Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Phyton (Buenos Aires)
versão On-line ISSN 1851-5657
Phyton (B. Aires) vol.79 no.2 Vicente López jul./dez. 2010
ARTÍCULOS ORIGINALES
Leaf angle and light interception in sunflower (Helianthus annuus L.). Role of the petiole's mechanical and anatomical properties
Ángulo e intercepción de luz en las hojas de girasol (Helianthus annuus L.). Rol de las propiedades mecánicas y anatómicas del pecíolo
Hernández LF1
1 Laboratorio de Morfología Vegetal. Depto. de Agronomía, Universidad Nacional del Sur. Bahía Blanca, 8000, CIC Provincia de Buenos Aires, La Plata, 1900. Argentina.
Address Correspondence to: Luis Hernández, e-mail: lhernan@criba.edu.ar Fax: +54 - 0291 - 4595127
Recibido - Received 19.V.2010.
Aceptado - Accepted 03.VI.2010.
Abstract. The relationships between (1) leaf biomass and morphology (lamina area and petiole and lamina inclination), (2) petiole's mechanical and structural properties, and (3) the vertical light gradient inside the crop's canopy were studied in field grown sunflower (Helianthus annuus L.) plants, maintained at optimum soil water and mineral levels. At flowering, incident photosynthetic active radiation (PAR) was measured at the top of the canopy and on individual leaves using a quantum sensor. The fraction of direct incident radiation which passed through the canopy reaching each individual leaf was then calculated. Individual petiole and lamina inclination angles (iaPetiole and iaLamina, respectively) were measured from sequential digital images. These were taken rotating plants and using a stationary digital photographic camera. Petiole length and lamina area were measured after detaching leaves from each plant. Leaves were separated in petiole, lamina and main veins, and their dry mass obtained. Petiole transverse cuts stained with acid fluoroglucinol were used to measure the relative area occupied by lignified and fibrous tissues. The petiole's structural Young's modulus (EPetiole) for different leaves was calculated from a three-point bending test. This was performed in petiole segments 4.0 to 8.0 cm length. Petiole flexural stiffness (EIPetiole) was calculated using elementary beam theory for homogenous materials. Intercepted PAR in the canopy for individual leaves decreased basipetally. The iaPetiole increased acropetally from -9.0° to +60.0°, while the iaLamina increased basipetally from +1.0° to -60.0° in concordance with increments in the intercepted PAR. Petiole specific weight per tranverse sections (g/cm2) did not change with leaf position whilst lamina specific weight decreased acropetally. Main veins dry weight increased basipetally. EPetiole and EIPetiole increased acropetally. There was a positive relationship between intercepted PAR and the ratio dry weightPetiole/ dry weightLamina. The relative area occupied by supporting tissue was significantly higher in upper petioles than in lower ones. These results suggest that, in order to optimize incident PAR interception, sunflower plants invest more energy in differentiating supporting tissues in the petioles of the upper canopy. This determines that the higher canopy strata has a preferentially planophyllous/erectophyllous leaf architecture.
Keywords: Biomechanics; Helianthus; Leaf angle; Light interception; Sunflower.
