Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.81 no.1 Vicente López ene./jun. 2012
ORIGINAL ARTICLES
Productive response of Nannochloropsis oculata, cultured in different media and their efficiency as food for the rotifer Brachionus rotundiformis
Respuesta productiva de Nannochloropsis oculata, cultivada en diferentes medios y su eficiencia como alimento para el rotífero Brachionus rotundiformis
Campaña-Torres A1, LR Martínez-Córdova2, M Martínez-Porchas3, JA López-Elías2, MA Porchas-Cornejo1
1 Centro de Investigaciones Biológicas del Noroeste (CIBNOR). Km 2,35 camino al Tular estero de Bacochibampo, C.P. 85465, Guaymas, Sonora, México.
2 Departmento de Investigaciones Científicas y Tecnológicas de la Universidad de Sonora (DICTUS). Luis Donaldo Colosio s/n, Colonia Centro, C.P. 83000. Hermosillo, México.
3 Centro de Investigación en Alimentación y Desarrollo, A.C. (CIAD) Km 0.6, carretera a La Victoria, C.P. 83304. Hermosillo, México.
Address Correspondence to: Luis R. Martínez-Córdova, e-mail: lmtz@guaymas.uson.mx; Marcel Martínez Porchas, e-mail: marcel@ciad.mx
Recibido / Received 30.XI.2011.
Aceptado / Accepted 14.I.2012.
Abstract. An experiment was conducted to evaluate the effect of different culture media on the productive response and proximate composition of microalgae, Nannochloropsis oculata, and the subsequent effect of such microalgae on the productive response and proximate composition of the rotifer Brachionus rotundiformis. Microalgae were cultured in different media: Guillard F/2 (control), an agricultural fertilizer, and an aquacultural fertilizer. Thereafter, such microalgae were used to feed rotifers. A better productive response was observed when microalgae were cultured in the agricultural fertilizer. In addition, the chemical proximate composition of microalgae was influenced by the type of culture medium used. When used as natural food for rotifers, higher productive response was observed in those rotifers fed with microalgae previously cultured in the aquacultural fertilizer. Also, the proximate composition of rotifers was influenced by the type of microalgae they consumed. In conclusion, the productive response and quality of microalgae depended on the medium in which they grew; also, the productive response and proximate composition of rotifers depended upon the nutritional quality of N. oculata.
Keywords: Alternative medium; Culture medium; Microalgae quality; Natural feed; Productive response; Proximate composition; Rotifer production.
Resumen. Se condujo un experimento para evaluar el efecto de diferentes medios de cultivo sobre la respuesta productiva y composición proximal de la microalga, Nannochloropsis oculata, y el efecto subsiguiente de dicha microalga sobre la respuesta productiva y composición proximal del rotífero Brachionus rotundiformis. La microalga fue cultivada en distintos medios: Guillard F/2 (control), un fertilizante agrícola, y un fertilizante acuícola. Posteriormente, estas microalgas fueron utilizadas para alimentar rotíferos. Se observó una mejor respuesta productiva en aquellas microalgas cultivadas con el fertilizante agrícola. Además, la composición química proximal de las microalgas fue influenciada por el medio de cultivo utilizado. Cuando se utilizaron las microalgas como alimento natural para rotíferos, se observó una mejor respuesta productiva en aquellos rotíferos alimentados con microalgas previamente cultivadas con fertilizante acuícola. La composición química proximal de los rotíferos también fue influenciada por el tipo de microalgas que consumieron. En conclusión, la respuesta productiva y calidad de las microalgas dependió del medio en que se desarrollaron; también, la respuesta productiva y composición proximal de los rotíferos dependió de la calidad nutricional de N. oculata.
Palabras clave: Alimento natural; Calidad de microalga; Composición proximal; Medios alternativos; Producción de rotíferos; Respuesta productiva.
