Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.80 no.2 Vicente López jul./dic. 2011
ARTÍCULOS ORIGINALES
Notes on the sexual condition of Myriophyllum aquaticum, Haloragaceae
Notas sobre la condición sexual de Myriophyllum aquaticum, Haloragaceae
Torres Robles SS1, G Peter1,2, NM Tur3
1 Universidad Nacional de Río Negro, Sede Atlántica, Colón 450 Of. 1, 8500, Viedma, Río Negro, Argentina.
2 CONICET/Centro Universitario Regional Zona Atlántica, Universidad Nacional del Comahue, Esandi y Ayacucho, Viedma, Río Negro, Argentina.
3 División Plantas Vasculares, Facultad de Ciencias Naturales y Museo, UNLP, Paseo del Bosque s/n, La Plata, Buenos Aires, Argentina.
Address Correspondence to: Silvia S. Torres Robles, Colón 450 Of. 1, 8500 Viedma, Río Negro, Argentina, e-mail: silviatorresrobles@gmail.com
Recibido - Received 30.VIII.2010.
Aceptado - Accepted 19.X.2010.
Abstract. Myriophyllum aquaticum (Vell.) Verdc. is native of South America and has a pantropical distribution. This species has been cited as dioecious, monoecious and polygamous. The purpose of this paper was to contribute to the discussion of its sexual condition, based on herbarium material, and supported by field observations. Herbarium material from Argentina was examined. Also, twenty branches from the Punta Lara Nature Reserve (Buenos Aires, Argentina) were periodically sampled to record the sex of flowers present on each whorl during the flowering period of 2002. Both in herbarium material and in field, we observed specimens with branches bearing either female or male, and specimens with female and male flowers on the same branch. Some of these materials have also fruits. Our observations support the idea that M. aquaticum is not a strictly dioecious species, at least in Argentina.
Keywords: Dioecy; Monoecy; Myriophyllum aquaticum; Punta Lara.
Resumen. Myriophyllum aquaticum (Vell.) Verdc. es nativa de Sudamérica con distribución pantropical. Esta especie ha sido citada como dioica, monoica y polígama. El objetivo de este trabajo es contribuir a la discusión sobre su condición sexual, basada en material de herbario, y apoyada por observaciones de campo. Se examinó material de herbario de Argentina. En la Reserva Natural de Punta Lara (Buenos Aires, Argentina), se muestrearon periódicamente veinte ramas para registrar el sexo de las flores presentes en cada verticilo durante el período de floración de 2002. Tanto en material de herbario, como en el campo, observamos especímenes con ramas con flores femeninas, especímenes con ramas con flores masculinas y especímenes con flores femeninas y masculinas en la misma rama. Algunos de estos materiales presentan frutos. Nuestras observaciones apoyan la idea que M. aquaticum no es una especie estrictamente dioica, al menos en Argentina.
Palabras clave: Dioecia; Monoecia; Myriophyllum aquaticum; Punta Lara.
INTRODUCTION
Myriophyllum aquaticum (Vell.) Verdc. is a native species of South America from Argentina, Brazil, Chile, Paraguay, Peru and Uruguay (Meijden, 1969; Meijden & Caspers, 1971; Boutique & Verdcourt, 1973; Ayres Fevereiro, 1975; Orchard, 1979, 1981; Li & Hsieh, 1996; Negritto & Anton, 1996; Zuloaga & Morrone, 1999). However, this species grows adventitiously in tropical and warm temperate regions of the world, where it is often cultivated as an ornamental (Meijden, 1969; Meijden & Caspers, 1971; Orchard, 1979, 1981; Lahitte et al., 1997; Hurrel et al., 2006).
Myriophyllum aquaticum is a submerged and emergent herb with stem branches up to 1 m long and emerging apexes, perpetuating growth after anthesis. The leaves are in whorls of four to six, 1-3 cm long, oblong in outline, pinnate, with thread-like segments. The flowers are unisexual, solitary, axillar, pedicellate and 4-merous. Male flowers have ovate-deltoid sepals, yellow petals and eight stamens. Female flowers have deltoid sepals, no petals, a pyriform ovary and a clavate style with four white densely fimbriate stigmas. Fruits are ovoid with four mericarps (Meijden & Caspers, 1971; Orchard, 1981; Lahitte et al., 1997; Tur et al., 2009).
