Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) v.77 Vicente López ene./dic. 2008
ARTÍCULOS ORIGINALES
Vernonia patensKunth, an Asteraceae species with phototoxic and pharmacological activity (With 3 Tables)
Vernonia patens Kunth, una especie de Asterácea con actividad fototóxica y farmacológica (Con 3 Tablas)
Pérez-Amador1 MC, V Muñoz Ocotero1, S Pérez Benitez1, F García Jiménez2
1 Facultad de Ciencias, Departamento de Biología.
2 Instituto de Química. Universidad Nacional Autónoma de México. Ciudad Universitaria. 04510. México, D.F. México.
Address Correspondence to: Dr. Maria Cristina Pérez-Amador; Facultad de Ciencias. UNAM. Circuito Exterior s/n Ciudad Universitaria. C.P. 04510. México D.F.; e-mail: perez_amador@yahoo.com, mcpa@hp.fciencias.unam.mx.
Recibido/Received 20.05.2008. Aceptado/Accepted 12.08.2008.
Abstract. The presence of phototoxic compounds in stems and leaves of young plants of Vernonia patens Kunth was confirmed by TLC. These compounds were in smaller amounts in younger than in adult plants of this species. Only the stems presented specific activity of these compounds from the two study plant organs. It included characteristic UV bands at 200 and 300nm, and phototoxic activity against Bacillus subtilis (ATCC-6633). Stems and leaves of Vernonia patens also showed anti-inflammatory activity and bactericide potential.
Key words: Asteraceae; Phototoxic compounds; Bactericide activity; Anti-inflammatory activity.
Resumen. Se estableció la presencia de compuestos fototóxicos en tallos y hojas de plantas jóvenes de Vernonia patens mediante TLC. Estos compuestos se encontraron en menor cantidad en plantas jóvenes que en plantas adultas de esta especie. De las dos partes estudiadas (tallos y hojas), sólo los tallos presentaron la actividad específica de estos compuestos: bandas características al UV entre 200 y 300 nm, y actividad fototóxica frente a Bacillus subtilis (ATCC-6633). Los tallos y hojas de Vernonia patens también mostraron actividad antiinflamatoria y potencial bactericida.
Palabras clave: Asteraceae; Compuestos fototóxicos; Actividad bactericida; Actividad antiinflamatoria.
INTRODUCTION
In our systematic study of species of the Asteraceae family, searching for phototoxic compounds which could be taxonomic markers, we analyzed Vernonia patens plants.
Since this species have a confirmed pharmacological activity, we also determined the biological activity of the extracts: anti-inflammatory and bactericidal potentials.
MATERIALS AND METHODS
Plant material. Plants were collected in Nauzontla, Puebla, Mexico, on 13 March 2006. This is the time when this species starts flowering. Harvested plants had a very small root system. From these plants, we studied the stems and the leaves. Voucher specimens were deposited in the National Herbarium, Instituto de Biología, UNAM (MEXU).
Preparation of extracts. Plants were divided in stems and leaves. Extracts were prepared from these plant parts using hexane, ethyl acetate and methanol; the solvents were eliminated at reduced pressure. The dry extracts were used for the different tests.
Phototoxic compounds. Presence of polyacetylenes and thiophenes was evident from the hexane extracts by TLC. This was confirmed by (1) the UV spectrum of the extract, with characteristic bands, and (2) by its bactericidal potential, after being irradiated with UV-light (Daniels, 1965).
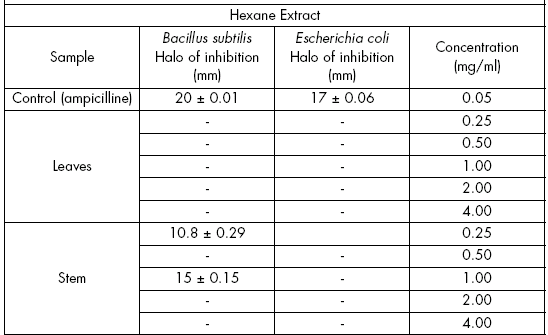
Antibacterial potential. From the three extracts of stems and leaves, the antibacterial activity was measured against Bacillus subtilis (ATCC-6633) and Escherichia coli (ATCC-6051).
