Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.80 no.1 Vicente López ene./jun. 2011
ARTÍCULOS ORIGINALES
Composition and abundance of phytoplankton ın relation to physical and chemical variables in The Kars River, Turkey
Composición y abundancia del fitoplacton en relación a variables físicas y químicas en el río Kars, Turquía
Özbay H
University of Kafkas, Faculty of Sciences, Biology Department, 36300, Kars, Turkey.
Address Correspondence to: Hanife Özbay, e-mail: hanifeozbay@gmail.com. Fax +90 474 2251179, phone: +90 474 2120201/3084.
Recibido - Received 2.XII.2010.
Aceptado - Accepted 3.II.2011.
Abstract. The phytoplankton of the Kars River was studied from May to October 2005 at five sampling stations. Sixty-six phytoplankton taxa were determined, consisting of Cyanophyta (9), Chlorophyta (25), Euglenophyta (18), Bacillariophyta (7), Cryptophyta (3), Dinophyta (1) and Chrysophyta (3). Total phytoplankton density increased from May to July and then decreased until October. The dominant phytoplankton group was Cyanophyta (36.5 - 64.4%) for most of the study period, followed by Bacillariophyta (20.4 - 38.7%) and Chlorophyta (20.9 - 28.9%). Temperature, pH and dissolved oxygen ranged from 9.6 °C to 21.6 °C; 7.6 to 8.0, and 5.9 to 7.4 mg/L, respectively. Chlorophyl a level changed from 0.0025 to 0.0059 mg/L. On the other hand, both phosphate and nitrogen levels tended to increase from May to July. The maximum and minimum densities of phytoplankton were 19781 (July) and 8851 (October) org/mL, respectively, and this variable correlated significantly with temperature. Soluble Reactive Phosphorus (SRP), Total Phosphate (TP), Dissolved Oxygen (DO) and abundance of Cyanophyta, Chlorophyta, Bacillariophyta and Cryptophyta.
Keywords: River Kars; Running water; Phytoplankton; Environmental factors.
Resumen. El fitoplacton del río Kars fue estudiado entre mayo y octubre de 2005, en cinco estaciones de muestreo. Se determinaron sesenta y seis taxa, pertenecientes a las Divisiones Cyanophyta (9), Chlorophyta (25), Euglenophyta (18), Bacillariophyta (7), Cryptophyta (3), Dinophyta (1) y Chrysophyta (3). La densidad total del fitoplacton aumentó desde mayo hasta julio, y luego disminuyó hasta octubre. El grupo de fitoplancton dominante fue Cyanophyta (36,5 - 64,4%) durante la mayor parte del período de estudio, seguido por Bacillariophyta (20,4 - 38,7%) y Chlorophyta (20,9 - 28,9%). La temperatura, el pH y el oxígeno disuelto varió de 9,6 °C a 21,6 °C; 7,6 a 8,0 y 5,9 a 7,4 mg/L, respectivamente. El nivel de clorofila a cambió de 0,0025 a 0,0059 mg/L. Por otra parte, los niveles de fosfato y nitrógeno tendieron a incrementar desde mayo a julio. Las densidades máxima y mínima de fitoplancton fueron 19781 (julio) y 8851 (octubre) org/mL, respectivamente, y esta variable se correlacionó significativamente con la temperatura, fósforo reactivo soluble (SRP), fosfato total (TP), Oxígeno disuelto (DO), y abundancia de Cianofitas, Clorifitas, Bacilorófitas y Criptófitas.
Palabras clave: Río Kars; Agua corriente; Fitoplacton; Factores ambientales.
INTRODUCTION
As a primary producer, phytoplankton uses abiotic factors and processes such as photosynthesis to produce energy; primary and secondary consumers depend on primary producers for energy. Therefore, phytoplankton is very important for the existence of all water ecosystems (Molles, 2005). Survey studies, such as physical, chemical and biological investigations on rivers, produce important baseline data which support further work on river systems. In general, however, phytoplankton composition and seasonal dynamics in rivers (Chessman, 1985; Marvan et al., 2004; Domitrovic et al., 2007) have received very little attention compared with lake phytoplankton. Although during the past twenty years riverine phytoplankton have been popular among the hydrobiologists, the river phytoplankton of Mediterranean Europe is still poorly known.