This species is commonly found in fresh-water bodies, forming patches in quiet, shallow water, usually associated with other free-emergent floating species (Orchard, 1981; Negritto & Anton, 1996). It is frequently found in an emergent form producing dense populations (the immersed leaves often deteriorate when plants grow in standing water).
This species, which has been sold commercially as an ornamental aquatic plant, is invasive on most continents (Moody & Les, 2010).
On the sexual issue, there are some disagreements that became worse because of the nomenclatural problem (Tur et al., 2009). Enydria aquatica Vellozo (1825), basionym of M. aquaticum, is described as a likely monoecious species. Myriopyllum brasiliense Cambess., a nomenclatural synonym of M. aquaticum, is cited as monoecious (Kanitz, 1882), mostly monoecious or more-or-less dioecious (Meijden & Caspers, 1971), and polygamous or dioecious (Ayres Fevereiro, 1975). Meijden & Caspers (1971) mentioned that all naturalized specimens are sterile or female but do not produce fruits (in Malaysia, male specimens are absent), and even fruits rarely occur in South America. In contrast to these authors, Ayres Fevereiro (1975) described male, female and bisexual flowers, and observed that this species flowers and fruits throughout the year in Brazil. On the other hand, M. proserpinacoides Gill. ex Hook. & Arn., another nomenclatural synonym of M. aquaticum, was cited as a subdioecious (Gillies, 1833; Schindler, 1905) or dioecious plant species (Kanitz, 1882; Reiche, 1898). Orchard (1979, 1981) reported that the lectotype of M. proserpinacoides is a monoecious specimen (Dr. Gillies s.n., K). Finally, M. aquaticum was cited as monoecious or mostly dioecious by Boutique & Verdcourt (1973) or as dioecious by Orchard (1979, 1981), Li & Hsieh (1996), Negritto & Anton (1996) and Moody & Les (2010). In revisions of the genus for South America and Australia, Orchard (1979, 1981) only found female plants of M. aquaticum outside South America, and concluded that reproduction is entirely vegetative. According to Meijden & Caspers (1971), male flowers and fruits are rare even in South America. Li & Hsieh (1996) cited entirely vegetative reproduction, with male plants absent, in Taiwan. Moody & Les (2010) studied the phylogenetic relationships in the genus Myriophyllum. They concluded that all the specimens of M. aquaticum collected in North America were exclusively female plants with identical genotypes.
The objective of this paper was to contribute to the discussion of the sexual condition of M. aquaticum, based on herbarium material and field observations.
MATERIALS AND METHODS
Herbarium material. Herbarium acronyms follow Holmgren et al. (1990). Herbarium material deposited at BA, LP and SI was examined to check the sex of the specimens, and to record the localities where the taxon had been collected. The study herbarium material is cited in three groups within Results: (1) specimens with only female flowers, (2) specimens with only male flowers, and (3) specimens with male and female flowers on the same branch. The presence of fruits had also been registered (fr). Some of these specimens are whole plants, whereas others are constituted only for branches or branch fragments.
Field observations. The study area was the Punta Lara Nature Reserve (34° 47´ 22´´ S, 58° 00´ 21´´ W), Buenos Aires province, Argentina, located in the Pampean biogeographical province (Cabrera, 1976). The climatic conditions are warm temperate, with frosts in winter and early spring; rainfall occurs throughout the year, but it is more intense in spring and autumn, scarce in winter and insufficient in summer. It ranges from 600 mm to 1100 mm annually (Cabrera, 1976).
In the field, M. aquaticum grows in dense patches with only female or male flowers at sight, which have a light difference in colour. We call them "female patches" or "male patches", in spite of the sex of all the flowers present in all branches. Twenty branches of M. aquaticum were selected from patches growing in the interior of the water bodies, and the last fertile whorl was marked on each branch. Of these whorls, ten of them came from a "male patch", and the other ten from a "female patch". We registered the sex of the flowers in the fertile whorls previous to its marking (before 6 October 2002). On each marked whorl, the number of new whorls and the sex of the flowers present on it were recorded during the flowering period of 2002 (18 October 2002, 1 November 2002 and 15 November 2002).
RESULTS
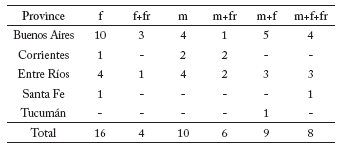
Herbarium material. The flowering period of Myriophyllum aquaticum ranges from August to December in Argentina; it rarely starts in June. Specimens with branches bearing either female or male, or female and male flowers on the same branch were recorded (Table 1). Some of the study material also had fruits (Table 1).