The bactericidal test was performed by the paper disc diffusion method (Cavalieri, 2005). Petri dishes with agar containing a bacterial concentration of 106 UFC were used with this purpose. Hexane, ethyl acetate and methanol were essayed each at concentrations of 0.25, 0.5, 1, 2, or 4 mg/ml. Petri dishes were incubated at 37 ºC during 24h. Anhydrous ampicilline (0.05 mg) (Sigma) was used as a positive control. Each assay was repeated three times.
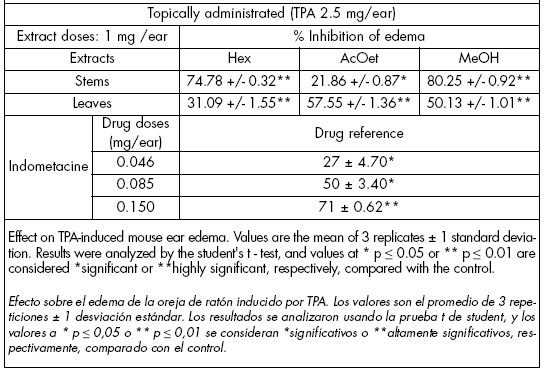
Anti-inflammatory test. The bioassay was conducted by the mouse ear edema test induced with TPA (12-O-tetradecanoyl-phorbol-13-acetate) (De Young et al., 1989). For each determination, three male CDI mice (25-30 g) were used; 10 μL of an ethanolic TPA solution (Table 3) were applied on the surface of the right ear. The same amount of ethanol (control) was applied on the left ear. Ten minutes after application of the TPA, 20 μL of the extracts were applied topically (1 mg dissolved in ethanol). After 4h, mice were sacrificed and an ear section (7 mm) was cut off and weighed. Increases in weight of the right compared to the left ears indicated swelling. Percentage swelling inhibition was calculated by comparison with the control (left ear). Indometacine (0.046, 0.085, 0.15 mg/ear) was used as a drug reference.
Table 3. Anti-inflammatory activity of V. patens.
Tabla 3. Actividad antiinflamatoria de V. patens.
Statistical analysis. When corresponding, results were analyzed using the Student's t test (Steel & Torrie, 1985). Differences were considered either significant or highly significant with respect to the control when p ≤ 0.05 or p ≤ 0.01, respectively.
RESULTS
Phototoxic compounds. The hexane extracts contained the phototoxic compounds, which were confirmed by TLC and developed with ceric sulphate. The UV spectrum gave the characteristic bands of these compounds at 200 and 300 nm (Table 1), and the phototoxic activity showed an inhibition halo at different concentrations. Only the stem extract was active, giving an inhibition halo of (1) 7.9 mm (39.5%) at a concentration of 0.25 mg/ml, and (2) 8.9 mm (44.5%) at a concentration of 1 mg/ml, compared to ampicilline (control). It gave an halo of 20 mm (100%) at a concentration of 0.05 mg/ml.
Table 1. Characteristic U.V. absorption of polyacetylenes.
Tabla 1. Absorción de U.V. característica de poliacetilenos.

Antibacterial potential. It was performed with hexane, ethyl acetate and methanol extracts against Bacillus subtilis (ATCC-6633) and Escherichia coli (ATCC-6051) (inoculation 106 UFC). Ampicilline was used as a control at a concentration of 0.05 mg/ml.
In the hexane extract, leaves showed no activity against Bacillus subtilis at concentrations of 0.25, 0.5, 1.0, 2.0 and 4.0 mg/ml. Stems had activity at 0.25 and 1 mg/ml, with an inhibition halo of 10.8 mm (54%) and 15 mm (75%), respectively, while the inhibition halo was 20 mm in the control. Stems and leaves did not present activity against Escherichia coli (Table 2).
Table 2. Antibacterial activity of leaf and stem extracts of V. patens.
Tabla 2. Actividad antibacteriana de extractos de hoja y tallo de V. patens.

The AcOEt extract from leaves presented activity against B. subtilis at a concentration of 4 mg/ml (halo of 7 mm, 35% inhibition), and against E. coli at concentrations of 1, 2 and 4 mg/ml (halo of 6, 7 and 8 mm, respectively, which determined 30, 35 and 40% inhibition of bacterial growth, respectively). Stems presented an inhibition halo of 7.2 mm (36%) at a concentration of 4 mg/ml against B. subtilis. The inhibition halo was of 6.3, 6.5, 7.6, 7.9 or 9.6 mm at concentrations of 0.25, 0.50, 1.0, 2.0 or 4 mg/ml, respectively, against E. coli. This determined 31.5, 32.5, 38, 39.5 and 48% growth inhibition, respectively (Table 2).