Turkish research on phytoplankton communities has also focused predominantly on lakes rather than rivers. Kars River is one of the important sources for irrigation purposes, fishing and recreational uses in the region. Dilber (2007), in a previous study, reported the river turbidity (Sechi depth), NO3-N, NH4-N, SRP, TP, silica and Chl a levels between: 32.5 cm (May) and 38.3 cm (July); 0.14 mg/L (June) and 0.542 mg/L (October); 31 μg/L (June) and 65μg/L (July); 47 μg/L (November) and 159 μg/L (August); 61 μg/L (October) and 237 μg/L (September); 1.96 μg/L (August) and 5.56 μg/L (November), 0.0033 mg/L (June) and 0.020 mg/L (September), respectively. No phytoplankton studies have been conducted to date in the Kars River. The present study, therefore, is the first attempt to describe the seasonal abundance of phytoplankton in this ecological system. The effects of physico-chemical parameters, such as temperature, DO, pH, conductivity, SRP, TP, NH4-H, NO3-N and chlorophyl a on the phytoplankton community were also evaluated.
MATERIALS AND METHODS
The Kars River, which is 93 km long, has its source in the mountains which lie near the town of Sarıkamıs, in north-eastern Turkey. The river is a tributary of the Aras River, and discharges into the Arpacay Dam via the Arpacay Creek. Based on previous records from the State Hydraulic Works Department (2005), the mean current speed of the river is 0.468 m/s. No information is available about the seasonal hydrological regime. The river is contaminated with urban, rural and industrial waste. The river basin is in a temperate zone with 3.9 °C mean annual temperature (State Meterological records, 2005), where the absolute minimum and maximum temperatures are -14.6 °C and 28.7 °C.
Samples were collected monthly, from May to October 2005, from the edge of the water, where the velocity is low, at five stations scattered along the river (Fig.1; beware of the waste- discharged point throughout the sampling). Sampling was not carried out during the winter because of severe weather conditions. At each sampling station, water temperature, dissolved oxygen (DO) and pH were measured in situ, with commercial meters. Water samples (1 liter) were also collected for analysis of soluble reactive phosphorus (SRP), total phosphate (TP), ammonium-nitrogen (NH4-N) and nitrate-nitrogen (NO3-N), following Mackereth et al. (1978). Single subsurface phytoplankton samples were taken with a Van Dorn sampler and preserved with Lugol's solution immediately after sampling. Subsamples were examined and enumerated with an inverted microscope at a magnification of X400, according to the method described by Lund et al. (1958). Standard texts were used for identification of phytoplankton species (Lind & Brook, 1980; Haris, 1986; Kramer & Lange-Bertalot, 1991; Canter-Lund & Lund, 1996). Chl a was extracted with acetone, and concentration calculated from absorbance reading at 663 nm (Talling & Driver, 1961). The Shannon-Wiener species diversity index (H') was calculated as follows:
where Pi is the proportion of each species in the sample. Principal Component Analysis (PCA) was performed using SPSS 16.0 to assess the influence of the physico-chemical variables on the abundance of phytoplankton.
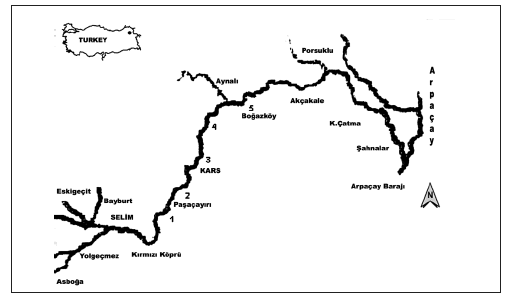
Fig. 1. Sampling stations in Kars River.
Fig. 1. Estaciones de muestreo en el río Kars.
RESULTS
A total of sixty-six phytoplankton taxa were identified in the Kars River (Table 1a y b ). Throughout most of the study period, the dominant group was Cyanophyta, except in June when it was overcome by Bacillariophyta. Within Cyanophyta, four species excedeed the 10% dominance: Anabaena circinalis, A. elenkinii, Aphanizomenon flos-aquae and Lyngbya concorta. For the second group (Bacillariophyta), only Fragillaria ulna reached such high values. Another important group was Chlorophyta (global dominance > 20% during all the study); only two taxa (Chlamydomonas sp. and Monoraphidium contortum) exceeded the 5% dominance. The Shannon-Wiener species diversity index, based on the number of individuals, ranged from 2.05 (October) to 4.20 (July) (Fig. 2).