Resumen. Se estudiaron (1) las relaciones entre la biomasa foliar y su morfología (área e inclinación de la lámina y ángulo de inclinación del pecíolo), (2) las propiedades mecánicas y estructurales de los pecíolos, y (3) el gradiente vertical de luz dentro del canopeo en plantas de girasol (Helianthus annuus L.) que crecieron a campo, en condiciones óptimas de agua edáfica y nutrientes. A la antesis, la radiación fotosintéticamente activa (RFA) incidente fue medida con un sensor cuántico al tope del canopeo y en hojas individuales. De esta forma se calculó la fracción de la radiación incidente que pasó a través del canopeo y llegó a cada hoja. La inclinación de los pecíolos y las láminas (iaPetiole y iaLamina, respectivamente) fúe medida sobre imágenes digitales tomadas con una cámara fotográfica digital sobre una secuencia de plantas giradas sobre su eje. La longitud de los pecíolos y el área de las láminas fueron medidos luego de remover las hojas de las plantas. Las hojas fueron separadas en pecíolos, láminas y nervaduras principales obteniéndose su peso seco. Cortes transversales de pecíolos fueron teñidos con fluoroglucinol para medir la superficie relativa ocupada por tejido lignificado. El módulo de elasticidad de Young en los pecíolos (EPetiole), para las diferentes hojas del canopeo, fue calculado mediante la prueba de flexión de tres puntos realizada en segmentos de pecíolo de 4,0 a 8,0 cm de longitud. La rigidez estructural de los pecíolos (EIPetiole) fue calculada utilizando los principios de la teoría elemental de vigas para materiales homogéneos. La RFA en el canopeo para las hojas individuales disminuyó basípetamente. El iaPetiole se incrementó acrópetamente de -9,0° a +60,0°, mientras que el iaLamina aumentó basípetamente de 1,0° a -60,0° acorde a los incrementos de la RFA interceptada. El peso específico por área transversal de los pecíolos (g/cm2) no varió con la ubicación de la hoja en el canopeo mientras que el peso específico de la lámina disminuyó acrópetamente. El peso seco de las nervaduras principales se incrementó basípetamente. El EPetiole y el EIPetiole aumentaron acrópetamente. Hubo una correlación positiva entre la RFA interceptada y el índice Peso seco del pecíolo/Peso seco de la lámina. El área relativa ocupada por tejido de sostén fue significativamente mayor en los pecíolos superiores que en los inferiores. Estos resultados indican que el girasol invierte más energía en la generación de tejido de sostén en los estratos altos del canopeo, lo que le permite mantener una arquitectura foliar plano-erectófila, y al mismo tiempo optimizar la intercepción de la RFA incidente.
Palabras clave: Biomecánica; Helianthus; Girasol; Inclinación foliar; Intercepción de luz.
INTRODUCTION
Plants can maximize canopy light interception by increasing both leaf surface area and the efficiency of light interception for each unit of leaf area (Huber & Stuefer, 1997; Knapp& Smith, 1997).
Interception efficiency of both direct and diffuse irradiance increases on leaves with horizontal laminae (planophyllous leaf architecture; Pearcy & Valladares, 1999; Valladares& Pearcy, 2000).
As leaves adapt to a light gradient inside the canopy, not only the inclination angles but also the morphology, anatomy, size and mass of their petioles and laminas are modified. Leaf inclination angles generally decrease with increasing leaf age. This is generally attributed to increases in both lamina weight and area (Hamerlynck & Knapp, 1996). These can alter the bending momentum exerted on the petiole, changing lamina and/or petiole inclination angles (Niinemets & Fleck, 2002; Huber at al., 2008).
Petiole mechanical properties, as a result of changes in its material properties, and/or by modifications in the cross-sectional shape and dimensions (Niklas, 1992), may change along the vertical light gradient, allowing modifications of the lamina inclination angle (Niklas, 1992; Niinemets & Fleck, 2002). There is evidence that petioles are stiffer at higher irradiance (Niinemets, 1998; Niinemets & Fleck, 2002) as the result of larger biomass investments in supporting tissue (Niklas, 1992; Weiijschedé et al., 2006).
In sunflower, biomass production and yield are positively coupled with solar radiation intercepted by the canopy (Connor& Hall, 1997). Fixing of a priori canopy parameters that could be associated with a better solar radiation interception is necessary to redesign a plant's ideotype if an increase in yield is expected after culturing sunflower in narrow rows or high densities (Andrade et al., 2002).
Objectives of this work were to study (1) the role of petiole mechanical properties on foliar inclination, and the relationships between (2) leaf and petiole angle variation patterns along the canopy, and (3) leaf and petiole biomass partitioning and morphology in field grown sunflower plants.
MATERIALS AND METHODS
Sunflower (Helianthus annuus L.) plants (hybrid cultivar Macón, Syngenta Seeds, Argentina), were grown at the experimental field of the Agronomy Department-UNSur, Bahía Blanca, Argentina (38°45'S; 62°11' W). The soil was a Typic Ustipsamment (Soil Survey Staff, 1999). At 4-leaf stage (Schneiter & Miller, 1981) plant density was adjusted to 5.6 plants/m2 (Fig. 1A). The crop was managed according to recommended conventional agronomical practices. Weeds and insects were adequately controlled. Soil water content and mineral nutrition were maintained at optimum levels by drip irrigation and fertilization (NPK: 30-30-30; 80 kg/ha) at sowing and anthesis (Schneiter & Miller, 1981).