The leaf methanolic extract produced an halo of inhibition of 9.3 and 10 mm (46.5 and 50%) at 2 and 4 mg/ml concentrations, respectively, against B. subtilis. At the same concentrations, the inhibition halo was of 8.9 (2 mg/ml) and 9.2 (4 mg/ml) mm with 44.5 and 46% of bacterial growth inhibition, respectively, against E. coli. The stem extract showed an halo of 7, 8 and 10.3 mm (35, 40 and 51% growth inhibition, respectively), at concentrations of 1, 2 and 4 mg/ml, respectively, against B. subtilis. Against E. coli, the halo was from 6.3 to 9.9 mm at concentrations from 0.25 to 4.0 mg/ml. Growth inhibition ranged from 31.5 to 49.5% (Table 2).
Anti-inflammatory test. It was performed with the stem and leaf extracts by the mouse ear edema test with TPA. Inhibition of the edema using plant stems were 74.8, 21.9 and 80.2% with the hexane, ethyl acetate and methanolic extracts, respectively (Table 3). In leaves, edema inhibition was 31.1% using the hexane extract; 57.5% using the ethyl acetate extract, and 50.1% utilizing the methanolic extract. These results highlight the anti-inflammatory activity of the extracts.
DISCUSSION
Phototoxic compounds. It is interesting the amount of phototoxic compounds that leaves and stems have developed at the flowering phenological stage.
Phototoxic compounds were found in stems and leaves, and their presence was made evident by TLC. The characteristic pale blue color of these compounds appeared when the plate was developed with ceric sulphate. It is more intense in stems than leaves. Their presence was also confirmed by the UV spectrum of the hexane extracts, and by the phototoxic activity against Bacillus subtilis, using the Petri dish agar method (Daniels, 1965).
The amount of phototoxic compounds at the initiation of the flowering stage was smaller than that in other Asteraceae at flowering time. This suggests that small changes at the study phenological stage may greatly influence the obtained results.
The only active parts were the stems; presence of the compounds was confirmed by their UV spectrum and phototoxic activity.
Antibacterial activity. Plant leaf and stem extracts (hexane, ethyl acetate and methanol) have different activity against Escherichia coli (ATCC-6051) or Bacillus subtilis (ATCC-6633).
The hexane extract from leaves did not have activity against both bacteria species. Although plant stems had a good activity against B. subtilis, they did not have any activity against E. coli.
Ethyl acetate extracts from leaves and stems inhibited growth of both bacteria species. Stems, however, showed more activity against E. coli than against B.subtilis. Leaf and stem methanolic extracts were active against E. coli and B.subtilis, although stems were more active than leaves.
These results indicate that leaf and stem methanolic extracts show the highest activity against the study bacteria. Ethyl acetate extracts from leaves and stems had also good activity against E. coli. While stem hexane extracts have little activity against bacteria, there was no effect when using leaf hexane extracts.
Anti-inflammatory activity. The three study extracts (hexane, ethyl acetate and methanol) were active. Extracts from the stem were the most active, showing a higher ear edema inhibition than indometacine (0.15 mg/ear) (drug reference). Leaf ethyl acetate and methanol extracts were more active than indometacine (0.085 mg/ear) (Table 3).
CONCLUSION
The high inhibitory values of the hexane, ethyl acetate and methanol extracts from the stems of Vernonia patens clearly demonstrate that this plant has a good pharmacological activity. In the near future, it could be used as a medicinal plant for the treatment of inflammatory processes.
REFERENCES
1. Cavalieri, S. J. (2005) Drug Resistance, Bacterial-Laboratory Manuals. Departments of Laboratory Medicine and Microbiology. University of Washington. Seattle, Washington. 248 p. [ Links ]
2. Daniels, F.J. (1965). A simple microbiological method for demonstrating phototoxic compounds. Journal of Investigative Dermatology 44: 259-263. [ Links ]
3. Steel, R.G. & J.H. Torrie (1985). Bioestadística: principios y procedimientos. Mc Graw-Hill, Bogotá. 622 p. [ Links ]
4. De Young, L.M., Kheifets, J.B., Ballaron, S.J. & Young, J.M. (1989). Edema and cell infiltration in the phorbol ester-treated mouse ear are temporarily separate and can be differentially modulated by pharmacological agents. Agents and Action 26: 335-341. [ Links ]