INTRODUCTION
The use of live feed in aquaculture is a key aspect for the success in nursery and larval stages of fish and crustacean; however, the use of such food source has high economical costs (Lin et al., 2009). Besides the common use of microalgae for most of the cultured species, the use of Artemia as live feed seems to be a universal practice in aquaculture (Faleiro & Narciso, 2009; Campaña-Torres et al., 2010; González et al., 2010). However, Artemia is expensive, sometimes scarce or may have doubtful quality (Watanabe et al., 1983). Herein, different systems to produce alternative organisms have been performed in order to replace Artemia at larviculture and nursery phases; among the alternative organisms to be used as live feed, stand out copepods (Rippingale & Payne, 2005; Farhadian et al., 2009; Martínez-Córdova et al., 2011), rotifers (Campaña-Torres et al., 2009) and cladocerans (Wiwattanapatapee et al., 2002; Martin et al., 2006).
The highest operative cost in the culture of these alternative organisms is the microalgae production, since they are used as the prime feed for such species. In particular, the media for microalgae culture based on highly purified compounds are too expensive. Successful attempts have been made in order to substitute conventional media for alternative and less expensive media. For instance, Voltolina & Lopez- Elías (2002) documented that agricultural fertilizers are viable from the technical and economical perspective, for the culture of diatoms. Simental-Trinidad et al. (2002) cultured benthic diatoms using agricultural fertilizers. Sanchez-Torres et al. (2008) used media enriched with fish isolates to culture Nannochloropsis oculata. Paniagua-Michel et al. (1987) evaluated the culture of marine microalgae using nutrients obtained from biodigestors.
The effect of the alternative media on the productive response and nutritional quality of microalgae, and on the effectiveness of such microalgae used as food source for diverse rotifer and zooplankton species (that are commonly used as live feed for fish and crustacean) have not yet been determined. Moreover, microalgae cultured in alternative media could have an effect on the proximate composition and nutritional value of such species. The use of rotifers as live feed has demonstrated to improve aquaculture yields, in particular Brachionus spp. (Campaña-Torres et al., 2009). Herein, N. oculata is one of the most important feed used to produce Brachionus sp. (Spolaore et al., 2006a); however, scarce information was found regarding the effect of culture media on microalgae response as well as the effect of such microalgae on the rotifer productive performance.
The objectives of this experiment were to evaluate two alternative media (one agricultural, one aquacultural) for culturing the microalgae Nannochloropsis oculata, and the efficacy of such microalgae on the production and proximate composition of the rotifer Brachionus rotundiformis.
MATERIALS AND METHODS
Experiment 1: Microalgae culture. Two alternative media and a control were used to culture the microalgae Nannochloropsis oculata. A simple experimental design was performed with four replicates per treatment. Microalgae inoculums (Nannochloropsis oculata) were obtained from a commercial laboratory (Maricultura del Pacifico, S.A.) at Sonora, México. Guillard F/2 medium was used to maintain the inoculums alive.
Different media were used to evaluate their effect on the productive response and proximate composition of N. oculata (Table 1). The control consisted in the conventional medium Guillard F/2 (GF/2; Guillard, 1975). Treatment 1 (T-Agri) was based on the use of an agricultural fertilizer (Monoammonium Phosphate, NH4H2PO4). To prepare the medium, 137 g of the fertilizer were diluted in 1 L of filtered and sterile water; thereafter, the medium was enriched with 1 mL/L of a mixture of minerals (ferric chloride, cupric sulfate, zinc sulfate, cobalt chloride, manganese chloride and sodium molybdate). Treatment 2 (T-Aqua) was based on an aquacultural fertilizer "Nutrilake with phosphorus" (Fertlizantes Tepeyac, Obregón, Sonora, México) enriched with vitamins for human consumption (Bedoyecta); the vitamin solution was added to the medium at a rate of 0.01 mL/L. Media were administered to each treatment at the beginning of the trial at a rate of 1 mL/L.
Table 1. Chemical content of the different media used to culture microalgae Nannochloropsis oculata.
Tabla 1. Contenido químico de diferentes medios utilizados para el cultivo de la microalga Nannochloropsis oculata.