Fig. 1. (A) Panoramic view of the crop at anthesis, time when leaf petiole and lamina angles measurements, and biomechanical analysis were done. (B) Plant removed from the planting site with the root system placed in a bucket with water, ready to start the photographic recording. Inserted in the image is a schematic detail showing the criteria used to measure the inclination angle of each lamina (iaLamina) and its petiole (iaPetiole) using the software Screen Protractor (SP).
Fig. 1. (A) Vista panorámica del cultivo a la antesis, momento en el cual se realizó la medición del ángulo de los pecíolos y las láminas, y el análisis biomecánico. (B) Una de las plantas removidas del cultivo con el sistema radical colocado en un balde con agua, lista para iniciar el registo fotográfico. Inserto en la imagen se presenta un detalle mostrando el criterio utilizado para medir la inclinación de cada lámina (iaLamina) y su pecíolo (iaPetiole), utilizando el programa Screen Protractor (SP).
Before first anthesis (Schneiter & Miller, 1981) a group of plants were selected for uniform size and developmental stage. PAR was measured at noon using a quantum sensor (LI-COR, Lincoln NE, USA) on different leaves in six of these selected plants (n=6). Measurements were made ten days before and at the anthesis phenological stage. The maximum incident PAR was 100% for the topmost leaves. The fraction of incident PAR (fIPAR) for each leaf inside the canopy was calculated (Sadras et al., 2000). fIPAR readings were averaged for both developmental stages.
Following the second PAR measurement and at predawn, selected plants were removed with a shovel from the experimental planting site one at a time, retaining almost 90% of the root plate (soil-root system close to the stem base; Sposaro et al., 2008). They were immediately transferred to a 15-L plastic bucket containing tap water. The submerged soil-root system allowed to maintain the whole plant in a vertical position while keeping a short-time optimum leaf water status (Fig. 1B). A 5.0 Mpixel digital camera mounted on a tripod was located at 7 m from the sampled plant, pointing at its centre and operated by a collaborator. The plant was then rotated clockwise 360° around its stem at 20° intervals, and three high resolution pictures of the whole plant were taken per rotating position (center, top and bottom). Time spent for each plant from removal to the last picture taken did not exceed 100 s.
Thereafter, all leaves for each plant were labeled, removed, packed in wet tissue paper, placed in plastic bags and transported immediately to the laboratory for biomechanical and morphological measurements.
In the laboratory each leaf lamina was quickly scanned using a desktop scanner. Afterwards the area of each leaf was measured on the scanned images using the software ImageJ (Rasband, 2008).
Leaf samples were separated with a razor blade in petiole, whole lamina, and laminar tissue with the main veins removed. These plant tissues were then oven-dried at 70 °C for 48 h, and dry weight obtained.
Digital micrographs of transverse hand cuts of petioles from leaves at two canopy levels (upper and lower) were stained with acid phluoroglucinol (Ruzin, 1999) to visualize lignified tissues. This allowed to calculate the relative area occupied by different biostructural components, mainly parenchymatic, vascular and supporting tissues (Fig. 2).

Fig. 2. Transverse cuts of petioles nearby the shoot node (shoot side) and laminar base (laminar side) showing its anatomy and distribution of supporting tissues (COL: collenchyma) for leaves 3 (A-C, upper) and 18 (B-D, lower). Note the increased number of collenchyma layers (CL) in upper petioles (A) than in the lower ones (B). Bars: A-D = 5.0 mm; E-F = 150 μm.
Fig. 2. Cortes transversales de los pecíolos cercanos al nudo y a la base de la lámina de las hojas 3 (A-C, superior) y 18 (B-D, inferior), mostrando su anatomía y la distribución de los tejidos de sostén (COL: colénquima). Nótese el mayor número de estratos de colénquima (CL) en los pecíolos superiores (A) con respecto a los inferiores (B). Escala: A-D = 5,0 mm; E-F = 150 μm.