Table 1. Number of herbarium specimens examined with female flowers (f), female flowers and fruits (f+fr), male flowers (m), male flowers and fruits (m+fr), male and female flowers (m+f), and male and female flowers and fruits (m+f+fr) in different provinces of Argentina.
Tabla 1. Número de especímes de herbario examinados con flores femeninas (f), flores femeninas y frutos (f+fr), flores masculinas (m), flores masculinas y frutos (m+fr), flores masculinas y femeninas (m+f), y flores masculinas, femeninas y frutos (m+f+fr) en diferentes provincias de Argentina.
Examined material
Specimens with only female flowers: ARGENTINA. Buenos Aires. Isla Santiago, 23 July 1906, Pastore 194 (SI); Campana, 01 November 1917, Parodi 1309 (BAA); Isla Martín García, 26 November 1923, Parodi 5278 (BAA); Dock Sud, 17 October 1926, Burkart 89 (SI); Río Santiago, 07 October 1928, Cabrera 425 (LP), 06 November 1932, Cabrera 2496 (LP); Punta Lara, 17 October 1949, Calderón s.n. (SI); 17 October 1949 (fr), Calderón 1666 (BAA); Dolores, Ea. Los Álamos, 23 November 1958 (fr), Grondona 6496 (BAA); Capital Federal, Fac. Agron. y Vet., 23 October 1962, Parodi 2896 (BAA); Capital Federal, Villa Ortúzar, Cult. Hort. Bot. Fac. Agr. y Vet., 20 October 1967 (fr), Ahumada & Boyle 5819 (BAA); Chascomús, 07 September 1973, Tur 1533 (SI); Boca Palermo, October 1973, Berg 221 (LP).
Corrientes. Mburucuyá, 15 August 1956, Pedersen 3946 (LP).
Entre Ríos. Delta del Paraná. Río Ceibo, 25 November 1932, Burkart 5086 (LP, SI); Arroyo Malo, Puerto Constanza a Ceibas, 18 September 1961, Burkart 22670 (SI); Gualeguaychú, Arroyo Salinas, 15 October 1971 (fr), Burkart 28756 (LP, SI); Gualeguaychú, entre Las Mercedes y Perdices, 24 October 1971, Burkart 28754 (LP, SI); 02 March 1973, Burkart 29410 (SI).
Santa Fe. Dep. Capital, Laguna Los Espejos, 27 November 1970, Tur 1363 (SI).
Specimens with only male flowers: ARGENTINA. Buenos Aires. La Plata, Los Talas, 14 November 1895, Alboff 238 (LP); Quilmes, October 1912, Rodríguez 60 (SI); Dock Sud, 01 October 1915 (fr), Parodi 5278 (BAA); City Bell, 04 November 1932, Cabrera 2476 (LP); Chascomús, 07 September 1973, Tur 1533 (LP).
Corrientes. Carlos Pellegrini, 01 November 1971, Krapovickas et al. s.n. (LP); Dep. San Martín, Carlos Pellegrini, 8 km al N de Estero Cambá Trapo, 01 November 1971, Krapovickas, Cristóbal, Ferraro, Yrigoyen, Maruñak & Tressens 20312 (BAA).
Entre Ríos. Delta del Paraná, Arroyo la Chilena, Brazo Largo, 26 February 1938, Burkart 8952 (SI); Arroyo Malo, Puerto Constanza a Ceibas, 18 September 1961, Burkart 22670 (LP); Gualeguaychú, embarcadero, Brazo Largo, 03 November 1965, Burkart 26006 (SI); La Paz, Distrito Tacuaras, Estancia Santa Cruz Cuí, 07 November 1965, Burkart 26007 (SI); Gualeguaychú, Arroyo Malo cerca de Paranacito, 30 December 1969 (fr), Burkart & Troncoso 27728 (SI); Gualeguaychú, Arroyo Salinas, 15 October 1971 (fr), Burkart 28755 (SI).