The experimental cultures were initiated in assay tubes (50 mL) using Guillard F/2 medium, until a density of 4 x 106 cells/mL was achieved. After that, microalgae were divided in the three different treatments; from that point on, microalgae were cultured and scaled to 80 L fiberglass columns, using the media described above (Control, T-Agri and T-Aqua) during three days. The initial density of microalgae cultured in columns was 1 x 105 cells/mL for each column.
A light intensity of 100 µmol/m2s, an environmental temperature of 21-23 °C, pH 8.2-8.5 and constant aeration were maintained during all the experimental period, as suggested by López-Elías et al. (2004). The water used for microalgae culture had a salinity of 34 and was previously filtered and sterilized.
Microalgae response. Growth rate was evaluated at the end of the bioassay; the cellular density was measured using a Neubauer® chamber (VWR Scientific, Los Angeles, CA, USA) and an optical microscope (Ward´s®, Rochester, N. Y., USA). To calculate the growth rate, the following equation was used:
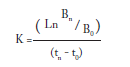
where K was growth rate, B0 the microalgae density (cell/mL) at the beginning, Bn the microalgae density at any time, and tn- t0 was the period of the culture from inoculation.
The generation time (gt) was estimated as ![]()
Final dry biomass was estimated after filtration with a 0.2 µm membrane average pore and dried in an oven until constant weight.
To analyze microalgae proximate composition (protein, lipid, carbohydrate and ash), samples were filtered using fiberglass filters (47 mm; Walkman® Maidstone UK) previously dried. The protein content was measured following the methodology described by Lowry et al. (1951), and modified by López-Elías & Voltolina (1993). The carbohydrate concentration was estimated by the "phenol-sulfuric acid" method suggested by Dubois et al. (1956). The lipid content was calculated by a colorimetric method described by Pande et al. (1963) and modified by Bustillos-Hurtado & López-Elías (1994). Ash was estimated by incineration of samples at 475 °C, as documented by Sánchez-Saavedra & Voltolina (2006).
Experiment 2: Use of microalgae to feed rotifers. Once high densities of microalgae cultured in different media were achieved, they were evaluated as food source for rotifers; the zooplankton species were reared and fed with microalgae during 15 days.
A simple and randomized experimental design was performed to evaluate the effect of microalgae (N. oculata) cultured in medium GF/2 (m-control), the agricultural fertilizer (mT-Agri) and the aquacultural fertilizer (T-Aqua), on the productive response and proximate composition of B. rotundiformis. Four repetitions per treatment were performed.
The experimental units consisted on 19-L plastic tanks provided with air stones; the rotifer culture was started with a density of 2.5 rotifers/mL. Microalgae were added daily and a density of 3 x 105 cells/mL was maintained within each experimental unit. At the end of the culture, rotifers were harvested with a net (60 mesh); thereafter, the number of rotifers/ mL, free eggs/mL and the fecundity index were calculated; subsamples (10 x 50 mL) were collected from each unit and counted by stereoscopic microscopy (50 x) (DelValls et al., 1996). Fecundity index was estimated by the method proposed by Ramírez-Sevilla et al. (1991), considering total of females and those females with eggs.
Finally, the protein, carbohydrate, lipid and ash contents of rotifers were estimated on a dry basis, by using the standard methods of the Association of Official Analytical Chemists (AOAC).
Statistical analysis. Production parameters of microalgae and rotifers were analyzed by a one-way ANOVA and a subsequent mean comparison test (Tukey). If data did not comply with normality (Shapiro-Wilk) and homoscedastic (Bartlett) tests, a non-parametric test was used (Kruskall-Wallis). Proximate composition of microalgae and rotifers was analyzed by a chi-square test. A significance level of a = 0.05 was considered.
RESULTS
Experiment 1: Microalgae response. Growth rate (K) and generation time (gt) appeared to be better in microalgae cultured with the agricultural fertilizer (T-Agri; Monoammonium Phosphate) (Fig. 1) although no significant differences (p>0.05) among treatments were registered. However, such responses were reflected on the final microalgae concentration and biomass. Microalgae concentration was 70% higher when cultured in the agricultural fertilizer (T-Agri; > 2.0 x 106 cells/ mL) than that observed in microalgae from T-Aqua and similar to those cultured in the control (1.76 x 106 cells/mL; Fig. 1, Table 2). A similar tendency was observed for biomass response (Table 2); the highest biomass was observed in T-Agri (14.3 mg/L), while the lowest was observed in T-Aqua (8.7 mg/L).