Petiole specific biomass (dry biomass per unit volume and per transverse surface area) and dry matter concentration (dry biomass per unit volume) were calculated from petiole dry mass, length, and cross-section areas. Lamina specific biomass (dry biomass per unit lamina surface area) was also calculated. The Young's elastic modulus of the petioles (EPetiole; Niklas, 1992) was calculated using a three-point bending method as described by Liu et al. (2007). E and I were not measured and calculated for the lamina because of the lack of a reliable model to approximate the load of lamina on the petiole (Niklas, 1992; Niinemets & Fleck, 2002). Sections of the medium part of the petiole (4 cm from the upper leaves to 8 cm from the lower leaves) were placed horizontally over two supports that were 2 to 4 cm apart. Vertical applied forces (F, N) and resulting deflections (d, m) were recorded using a testing machine as described in Hernández & Bellés (2006). Young's modulus was calculated as follows (Gere& Timoshenko, 1997):
where L is the length between the supports (m) and IPetiole the axial second moment of area (m4) (Gere & Timoshenko, 1997). For all leaves, IPetiole was calculated from the petiole cross-sectional dimensions assuming it was a sector of a circular ring (see Fig. 1C):
where R, the radius of irregular petioles (see Fig. 2 A-D), was estimated using a digital caliper as an average of three perpendicular measurements.
The flexural stiffness of the petiole was calculated as the product of EPetiole and IPetiole (MN m2; Niklas, 1992; Vogel, 1992). The petiole and lamina inclination angles (iaPetiole and iaLamina, respectively; Fig. 3) for each leaf of each plant was obtained using the digital images of the rotated plants [registered as described above (Fig. 1B)] using the software Screen Protractor (Iconico Inc.; http://www.iconico.com/protractor/). The 3° to 4° parallax error generated in each picture between the upper and lower ends of each plant was corrected for each reading of lamina or petiole inclination angle straightening each set of three pictures per rotating position with the software PTGui (New House Internet Services B.V.; http://www.ptgui.com/). Linear regression analyses between different parameters were performed using the software Kaleidagraph v.4.1 (Synergy Software).

Fig. 3. Example of the measurements of petiole (iaPetiole) and lamina (iaLamina) inclination angles from digital images of each plant's leaf.
Fig. 3. Ejemplo de las mediciones del ángulo de inclinación del pecíolo (iaPetiole) y de la lámina (iaLamina) en las imágenes digitales de cada hoja.
RESULTS AND DISCUSSION
There was a decreasing vertical light gradient along the plant canopy (Fig. 4A). Leaf area and lamina weight increased almost linearly from top nodes (leaf No. 1) to central canopy nodes (leaf No. 13; Fig. 4B). Thereafter, lamina dry weight (excluding petiole) slightly decreased towards the bottom canopy nodes (Fig. 4B).

Fig. 4. (A) Fraction of incident PAR (μmol/m2/s) measured at each leaf level inside the crop with a quantum sensor. (B) Individual leaf mass and leaf area in the plants used in this work. Bars for each point (mean of n=6): ± 1S.E.
Fig. 4. (A) Fracción de la RFA incidente (μmol/m2/s) medida al nivel de cada hoja dentro del cultivo con un sensor cuántico. (B) Biomasa y área de cada hoja en las plantas utilizadas en este trabajo. Las barras en cada punto (promedio de n=6) indican ± 1E.E.
The upper leaves had shorter petioles (Table 1). These petioles also had a higher Young's elastic modulus (Fig. 5A; Table 1). The aiPetiole increased acropetally (more PAR intercepted) from -9° to +60° while the aiLamina increased basipetally from +1° to -60° (Fig. 5B). The petioles specific biomass (BspPetiole) did not change along the canopy levels while the lamina specific biomass (BspLamina) decreased acropetally (Fig. 5C). Main veins biomass increased basipetally (Fig. 5D). Magnitudes of EPetiole and EIPetiole increased acropetally (Fig. 5A,E). The relationship BspcPetiole/BspcLamina was positively correlated with the fIPAR (Fig. 5) being significantly higher from leaf 7 upwards (Fig. 5F).