Specimens with male and female flowers: ARGENTINA. Buenos Aires. San Isidro, F.C.C.A., 21 October 1932, Burkart 4617 (SI); Punta Lara, 25 September 1973, Zardini 195 (LP); Ca. por el camino Boca Cerrada a Villa Elisa, 24 October 1986 (fr), Tur 1840 (LP); Villa Elisa, 20 November 1993, Tur 2059 (LP); 28 September 1993 (fr), Tur 2058 (LP); Magdalena, Ea. San Isidro, 06 June 2001, Torres Robles 410 (LP); 12 September 2001, Torres Robles 446 (LP); Punta Lara 06 October 2002 (fr), Tur & Torres Robles 2155 (LP); Punta Lara, Camino a Villa Elisa, 18 October 2002 (fr), Tur & Torres Robles 2157 (LP). Corrientes. Mburucuyá, Ea. Santa Teresa, 07 September 1956, Pedersen 3977 (LP); Dep Capital, Riachuelo, 19 September 1970, Benitez 18 (BAA).
Entre Ríos. Concordia, Yuquerí Chico, 03 November 1949, Job s.n. (LP); Ayuí 3°, 13-IX-1968, Tur 1099 (SI); Gualeguaychú, 30 November 1969, Burkart & Troncoso 27746 (LP); Gualeguaychú, Arroyo Malo cerca de Paranacito, 30 December 1969 (fr), Burkart & Troncoso 27728 (LP); Gualeguaychú, Arroyo Salinas, 15 October 1971 (fr), Burkart 28755 (LP); Colón, El Palmar, Arroyo El Borracho, 24 September 1977 (fr), Troncoso, Bacigalupo & Nicora 2121 (SI). Santa Fe. Dep. Capital, Laguna Los Espejos, 27 November 1970 (fr), Tur 1363 (BAA); Tucumán. Leales, Los Gomez, 17 September 1919, Venturi 629 (LP).
Field observations. At the time of marking, nine out of 10 branches marked as male presented two or more whorls of female flowers at the base (Table 2, branches 1 to 9). All branches marked as female later developed whorls of male flowers (Table 2, branches 11 to 20).
Table 2. Sex of the flowers present on each whorl of 20 branches (10 in a male patch and 10 in a female patch at sight) registered at the field during the flowering period of 2002. The highlighted row shows the whorl used to mark the branches in the patch identified at sight as either male or female. Abbreviations: m: whorl with male flowers; f: whorl with female flowers; mf: whorl with male and female flowers; -: sterile whorl.
Tabla 2. Sexo de las flores presentes en cada verticilo de veinte ramas (10 en un parche masculino y 10 en un parche femenino a simple vista) registradas a campo durante el período de floración de 2002. La fila resaltada muestra el verticilo usado para marcar las ramas en el parche identificado a simple vista como masculino o femenino. Abreviaturas: m: verticilo con flores masculinas; f: verticilo con flores femeninas; mf: verticilo con flores masculinas y femeninas; -: verticilo estéril.


Four out of the 20 study branches presented alternate whorls of female and male flowers (Table 2, branches 3, 6, 8 and 20). At the same time, five of them presented both female and male flowers on the same whorl (Table 2, branches 1, 3, 8, 18 and 19).
All branches developed whorls of male flowers at the end of the flowering period, and all presented fruits on the submerged parts. The branches that survived drought and herbivory followed their flowering period developing vegetative whorls.
DISCUSSION
Is Myriophyllum aquaticum dioecious or monoecious? By analyzing herbarium material we noted the presence of monoecious specimens in a broad range of distribution of M. aquaticum in Argentina. This indicates that M. aquaticum is not strictly dioecious, at least in this part of the world. This conclusion was supported by field observations.
Our observations demonstrated that there were herbarium specimens and field grown plants that showed male flowers and/or fruits (Table 1; Table 2; Fig. 1). These results disagree with findings of Meijden (1969), Meijden & Caspers (1971) and Orchard (1979, 1981), who reported that male flowers and fruits were rare in South America. Furthermore, it was verified that this species developed abundant fruits, although they are not easily seen because they promptly become submerged. These findings agree with those of Ayres Fevereiro (1975), who pointed out that M. aquaticum flowers and fruits during almost the whole year.

Fig. 1. Flowers and fruits in Myriophyllum aquaticum. A: Branch with male and female flowers in different whorls. B: branch showing whorl with mature fruits. C: detail of a whorl with fruits. Abbreviations: m: male flower; f: female flower; fr: fruit. B, C, from Tur 1840 (LP).
Fig. 1. Flores y frutos en Myriophyllum aquaticum. A: rama con flores masculinas y femeninas en verticilos diferentes. B: rama mostrando verticilo con frutos maduros. C: detalle de un verticilo con frutos. Abreviaturas: m: flor masculina; f: flor femenina; fr: fruto. B, C, de Tur 1840 (LP).