Table 1. Species composition, dominance values and diversity (H': Shannon-Wiener diversity index) of phytoplankton in Kars River.
Tabla 1. Composición específica, dominancia y diversidad (H': índice de diversidad de Shannon-Wiener) del fitoplancton en el río Kars.
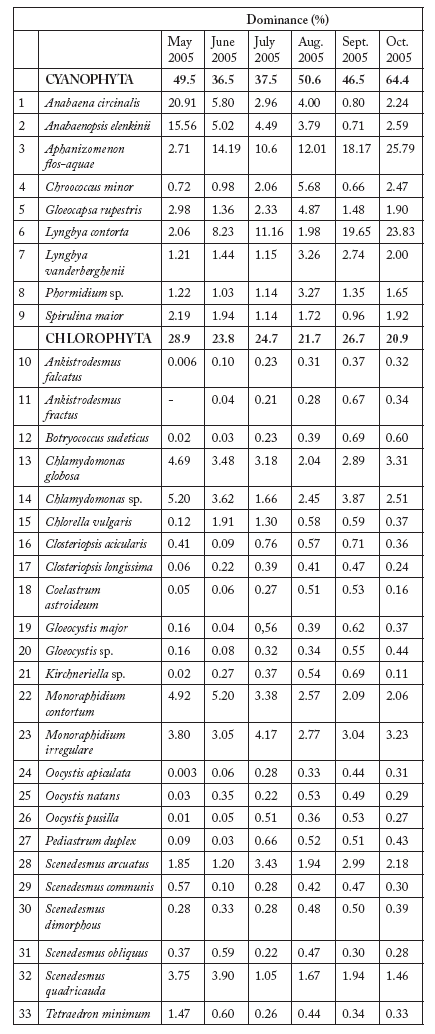
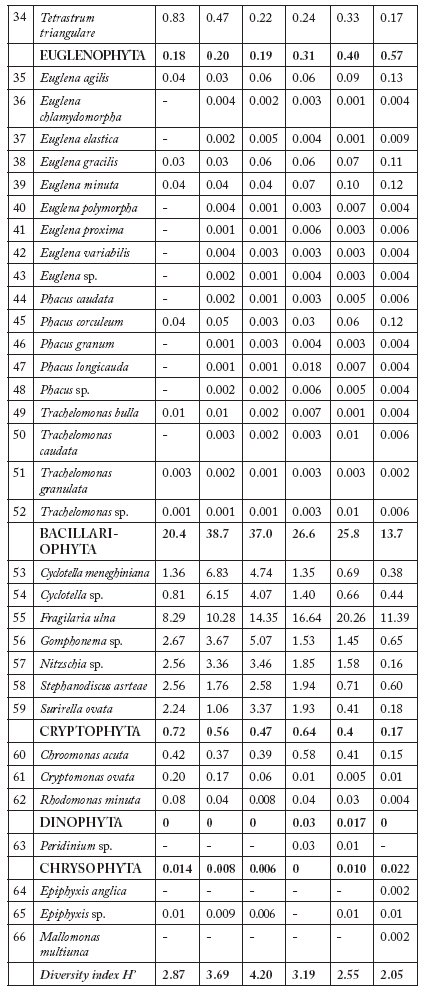
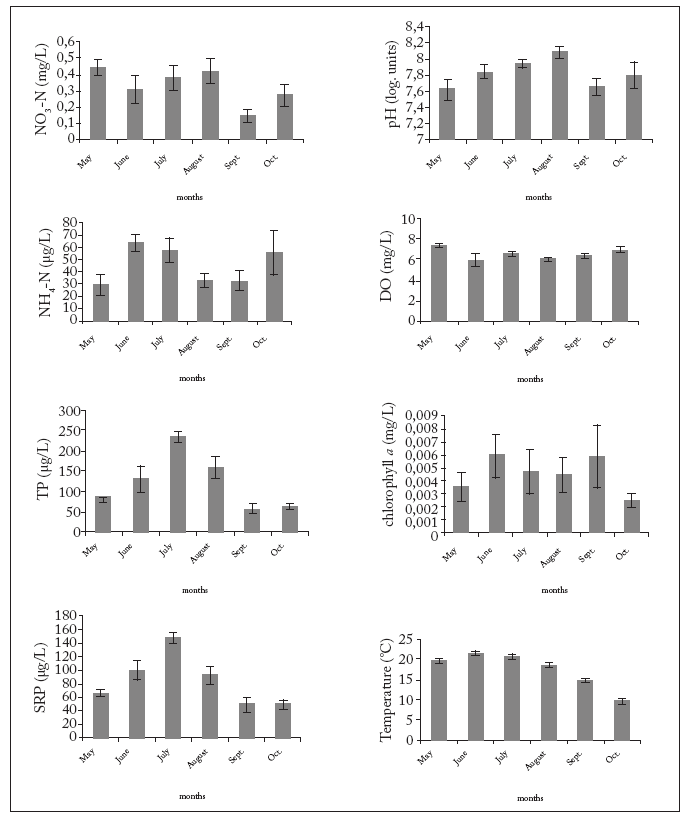
Fig. 2. Average values ± 1 S.D. of physico-chemical variables and Chl a concentration in river water during the study. See text for abreviations.
