylene glycol solution: effects of filter paper exclusion and water vapor loss. Crop Science 31:454-458. [ Links ]
6. Evans, R.A. & J.A. Young (1983). `Magnar´ Basin Wildrye-germination in relation to temperature. Journal of Range Management 36:395-398. [ Links ]
7. Giusti, L. & A. Grau (1983). Inhibidores de la germinación en Atriplex cordobensis Gand. Et Stucker (Chenopodiaceae). Lilloa 36:143-149. [ Links ]
8. Grossi Gallegos, H. (1998a). Distribución de la radiación solar global en la República Argentina. I. Análisis de la información. Energías Renovables y Medio Ambiente 4:119-123. [ Links ]
9. Grossi Gallegos, H. (1998b). Distribución de la radiación solar global en la República Argentina. II. Cartas de radiación. Energías Renovables y Medio Ambiente 5:33-42. [ Links ]
10. Hardegree, S.P. & W.E. Emmerich (1990). Partitioning water potential and specific salt effects on seed germination of four grasses. Annals of Botany 66:587-595. [ Links ]
11. Hull, A.C. (1974). Species for seeding arid rangeland in southern Idaho. Journal of Range Management 27:216-218. [ Links ]
12. Jones, T.A. & D.C. Nielson (1994). Indian ricegrass (Oryzopsis hymenoides) germination affected by irrigation and bagging during seed production. Arid Soil Research and Rehabilitation 8:269-275. [ Links ]
13. Kania, S. & G. Giacomelli (2005). Solar radiation availability for plant growth in Arizona controlled environment agriculture systems. http://ag.arizona.edu/ceac/research/archive/solar-radiation_Kania.pdf. [ Links ]
14. Knipe, O.D. (1968). Effects of moisture stress on germination of alkali sacaton, galleta, and blue grama. Journal of Range Management 21: 3-4. [ Links ]
15. Koller, D. & A. Hadas (1982). Water relations in the germination of seeds. In: Lange, O.L., Nobel, P.S., Osmond, C.B. and Ziegler, H. (eds), pp. 401-431. Encyclopedia of Plant Physiology. New Series, Vol. 12B. Physiological Plant Ecology II. Water Relations and Carbon Assimilation. Springer-Verlag, Berlin. [ Links ]
16. Linsdstrom, M.J., R.I. Papendick & F.E. Koehler (1976). A model to predict winter wheat emergence as affected by soil temperature, water potential, and depth of planting. Agronomy Journal 68:137-141. [ Links ]
17. Mac Mahon, J.A. & D.J. Schimpf (1981). Water as a factor in the biology of North American plants. In: Evans, D.D. and Thames, J.L. (eds), pp. 114-171. Water in Desert Ecosystems. USIBP Synthesis Series 2. Hutchinson and Ross, Inc., Dowden. [ Links ]
18. McDonough, W.T. & R.O. Harniss (1974). Effects of temperature on germination in three subspecies of big sagebrush. Journal of Range Management 27:204-205. [ Links ]
19. Mc Ginnies, W.J. (1960). Effects of moisture stress and temperature on germination of six range grasses. Agronomy Journal 52:159-162. [ Links ]
20. Meyer, S.E., J. Beckstead, P.S. Allen & H. Pullman (1995). Germination ecophysiology of Leymus cinereus (Poaceae). International Journal of Plant Sciences 156:206-215. [ Links ]
21. Michel, B.E. & M.R. Kaufmann (1973). The osmotic potential of polyethylene glycol 6000. Plant Physiology 51:914-916. [ Links ]
22. Owens, D.W. & C.A. Call (1985). Germination characteristics of Helianthus maximilianai Schard. and Simsia calva (Engelm & Gray). Journal of Range Management 38:336-339. [ Links ]
23. Peretti, A. (1994). Manual para el análisis de semillas. INTA-UNMar del Plata. 1ª Ed. Editorial Hemisferio Sur, Buenos Aires. [ Links ]
24. Raven, P.H., R.F. Evert & S.E. Eichhorn (1986). Biology of plants. Worth Publishers, Inc., New York. [ Links ]
25. Romo, J.T. & L.E. Eddleman (1988). Germination of green and gray rubber rabbitbrush and their establishment on coal mined land. Journal of Range Management 41:491-495. [ Links ]
26. Romo, J.T., P.L. Grilz, C.J. Bubar & J.A. Young (1991). Influences of temperature and water stress on germination of plains rough fescue. Journal of Range Management 44:75-81. [ Links ]
27. Roundy, B.A. (1985). Emergence and establishment of Basin wildrye and tall wheatgrass in relation to moisture and salinity. Journal of Range Management 38:126-131. [ Links ]
28. Sharma, M.L. (1976). Interaction of water potential and temperature effects on germination of three semi-arid plant species. Agronomy Journal 68:390-394. [ Links ]
29. Wright, L.N. (1975). Improving range grasses for germination and seedling establishment under stress environments. In: Campbell, R.S. and Herbel, C.H. (eds), pp. 3-22. Improved Range Plants. Range Symp. Series No. 1. Society for Range Management, Colorado. [ Links ]
30. Young, J.A., C.D. Clements & T.A. Jones (2003). Germination of seeds of robust needlegrass. Journal of Range Management 56:247-250. [ Links ]