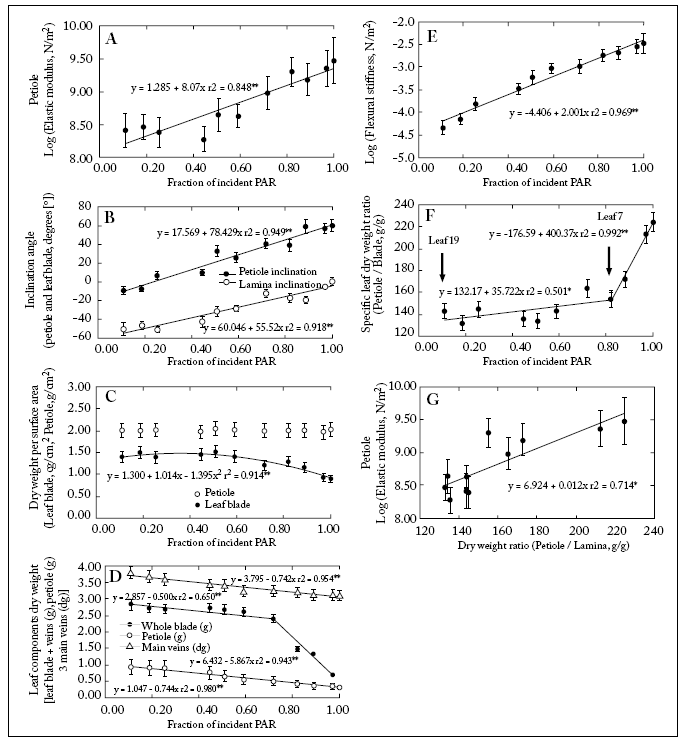
Fig. 5. (A) Log of EPetiole vs. fIPAR; (B) Lamina and petiole inclination (°) vs. fIPAR; (C) Lamina and petiole specific biomass vs. fIPAR; (D) Biomass of leaf components blade, petiole and main veins vs. fIPAR; (E) Log of EIPetiole vs. fIPAR; (F) relationship between the petiole mass (g)/ lamina specific biomass (g/g) vs. fIPAR; (G) Log of EPetiole vs. the relationship petiole biomass/lamina biomass. Bars for each point (mean of n=6):± 1S.E. Linear fits are included.
Fig. 5. (A) Logaritmo del EPetiole vs. fIPAR; (B) Inclinación de la lámina y del pecíolo (°) vs. fIPAR; (C) Biomasa específica de la lámina y del pecíolo vs. fIPAR; (D) Biomasa de los componentes foliares lámina, pecíolo y nervaduras principales vs. fIPAR; (E) Log del EIPetiole vs. fIPAR; (F) relación entre el cociente biomasa del pecíolo (g)/ biomasa específica de la lámina (g/g) vs. fIPAR; (G) Log del EPetiole vs. la relación biomasa del pecíolo (g)/ biomasa de la lámina (g). Las barras en cada punto (promedio de n=6) indican ± 1S.E. Se incluyen los ajustes lineales.
Table 1. Main anatomical parameters of petioles on leaves number 3 (upper canopy) and 18 (lower canopy). These can be associated with its mechanical properties and were used to calculate its flexural stiffness (EIPetiole). For petioles located at intermediate levels within the canopy, E was extrapolated assuming a linear relationship.
Tabla 1. Principales caracteres anatómicos de los pecíolos en la hoja número 3 (canopeo superior) y 18 (canopeo inferior). Estos pueden asociarse con sus propiedades mecánicas y fueron utilizados para calcular su rigidez estructural (EIPetiole). Para los pecíolos ubicados en los estratos intermedios del canopeo, E fue extrapolado asumiendo la existencia de una relación lineal.

A positive correlation was also found between lamina and petiole inclination angle and the fIPAR. However, leaves in the upper canopy were more upwardly inclined, whilst those in the lower canopy were bent downwards (Fig. 5B). This suggests that the light interception efficiency did decrease with decreasing light availability towards the lower canopy strata. This allows to speculate that a limited investment of biomass was taking place in lower petioles for mechanical support (Fig. 5G). In fact, transverse sections of upper petioles showed a higher proportion of supporting tissue (collenchyma and schlerenchyma) than lower ones (Fig. 2C-D).