Fig. 1. Weekly concentration of N. oculata cultured in different media based on an agricultural fertilizer (T-Agri), an aquacultural fertilizer (T-Aqua) and a conventional medium (Guillard F/2).
Fig 1. Concentración semanal de N. oculata cultivada en diferentes medios basados en un fertilizante agrícola (T-Agri), un fertilizante acuícola (T-Aqui) y un medio convencional (Guillard F/2).
Table 2. Production parameters of N. oculata cultured in different media.
Tabla 2. Parámetros de producción de N. oculata cultivada en diferentes medios.

Regarding the proximate composition, higher carbohydrate concentrations were observed for microalgae cultured in T-Agri (24.15%), followed by T-Aqua (20.8%) and the control (Guillard F/2; <18.8%) (Table 3). Contrarily, the lipid concentration was higher in microalgae from control (>30%) compared to both treatments (<28%) (Table 3). The protein concentration was higher in microalgae from T-Aqua (>23%) compared to the other treatments (<21%). No differences were observed for ash concentrations which ranged from 26 to 27%.
Table 3. Chemical proximate composition of N. oculata cultured in three different media.
Tabla 3. Composición química proximal de N. oculata cultivadas en tres diferentes medios.

Experiment 2: Zooplankton response. Higher productive responses were observed in those rotifers fed with microalgae previously cultured in T-Aqua (mT-Aqua), followed by those fed with microalgae from T-Agri (mT-Agri) and the control (m-control) (Table 4). Rotifers fed with mT-Aqua showed the highest density (>130 rotifers/mL), number of eggs (>20 eggs/ mL), free eggs (>14 eggs/mL) and fecundity index (>1.2). No differences in density were observed among rotifers fed with mT-Agri and m-control; however, higher number of eggs, free eggs and fecundity index were observed in the treatment where mT-Agri was used, compared to m-control (Table 4).
Table 4. Production parameters of rotifer, Brachionus rotundiformis fed on N. oculata previously cultured in different media.
Tabla 4. Parámetros de producción del rotífero, Brachionus rotundiformis alimentados con N. oculata previamente cultivada en diferentes medios.

The proximate composition of rotifers was affected by the different treatments (Table 5). Rotifers fed on mT-Agri showed the highest carbohydrate concentration (22.7%) followed by those fed on m-control (15.9%) and mT-Aqua (10.3%). Lipid concentration was higher in rotifers fed on mT-Agri (>24%) compared to those fed on mT-Aqua and m-control (<22%). The protein concentration was the lowest in rotifers fed on mT-Agri (>46%), while no differences were assessed among the other treatments (>55%) (Table 5).
Table 5. Chemical proximate composition of B. rotundiformis fed on N. oculata previously cultured in different media.
Tabla 5. Composición química proximal de B. rotundiformis alimentada con N. oculata previamente cultivada en diferentes medios.

DISCUSSION
The experimental conditions maintained during the study were similar to those reported by Spolaore et al. (2006b) as suitable for N. oculata.
The production of N. oculata observed in all treatments was one order of magnitude lower than that obtained in photobioreactors using F/2 medium. However, it was similar to the production achieved in commercial laboratories which cultivate their microalgae using the same methodology of this experiment (Chini Zittelli et al., 2003). It is remarkable that microalgae cultured in the agricultural fertilizer had a similar productive response than those cultured in the conventional medium (control). This could represent a better alternative to substitute the conventional medium, which is more expensive and less accessible to consumers compared to the agricultural fertilizer. Regarding the aquacultural fertilizer, it was demonstrated that N. oculata can somehow use it as nutrient source, but the productive response was not the expected; Leal et al. (2004) reported that the excessive use of aquacultural fertilizer (Nutrilake) resulted in lower production of the microalgae Tetraselmis suecica, compared to other nutrient sources. It is possible that the lower content of metasylicate in the aquacultural fertilizer compared to the other treatments could have a negative effect on the growth of microalgae. Silicate is an essential nutrient and a deficiency negatively affects the growth process of diatoms (Wen & Chen, 2000).