Fig. 2. Valores promedio ± 1 D.S. de variables fisicoquímicas y concentraciones de clorofila a en el agua de río durante el estudio. Abreviaturas en el texto.
The density of phytoplankton ranged from 19781 (July) to 8851 (October) org/mL (Fig. 3). This variable correlated significantly with temperature, SRP, TP, DO, and Cyanophyta, Chlorophyta, Bacillariophyta and Cryptophyta abundances (p<0.03 in all cases). However, the density of phytoplankton did not correlate significantly with the other variables (p>0.05 in all cases). Cyanophyta, Bacillariophyta and Chlorophyta, the three most abundant groups in the river, strongly correlated with SRP (p<0.001), TP (p<0.001) and temperature (p<0.001). Correlation with NO3-N was also significant (p=0.047) for Cyanophyta, and significant correlation (p=0.039) with NH4-N was found for Bacillariophyta. On the other hand, no significant association with NO3-N was determined for either Chlorophyta (p=0.127) or Bacillariophyta (p=0.235). Likewise, no significant relation with NH4-N was found for either Chlorophyta (p=0.145) or Cyanophyta (p=0.215).
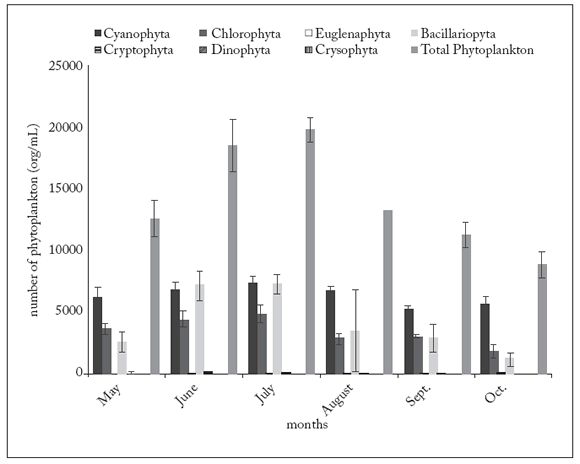
Fig. 3. Average density of phytoplankton groups (org/mL) in Kars River during the study.
Fig. 3. Densidad promedio de los grupos de fitoplacton (org/mL) en el río Kars durante el estudio.
The influence of the measured physico-chemical variables on the abundance of phytoplankton groups was assessed using Principal Component Analysis (PCA). PCA extracted two main factors that explaned 52% of the total variance at the river. The first axis, was closely related to Cryptophyta and Chl a, and the second axis, was strongly associated with Crysophyta (Fig. 4).