Mechanical properties of petioles and other plant tissues can also be determined by their hydrostatic pressure (Niklas, 1992). Water stress in sunflower increases with increasing height in the canopy (Black, 1979; Rawson, 1979; LoGullo et al., 2004) driving to lower water potentials in the intensively transpiring, upper canopy leaves. This subsequently reduces the elastic modulus of all involved tissues, lamina and petiole (Niklas, 1992; Smith & Ennos, 2003). Even though leaf water potential was not measured in this work, a comparison of the number and diameter of xylem vessels between the upper and lower petioles did not show relevant differences. Lamina surface areas increased downwards. This suggests that tissue turgor and the tissue elastic modulus could not be maintained at the same magnitude in the lower as in upper leaves. This could help to explain the higher values of elastic modulus for petioles from the upper canopy leaves (Table 1, Fig. 5A).
CONCLUSION
It was demonstrated that with increasing light availability, steeper inclination angles in the upper canopy were more convenient for the plant photosynthetic balance in a broad leaf crop plants like sunflower (Hernández & Orioli, 1983; Connor & Hall, 1997). Such canopy architecture gives more uniform distribution of light within the canopy, allowing a greater exposure of the photosynthetic leaf surface area to light (Valladares & Pearcy, 2000; Rey et al., 2008).
Steeper leaf angles in the upper canopy also lead to a reduction in midday heat-loads, thereby increasing water use efficiency and decreasing the risk of overheating (King, 1997).
Results of this study suggest that to optimize PAR interception, sunflower plants invest part of the captured energy in synthesizing petiole structural, supporting material. Petiole mechanical properties changed along the vertical light gradient, allowing modification of the lamina inclination angle for a common lamina load and petiole length. Vertical deflection of the leaf lamina was reduced by increases in the petiole elastic modulus via changes of the petiole material properties.
This investment has a multiplicative component from base to apex. Thus, petioles of the upper canopy showed more supporting tissue (collenchyma and vascular fibers) than lower ones. As a result of this the upper canopy showed a preferentially planophyllous to erectophyllous architecture.
ACKNOWLEDGEMENTS
This work was funded by grants to L.F.H. of the Secretaría Gral. de Ciencia Tecnología (SeGCyT-UNS, Bahía Blanca) and the Comisión de Investigaciones Científicas (CIC, La Plata), both from Argentina. The author thanks Mrs. M. Abrego for field assistance, Ms. M.C. Franchini for laboratory assistance and Ms. M.C. Moreno (CIC-PBA) for reviewing the manuscript.
REFERENCES
1. Andrade, F.H., P. Calviño, A. Cirilo & P. Barbieri (2002). Responses to narrow rows depend on increased radiation interception. Agronomy Journal 94: 975-980. [ Links ]
2. Black, C.R. (1979). The relative magnitude of the partial resistances to transpirational water movement in sunflower (Helianthus annuus L.). Journal of Experimental Botany 30: 245-253. [ Links ]
3. Connor, D.J. & A.J. Hall (1997). Sunflower physiology. In: Sunflower Techonology and Production. A.A. Schneiter (Ed.), ASA, CSSA, SSSA, Wisconsin, p.113-182. [ Links ]
4. Gere, J.M. & S.P. Timoshenko (1997). Mechanics of Materials. 4th edition. PWS, Boston. [ Links ]
5. Hamerlynck E.P. & A.K. Knapp (1996). Early season cuticular conductance and gas exchange in two oaks near the western edge of their range. Trees 10: 403-409. [ Links ]
6. Hernández, L. F. & P. M. Bellés (2006). A 3-D finite element analysis of the sunflower (Helianthus annuus L.) fruit. Biomechanical approach for the improvement of its hullability. Journal of Food Engineering 78: 861-869. [ Links ]
7. Hernández, L.F. & G.A. Orioli (1983). Estudio comparativo de la estructura foliar, intercepción de luz y rendimiento en girasol. Anales de Edafología y Agrobiología (Madrid) 42: 2137-2148. [ Links ]
8. Huber, H. & J.F. Stuefer (1997). Shade-induced changes in the branching pattern of a stoloniferous herb: functional response or allometric effect? Oecologia 110: 478-486. [ Links ]