The effect of culture media was also reflected on the proximate composition of microalgae. Carbohydrate and protein concentrations were similar or near the values reported for Nannochloropsis sp. (Rebolloso-Fuentes et al., 2001), except for the lipid content, which was quite higher. However, other authors have reported similar lipid concentrations for this species (Renaud et al., 1991). The changes on proximate composition of microalgae produced by the different culture media appeared to have a subsequent effect on the productive response of rotifers. Some authors have reported that the type and quality of feed consumed by rotifers Brachionus sp. have an effect on their chemical proximate composition (Whyte et al., 1990; Forlov et al., 1991).
In spite that the better productive responses were achieved in microalgae cultured with the conventional medium and the alternative agricultural fertilizer, the better productive responses of rotifers were observed when mT-Aqua was used as feed. Thus, the quality of microalgae produced by agricultural fertilizers could not be adequate at all for rotifer production; however, the nutritional quality should also be estimated by measuring the fatty acids and amino acids profiles.
Considering the better productive response of microalgae cultured in T-Agri, the agricultural fertilizer must be studied as potential medium to produce N. oculata destined as food source for other species or other purposes. For instance, N. oculata has been identified as one of the most promising photoautotrophic producers of eicosapentaenoic acid; also it has been used to produce biodiesel, and has many other applications (Spolaore et al., 2006b; Converti et al., 2009). The Guillard F/2 medium has been used for massive production of Nannocloropsis sp. in photobioreactors (Briassoulis et al., 2009); thus it can be hypothesized that the use of agricultural fertilizers could provide similar or better results at a lower cost.
The higher productive response obtained in rotifers fed on microalgae produced with mT-Aqua, may be attributed to the higher protein content found in such microalgae. Yamamoto et al. (2009) documented that rotifers require high amounts of protein to have an adequate growth performance. In addition, the quality of macronutrients could have an effect on the production of rotifers; however, no measurements were done about these parameters.
The rotifer production was quite lower than that obtained in technified systems (novel culture; Yoshimura et al., 2003); however, the production of rotifers fed on mT-Aqua was similar to that observed in traditional systems (Rueda-Jaso, 1991). Regarding the number of eggs and fecundity index of rotifers fed on mT-Agri and mT-Aqua, similar results were obtained by Yufera & Navarro (1995) after feeding B. plicatilis with microalgae powder (N. oculata). However, rotifers from m-control had lower productive responses, which suggests that microalgae cultured in the conventional medium were less adequate to produce B. rotundiformis, compared to the aquacultural fertilizer. No reports were found concerning the nutrient requirement of rotifers for egg reproduction.
The proximate composition of rotifers was also affected by the nutritional quality of microalgae. Carbohydrate concentrations were within the range reported for Brachionus sp. (Guisande & Serrano, 1989). Lipid and protein concentrations in rotifers from mT-Agri and m-control were similar to those reported for rotifers fed with Chlorella sp. (Rueda-Jaso, 1991; Srivastava et al., 2006). However, rotifers from mT-Aqua had greater concentrations of protein. The quality of rotifers was different according to their food source; it could be inferred that rotifers from mT-Aqua could be useful to feed carnivorous species due to the high protein and lower carbohydrate concentrations. Rotifers produced in the other treatments may be useful for production of omnivorous species; nevertheless, further studies about the effect of such rotifers on the productive responses of fish and/or crustacean are needed.
It can be concluded that the agricultural fertilizer is an adequate alternative to produce N. oculata, while the aquacultural fertilizer are useful to produce microalgae destined for rotifer production. Also, the productive response and the quality of rotifers as food source depend not only upon the microalgae species they consume, but on the nutritional quality of such species.