The disagreement about the presence or absence of male flowers and fruits may be because of the timing of collection. In agreement with Ayres Fevereiro (1975), our results demonstrated that branches with female flowers at the base later developed male flowers in the upper part (Table 2). Depending on the timing of sampling, it is possible to find specimens with only female flowers, female and male flowers, or male flowers and submerged fruits. More fertile whorls at the moment of marking were found on branches marked as male than female (Table 2). We can conclude that "male patches" began to flower earlier than "female patches". Thus, a "female patch" will be seen later than a "male patch".
In his work, Orchard (1981) mentioned that "all specimens examined of M. aquaticum, with one exception, are strictly dioecious, bearing either male or female flowers", recognizing only the specimen Gillies s.n. (K) as monoecious. Among the other five specimens from Argentina that he cited as dioecious, two were cited as monoecious in this study (Pedersen 3977 and Venturi 629). We observed the original material deposited in LP, and Orchard studied its duplicates deposited at F, MO, NY, P, and US. It is likely that the branches present in the duplicates had only female flowers, whereas the original material that we observed had both types.
The absence of male flowers in the region where M. aquaticum is adventitious, and the conclusion that its reproduction is sexual in its natural distribution area, and vegetative in the region where it is adventitious, has previously been reported (Meijden, 1969; Meijden & Caspers, 1971; Orchard, 1979, 1981; Li & Hsieh, 1996; Moody & Les, 2010). This conclusion is reinforced by the results of Moody & Les (2010); these authors remarked that all their collected plants had the same genotype. Moreover, there is some evidence of different sexual strategies on aquatic plants depending on competition or environmental conditions (Dorken & Barrett, 2004). Aquatic plants display a remarkable range of reproductive strategies, including diverse sexual systems and clonal propagation (reviewed by Barrett et al., 1993). Variation in reproductive traits will influence the ability of populations to colonize and persist in different types of habitats (Dorken & Barrett, 2004). These authors suggested that monoecy represents a flexible reproductive strategy to adjust allocation to female vs. male sex function in response to environmental heterogeneity, i.e. reproduction might be sexual in competitive habitats, whereas vegetative reproduction might be common under favorable environmental conditions.
Nomenclatural issue? Our results demonstrated that Myriophyllum aquaticum is monoecious in Argentina, whereas other authors (Orchard, 1979, 1981; Li & Hsieh, 1996; Negritto& Anton, 1996; Moody & Les, 2010) affirmed that the species is dioecious. Only the type specimen of M. proserpinacoides (nomenclatural synonym of M. aquaticum) is accepted as monoecious by Orchard (1981). Considering the list of monoecious specimens cited here, and that this material was collected in Argentina, another question arises: Might monoecy vs. dioecy define in fact the existence of two species?
Reiche (1898) alluded in the Flora of Chile that M. proserpinacoides is identical to M. verticillatum var. limosum (in spite of the dioecy ocurring in the former). Orchard (1981) mentioned that M. verticillatum was listed in Chile, but the specimens examined by him were in fact M. aquaticum or M. quitense. Then, he excluded M. verticillatum from the flora of South America. Very likely, there has been confusion among these species most of the times.
These examples suggest that the disagreements on the sexual issue could be taxonomic in nature. A much broader analysis should be made to examine the distribution of monoecious and dioecious populations, in combination with general morphological studies of monoecious and dioecious plants in the context of a nomenclatural revision.
ACKNOWLEDGEMENTS
We thank curators of the Herbaria; Daniel H. Bozzano for the photography of figure 1. A, and Rosemary Scoffield for corrections of the text in English. This study was part of a Doctoral Fellowship (S.S.T.R.) of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and it was made at the Universidad Nacional de La Plata. G.P. is researcher of CONICET.