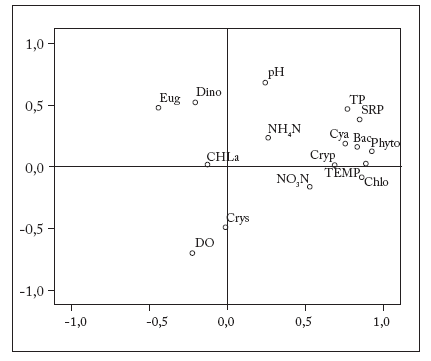
Fig. 4. Principal component analysis (PCA) of physico-chemical variables and main phytoplankton groups. Abbreviations: CHLa, chlorophyll a; DO, dissolved oxygen; NH4N, ammonia nitrogen; NO3N, nitrate nitrogen; TP, total phosphate; SRP, soluble reactive phosphorus; TEMP, temperature; Cya, Cyanophyta; Chlo, Chlorophyta; Eug, Euglenophyta; Bac, Bacillariophyta; Cryp, Cryptophyta; Dino, Dinophyta; Crys, Chrysophyta; Phyto, total phytoplankton.
Fig. 4. Análisis de componentes principales (ACP) de variables fisicoquímicas y principales grupos de fitoplancton. Abreviaturas: CHLa, clorofila a; DO, oxígeno disuelto; NH4N, nitrógeno amoniacal; NO3N, nitrato; TP, fosfato total; SRP, fósforo reactivo soluble; TEMP, temperatura; Cya, Cyanophyta; Chlo, Chlorophyta; Eug, Euglenophyta; Bac, Bacillariophyta; Cryp, Cryptophyta; Dino, Dinophyta; Crys, Chrysophyta; Phyto, fitoplancton total.
DISCUSSION
In the present study, total phytoplankton density increased from May to July in the Kars River, and then decreased until October (Fig. 3). Throughout most of the study period the dominant phytoplankton group in the river was Cyanophyta. Thıs disagrees with a number of studies performed in England and Africa, which have identified diatoms (Bacillariophyta) as a dominant group (Talling & Rzoska, 1967; Lack, 1971; Aykulu, 1978). Results similar to ours have been reported by Bennett et al. (1986) who found that Cyanophyta form dense blooms in the Nile and temperate rivers during the summer. On the other hand, Sabater (1990) has identified the green algae as a dominant group in the River Ter in Spain.
It is largely known that phytoplankton density increases due to eutrophication (Seaborn, 1997; Petr et al., 2004). In general, the Kars River had sufficient nutrients for phytoplankton growth during the study period. Nutrient levels in the Kars River increased from May to July, owing to the increasing amount of waste discharged into the river (Fig. 2). In the present study, total phytoplankton density tended to rise concomitantly with nutrient level increase in the river from May to August. Seaborn (1997) also found very high phytoplankton concentration in a nutrient-enriched river. Furthermore, Marvan et al. (2004) reported that phytoplankton abundance in the Morava River increased in 2002, when compared to the late 1950s, due to the increasing organic pollution. Similarly, some studies have found low phytoplankton concentration in low-nutrient level rivers (Chessman, 1985; Domitrovic, 1988).
Lauren (2007) found that phytoplankton abundance was more strongly affected by nitrate than by phosphate levels in the Duwamish River, USA This author suggested that nitrate was the limiting factor for phytoplankton growth in that system. In the present study, although no nutrient limitation was determined, it seems likely that phosphate was the main nutrient for phytoplankton growth. This is in agreement with the majority of studies that indicate that phosphorus rather than nitrogen is the limiting factor in freshwater ecosystems (Wheeler & Neushul, 1981; Hecky & Kilham, 1988).
Dissolved oxygen and Crysophyta yielded negative scores for all the variables tested in this study. Although chl a levels increased with the increase in nutrients, the lack of correlation between chlrophyll a level and (1) total phytoplankon density or (2) any of the phytoplankton groups may be explained by other factors. The chlorophyll content of each group may have remained at levels lower than the other pigments due to environmental factors such as high turbidity, which involves low light penetration. The grazing effect of zooplankton on algae species, as a regulating factor of the phytoplankton community structure, needs to be taken into account. It seems that predation of zooplankton on Cyanophyta is low in the Kars River. It is known that many blue-green algae are poor food, and that the filaments of blue-green algae may be too large for ingestion (Moss, 1998). Therefore, blue-green algae is generally not a primary food source for either zooplankton or fish. If this is the case, it is possible that many zooplankton and fish consumed diatoms as a primary food source. Furthermore, the majority of the zooplankton species in the river have been previously found to be benthic (Özbay & Altındag, 2009). This could explain in part why Cyanophyta, rather than Bacillariophyta, was the dominant group in the river. On the other hand, the structure of phytoplankton communities depends not only on grazing pressure but also on nutrient and light availability (Reynolds, 1988). Therefore, species belonging to similar functional groups can in turn be classified in three basic adaptive strategies: (1) C (colonist-invasives), (2) S (stress-tolerant) and (3) R (ruderals). The succession of phytoplankton in Kars River began with S-Strategists (Cyanophyta) in May. This group was followed by R-Srategists (Bacillariophyta), but later on S-Strategists became dominant again. Within the Cyanophyta, Bacillariophyta and Chlorophyta, seven species excedeed the 5-20% dominance: Anabaena circinalis, A. elenkinii, Aphanizomenon flos-aquae and Lyngbya concorta, Fragillaria ulna, Chlamydomonas sp. and Monoraphidium contortum.