9. Huber, H., J. De Brower, H. De Caluwe, J. Wijschedé & N.P.R. Anten (2008). Shade induced changes in biomechanical petiole properties in the stoloniferous herb Trifolium repens. Evolution Ecology 22: 399-416. [ Links ]
10. King, D.A. (1997). The functional significance of leaf angle in Eucalyptus. Australian Journal of Botany 45: 619-639. [ Links ]
11. Knapp, A.K. & D.L. Smith (1997). Leaf angle, light interception and water relations. Demonstrating how plants cope with multiple resource limitations in the field. American Biology Teacher 59: 365-368. [ Links ]
12. Liu, Y., F. Schieving, J.F. Stuefer & N. P. R. Anten (2007). The effects of mechanical stress and spectral shading on the growth and allocation of ten genotypes of a stoloniferous plant. Annals of Botany 99: 121-130. [ Links ]
13. LoGullo, M.A., L. Castro Noval, S. Salleo & N. Nardini (2004). Hydraulic architecture of plants of Helianthus annuus L. cv. Margot: evidence for plant segmentation in herbs. Journal of Experimental Botany 55: 1549-1556. [ Links ]
14. Niinemets, Ü. (1998). Adjustment of foliage structure and function to a canopy light gradient in two co-existing deciduous trees. Variability in leaf inclination angles in relation to petiole morphology. Trees 12: 446-451. [ Links ]
15. Niinemets, Ü. & S. Fleck (2002). Petiole mechanics, leaf inclination, morphology, and investment in support in relation to light availability in the canpy of Liriodendron tulipifera. Oecologia 132: 21-33. [ Links ]
16. Niklas, K.J. (1992). Plant Biomechanics. Univ. Chicago Press, 607 pp. [ Links ]
17. Pearcy, R.W. & F. Valladares (1999). Resource acquisition by plants: the role of crown architecture. In: Press M.C., J.D. Scholes and M.G. Baker (eds.), Physiological Plant Ecology. Blackwell, MPG, Cornwall, pp. 45-66. [ Links ]
18. Rasband, W.S. (2008). ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. http://rsb.info.nih.gov/ij/ [ Links ]
19. Rawson, H.M. (1979). Vertical wilting and photosynthesis, transpiration, and water use efficiency of sunflower leaves. Australian Journal of Plant Physiology 6: 109-120. [ Links ]
20. Rey, H., J. Dauzat, K. Chenu, J.-F. Barczi, G.A. A. Dosio & J. Lecoeur (2008). Using a 3-D virtual sunflower to simulate light capture at organ, plant and plot levels: contribution of organ interception, impact of heliotropism and analysis of genotypic differences. Annals of Botany 101: 1139 - 1151. [ Links ]
21. Ruzin, S.E. (1999). Plant Microtechnique and Microscopy. Oxford University Press, Oxford, New York, 322 p. [ Links ]
22. Sadras, V.O., L Echarte & F.H. Andrade (2000). Profiles of leaf senescence during reproductive growth of sunflower and maize. Annals of Botany 85: 187-195. [ Links ]
23. Schneiter, A.A. & J.F. Miller (1981). Description of sunflower growth stages. Crop Science 21: 901-903. [ Links ]
24. Smith, V.C. & A.R. Ennos (2003). The effects of air flow and stem flexure on the mechanical and hydraulic properties on the stems of sunflowers (Helianthus annuus L.). Journal of Experimental Botany 54: 845-849. [ Links ]
25. Soil Survey Staff, USDA (1999). Soil Taxonomy: A Basic System for Classifying Soils. Soil Survey Staff, Agriculture, Homework, 436 p. [ Links ]
26. Sposaro, M.M., C.A. Chimenti & A.J. Hall (2008). Root lodging in sunflower. Variations in anchorage strength across genotypes, soil types, crop population densities and crop developmental stages. Field Crops Research 106: 179-186. [ Links ]
27. Valladares, F. & R.W. Pearcy (2000). The role of crown architecture for light harvesting and carbon gain in extreme light environments assessed with a realistic 3-D model. Anales de Jardinería y Botánica (Madrid) 58: 3-16. [ Links ]
28. Vogel, S. (1992). Twist-to-bend ratios and cross-sectional shapes of petioles and stems. Journal of Experimental Botany 43: 1527- 1532. [ Links ]
29. Weiijschedé, J., J. Martinkova, H. De Kroon & H. Huber (2006). Shade avoidance in Trifolium repens: costs and benefits of plasticity in petiole length and leaf size. The New Phytologist 172: 655- 666. [ Links ]