REFERENCES
1. Ayres Fevereiro, P.C. (1975). Haloragáceas. In: P.R. Reitz (Ed.), Flora Ilustrada Catarinense, pp. 1-17. Itajaí, Santa Catarina: Conselho Nacional de Pesquisas. [ Links ]
2. Barrett, S.C.H., C.G. Eckert & B.C. Husband (1993). Evolutionary processes in aquatic plants. Aquatic Botany 44: 105-145. [ Links ]
3. Boutique, R. & B. Verdcourt (1973). Myriophyllum. In: R.M. Polhill (Ed.). Flora of Tropical East Africa, pp. 7-9. London: Crown Agents for the Colonies. [ Links ]
4. Cabrera, A.L. (1976). Regiones fitogeográficas Argentinas. In: L.R. Parodi (Ed.) Enciclopedia Argentina de Agricultura y Jardinería 2 (1), pp. 1-85. Buenos Aires: Acme. [ Links ]
5. Dorken, M.E. & S.C.H. Barrett (2004). Phenotypic plasticity of vegetative and reproductive traits in monoecious and dioecious populations of Sagittaria latifolia (Alismataceae): a clonal aquatic plant. Journal of Ecology 92: 32-44. [ Links ]
6. Gillies, J. (1833). Myriophyllum proserpinacoides. In: W.J. Hooker, Bot. Misc. 3: 313. [ Links ]
7. Holmgren, P.K., N.H. Holmgren & L.C. Barnett (1990). Index Herbariorum. Part 1: The Herbaria of the World (8th edn). Bronx, N. Y.: International Association for Plant Taxonomy, New York Botanical Garden. [ Links ]
8. Hurrel, J.A., D.H. Bazzano & G. Delucchi (2006). Dicotiledóneas Herbáceas I Nativas y Exóticas. In: J.A. Hurrel (Ed.). Colección Biota Rioplatense, vol. 11, pp. 122-123. Buenos Aires: LOLA. [ Links ]
9. Kanitz, A. (1882). Myriophyllum. In: A.W. Eichler (Ed.). Flora Brasiliensis 13 (2): 379-380. Munich: Apud R. Oldenbourg in comm. [ Links ]
10. Lahitte, H.B., J.A. Hurrel, K. Mehltreter, M.J. Belgrano, L.S. Jawkowski, M.P. Haloua & G. Canda (1997). Plantas de la Costa, pp. 46-47. Buenos Aires: LOLA. [ Links ]
11. Li, Z-Y. & Ch-F. Hsieh (1996). New materials of the genus Myriophyllum L. (Haloragaceae) in Taiwan. Taiwania 41: 322-328. [ Links ]
12. Meijden, R. van der. (1969). An annotated key to the South-East Asiatic, Malesian, Mascarene, and African species of Myriophyllum (Haloragaceae). Blumea 17: 303-311. [ Links ]
13. Meijden, R. van der. & N. Caspers (1971). Haloragaceae. In: C.G.G.J. van Steenis (Ed.). Flora Malesiana 7, pp. 239-263. Groningen: Wolters-Noordhoff Publishing. [ Links ]
14. Moody, M.L. & D.H. Les (2010). Systematics of the Aquatic Angiosperm Genus Myriphyllum (Haloragaceae). Systematic Botany 35: 121-139. [ Links ]
15. Negritto, M.A. & A.M. Anton (1996). Haloragaceae. In: A. Hunziker (Ed.). Flora Fanerogámica Argentina 27, pp. 1-4. Córdoba: Programa PROFLORA (CONICET). [ Links ]
16. Orchard, A.E. (1979). Myriophyllum (Haloragaceae) in Australasia. I. New Zeland: A revision of the genus and a synopsis of the family. Brunonia 2: 247-287. [ Links ]
17. Orchard, A.E. (1981). A revision of South American Myriophyllum (Haloragaceae), and its repercussions on some Australian and North American Species. Brunonia 4: 27-65. [ Links ]
18. Reiche, K. (1898). Estudios críticos sobre la flora de Chile 2, pp. 269-270. Santiago: Universidad de Chile. [ Links ]
19. Schindler, A.K. (1905). Halorrhagaceae. In : H.G.A. Engler (Ed.). Das Pflanzenreich 23: 89. Leipzig: Engelmann. [ Links ]
20. Tur, N.M., S.S. Torres & G. Peter (2009). About the Typification of Myriophyllum aquaticum (Haloragaceae). Novon 19: 127-129. [ Links ]
21. Vellozo, J.M. da C. (1825) [1829]. Enydria., In: J.M. da C. Vellozo, Florae fluminensis 1: 56-57. Rio de Janeiro: Typographia nationali. [ Links ]
22. Zuloaga F.O. & O. Morrone (1999). Catálogo de las plantas vasculares de la República Argentina II. Fabaceae-Zygophylaceae (Dicotyledoneae). Monogr. Syst. Bot. Miss. Bot. Gard. 74: 761-762. [ Links ]