Mitrovic et al. (2001) suggested that buoyancy ability may offer considerable advantage to A. circinalis to be dominant in turbid freshwater rivers. Similarly, in the present study A. circinalis was the dominant Cyanophyta in May (20.9%), when the turbidity might be expected to be high due to the spring flooding in that month. On the other hand, Kemp (2009) indicated that in a Cyanophyta community, the abundance of non-heterocytic (non N-fixing) species decrease with the decreasing inorganic N. This is in contrast to heterocytic (N-fixing) species such as A. circinalis and A. elenkinii in both Yangebup and Bibra Lakes. In the present study, abundance of both A. circinalis and A. elenkinii were also high in May, when the inorganic N content (especially NH4-N) was low. Field observations indicate that large colonies of A. flos-aquae are associated with an increase in water clarity, low nanophytoplankton crops, and dense populations of Daphnia plux (Holm et al., 1983). In Kars River, dominance of A. flox-aquae started to increase from June onwards with the increasing Secchi depth (see introduction). Some research on Lake Shira (Siberia) suggested that the photoheterotrophic capability of L. contorta might help explain its development in deeper waters, where light availability is near the light compensation point (Quesada et al., 2002; Degermendzhy & Gulati, 2002). The photoheterotrophic communities are able to assimilate organic compounds, thus supplementing their carbon and energy requirements. An inverse relationship was found between the uptake of organic compounds and light intensity in Lake Shira (Quesada et al., 2002). L. contorta reached its the maximum dominance (23.83%) in October in Kars River, probably due to the increasing organic compounds (e.g. detritus).
Fernandez & Galvan (2008) have concluded that Chlamydomonas is an attractive system for nitrate assimilation in photosynthetic eukaryotes. This ability could foster growth of Chlamydomonas sp. (also Chlamydomonas globosa) which reached aproximately 5% dominance in May, when the NH4-N was at the lowest level in Kars River.
Monoraphidium contortum is able to cope with rapid environmental changes due to their short reproduction time (Negro et al., 2000). Therefore the dominance of M. contortum was highest in May (4.92%) and June (5.20%), when the rapid increase for SRP, TP, NH4-N and temperature was observed in the Kars River.
Fragillaria ulna has been reported as a characteristic species of waters affected by sewage inputs; this organism was reported to be dominant in many eutrophic Turkish rivers (Albay & Aykulu, 1994; Temel, 2006). Kars River is also affected by sewage inputs, as many other Turkish rivers, and therefore dominance of F. ulna has increased with the nutrient rising (especially SRP and NH4-N).
In conclusion, Cyanophyta was the dominant group in phytoplankton of the Kars River throughout most of the study period. No doubt that there is lack of information about phytoplankton diversity and abundance in Turkish river systems. This means that more studies are needed to understand and compare the structure and ecology of the phytoplankton communities in both the Kars River and other river systems of the country.
ACKNOWLEDGEMENTS
The author thanks Dr. İsmail Çakmak, Kafkas University, for his help in statistical analyses, and Elif Öztürkkan for her help on the map drawing.
REFERENCES
1. Aykulu, G. (1978). A quantitative study of the phytoplankton of the River Avon, Bristol. Br.Phycol J. 13: 91-102. [ Links ]
2. Albay, M. & G. Aykulu (1994). Algological characteristics of Göksu River (Istanbul). I. Planktonic algae. XIIth Biological Congress, 6-8 July, Edirne, Turkey. Section of Hydrobiology 6: 157-165. [ Links ]
3. Balbi, D. M. (2000). Suspended chlorophyll in the River Nene, a small nutrient-rich river in eastern England: long-term and spatial trends. Science of the Total Environment 251: 401-421. [ Links ]
4. Bansu, B. K., J. Kalff & B. Pinel-Alloul (2000). Midsummer plankton development along a large temperature river: the St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 57: 7-15. [ Links ]
5. Bennett, J.P., J.W. Woodward & D.J. Shultz (1986). Effect of discharge on the chlorophyll a distribution in the tidally-influeced Potomac River. Estuaries 9: 250-260. [ Links ]
6. Canter-Lund, H. & J.W.G. Lund (1996). Freshwater algae, their microscopic world explored. Bristol, Biopress Ltd., 360 p. [ Links ]
7. Chessman, B. C. (1985). Phytoplankton of the La Trobe River, Victoria. Australian Journal of Marine and Freshwater Research 36: 115-122. [ Links ]
8. Degermedzhy, A. G. & R. D. Gulati (2002). Understanding the mechanisms of bloming of phytoplankton in Lke Shira, a saline lake in Siberia (the Republic of Khakasia). Aquatic Ecology 36: 331-340. [ Links ]
9. Dilber, E. (2007). Seasonal Determination of algal chlorophyll level in Kars River (in Turkish). MSc Thesis, University of Kafkas, 35 p. [ Links ]
10. Domitrovic, Z.Y., A. S. G. Neiff & S.L. Casco (2007). Abundance and diversity of phytoplankton in the Parana River (Argentina) 220 km downstream of the Yacyreta reservoir. Brazilian Journal of Biology 67: 53-63. [ Links ]
11. Fernandez, E. & A. Galvan (2008). Nitrate Assimilation in Chlamydomonas. Eukaryotic Cell 7: 555-559. [ Links ]
12. Haris, G.P. (1986). Phytoplankton ecology; structure, function and fluctuation. London, Chapman & Hall., 384 p. [ Links ]
13. Hecky, R.E. & P. Kilham (1988). Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnology and Oceanography 33: 796-822. [ Links ]
14. Holm, N. P., G.G. Ganf & J. Shapiro (1983). Feeding and assimilation rates of Daphnia plux fed Aphanizomenon flos-aquae. Limnology and Oceanography 28: 677-688. [ Links ]
15. Kopf, W., W. Pöhlmann & K. Reimann (1988). Grundlagen der Eutrophierung von Fliessgewässern, dargestellt am Beispiel von Main und Regnitz, 2 parts, Bayerische Landesanstalt Wasserforschung, München, 623 p. [ Links ]
16. Krammer, K. & H. Lange-Bertalot (1991). Bacillariophyceae 4 Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis. In: Ettl H., Gärtner G., Gerloff J., Heynig H., Mollenhauer D. (eds). Süßwasserflora von Mitteleuropa, Stuttgart, Gustav Fischer Verlag, vol. 2/4, pp. 1-437. [ Links ]
17. Lack, T. J. (1971). Quantitative studies on the phytoplankton of the Rivers Thames and Kenet at Reading. Freshwater Biology 1: 213-224. [ Links ]
18. Lauren, H. (2007). Nitrate limiting factor of phytoplankton growth in Duwamish River, affected by restoration conditions. Journal of Ecological Stuff 1: 1-8. [ Links ]
19. Lind, E.M. & A.J. Brook (1980). Desmids of the English Lake District, Ambleside, U.K. Freshwater Biological Association Scientific Publication no. 42. [ Links ]
20. Lund, J.W.G., C. Kipling & D.E. Lecren (1958). The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11: 143-170. [ Links ]
21. Mackereth, F.J.H., J. Heron & J.F. Talling (1978). Water analysis: some rivesed methods for limnologists. Freshwater Biological Association Scientific Publication no. 36. Titus Wilson & Son Ltd. Kendall, 117 p. [ Links ]
22. Marvan, P., J. Hetésa, F. Hindák & A. Hindáková (2004). Phytoplankton of the Morava River in the Czech Republic and Slovakia: Past and Present. Oceanological and Hydrobiological Studies 33: 41-60. [ Links ]
23. McCullough, D. A., G. W. Minshall & C. E. Cushing (1979). Biogenergetics of lotic filter-feeding insects Simulium spp. (Dipteria) and Hydropsyche occidentalis (Trichoptera) and their function in controlling organic transport in streams. Ecology 60: 585-596. [ Links ]
24. Mitrovic, S. M., L. C. Bowling & R. T. Buckney (2001). Vertical disentrainment of Anabaena circinalis in the turbid, freshwater Darling River, Australia: quantifying potential benefits from buoyancy. Journal of Plankton Research 23: 47-55. [ Links ]
25. Molles, M. (2005) Ecology: Concepts and Applications. Boston, McGraw Hill. 608 p. [ Links ]
26. Moss, B. (1998). Ecology of Freshwaters: Man and Medium, Past to Future. UK, Blackwell, 557 p. [ Links ]
27. Negro, A. I., C. De Hoyes & J.C. Vega (2000). Phytoplankton structure and dinamics in lake Sanabria and Valparaiso reservoir (NW Spain). Hydrobiologia 412: 25-37. [ Links ]
28. Noppe, K., J. Prygiel, M. Coste & A. Lepretre (1999). Phtoplankton of the canalized Scarpe River downstream from the Douai sewage treatment plant. Journal of Freshwater Ecology 14: 167-178. [ Links ]
29. Özbay, H. & A. Altindag (2009). Zooplankton abundance in the River Kars, Northeast. Turkey: Impact of environmental variables. African Journal of Biotechnology 8: 5814-5818. [ Links ]
30. Quesada, A., F. Jüttner, T. Zotina, A.P. Tolomeyev & A.G. Degermendzhy (2002). Heterotrophic capability of a metalimnetic plankton population in saline Lake Shira (Siberia, Khakasia). Aquatic Ecology 36: 219-227. [ Links ]
31. Reynolds, C.S. (1988). Potamoplankton: Paradigms, paradoxes and prognoses. In: Algae and the Environment: Contribution in honour of J.W.G. Lund, Round F.E. (eds). Bristol, Biopress Ltd., pp. 283-311. [ Links ]
32. Sabater, S. (1990). Phytoplankon composition in a medium-sized medditerranean river: The Ter (Spain). Limnetica 6: 47-56. [ Links ]
33. Schol, A., V. Kirchesch, T. Bergfeld & D. Muller (1999). Model-based analysis of oxygen budget and biological processes in the regulated rivers Moselle and Saar: modelling the influence of benthic filter feeders on phytoplankton. Hydrobiologia 410: 167-176. [ Links ]
34. Seaborn, D.W. (1997). Seasonal phytoplankton composition in the Pagan River, Virginia: A nutrient enriched river. Virginia Journal of Science 48: 265-274. [ Links ]
35. State Hydrolic Work Recods (2005). Kars, Turkey. [ Links ]
36. State Meterological Records (2005). Kars, Turkey. [ Links ]
37. Talling, J.F. & J. Rzoska (1967). The development of phytoplankton in relation to hydrological regime in the Blue Nile. Journal of Ecology 55: 637-662. [ Links ]
38. Talling, J.F. & D. Driver (1961). Some problems in the estimation of chlorophyll a in a phytoplankton. Proceedings of a conference on a primary productivity measurement in marine and frehwaters. MS. Doty. University of Hawaii. US Atomic Energy Commission Publication, TID 7633: 142-146. [ Links ]
39. Temel, M. (2006). A study of Prokaryota (Cyanobacteria, Cyanoprokaryota) and Eukaryota algae in the Riva (Durusu) Stream, Istanbul, Turkey. Supplementa ad Acta Hydrobiologica 8: 79-90. [ Links ]
40. Welker, M. & N. Walz (1998). Can mussels control the plankton in river? A planktological approach applying a Lagrangian sampling strategy. Limnology and Oceanography 43: 753-762. [ Links ]
41. Wheeler, W.N. & M. Neushul (1981). The aquatic environment. In: Lange O.L., Nobel P.S., Osmond C.B. & Ziegler H. (eds). Encyclopedia of Plant Physiology, New Series, Vol. 12 A: Physiological Plant Ecology I, Responses to the Physical Environment. Springer-Verlag, New York, pp. 229-247. [ Links ]