REFERENCES
1. Briassoulis, D., P. Panagakis, M. Chionidis, D. Tzenos, A. Lalos, C. Tsinos, K. Beberidis & A. Jacobsen (2010). An experiment al helical-tubular photobioreactor for continuous production of Nannochloropsis sp. Bioresource Technology 101: 6768-6777. [ Links ]
2. Bustillos-Hurtado, C.A. & J.A. López-Elías (1994). Composición química de dos especies de microalgas en dos fases de crecimiento, cultivadas en medios simplificados. Oceanología 2: 133-148. [ Links ]
3. Campaña-Torres, A., L.R. Martínez-Córdova, H. Villarreal-Colmenares, J. Hernández-López, J.M. Ezquerra-Brauer & E. Cortés-Jacinto (2009). Efecto de la adición del rotífero Brachionus rotundiformis (Tschugunoff, 1921) sobre la calidad del agua y la producción, en cultivos superintensivos de camarón blanco del Pacífico Litopenaeus vannamei (Boone, 1931). Revista de Biología Marina y Oceanografía: 44: 335-342. [ Links ]
4. Campaña-Torres, A., L.R. Martínez-Córdova, H. Villarreal-Colmenares & E. Cortés-Jacinto (2010). Evaluation of different concentrations of adult live Artemia (Artemia franciscana, Kellogs 1906) as natural exogenous feed on the water quality and production parameters of Litopenaeus vannamei (Boone 1931) intensively pre grown. Aquaculture Research 42: 40-46. [ Links ]
5. Chinni-Zitelli, G., L. Rodolfi & M.R. Tredici (2003). Mass cultivation of Nannochloropsis sp. in annular reactors. Journal of Applied Phycology 15: 107-114. [ Links ]
6. Converti, A., A.A. Casazza, E.Y. Ortiz, P. Perego & M. Del Borghi (2009). Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chemical Engineering Process 48: 1146-1151. [ Links ]
7. Del Valls, T.A., L.M. Lubián, M. González del Valle & J.M. Forja (1996). Evaluating decline parameters of rotifer Brachionus plicatilis populations as an interstitial wáter toxiticy bioassay. Hydrobiologia 341: 159-167. [ Links ]
8. Dubois, M., K.A. Gilles, K. Hamilton, P.A. Rebers & F. Smith (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350-356. [ Links ]
9. Faleiro, F. & L. Narciso (2009). Brachionus vs Artemia duel: Optimizing first feeding of Upogebia pusilla (Decapoda: Thalassinidea) larvae. Aquaculture Research 295: 205-208. [ Links ]
10. Farhadian, O., F. MdYuso & S. Mohamed (2009). Nutritional values of Apocyclops dengizicus (Copepoda: Cyclopoida) fed Chaetocerous calcitrans and Tetraselmis tetrathele. Aquaculture Research 40: 74-82. [ Links ]
11. Forlov, A.V., S.L. Pankov, K.N. Geradze, S.A. Pankova & L.V. Spektorova (1991). Influence of the biochemical composition of food on the biochemical composition of the rotifer Brachionus plicatilis. Aquaculture 97: 181-202. [ Links ]
12. González, R., J.D. Celada, A. González, V. García, J.M. Carral & M. Sáez-Royuela (2010). Stocking density for the intensive rearing of juvenile crayfish, Pacifastacus leniusculus (Astacidae), using Artemia nauplii to supplement a dry diet from the onset of exogenous feeding. Aquaculture International 18: 371-378. [ Links ]
13. Guisande, C. & L. Serrano (1989). Analysis of protein, carbohydrate and lipid in rotifers. Hidrobiológica 186-187: 339-346. [ Links ]
14. Lin, Q., J. Lin, D. Zhang & Y. Wang (2009). Weaning of juvenile seahorses Hippocampus erectus Perry, 1810 from live to frozen food. Aquaculture 291: 224-229. [ Links ]
15. Leal, S., G. Delgado & Y. Almaguer (2004). Efecto de cinco tipos de productos zeolíticos sobre el crecimiento de la microalga Nannocloropsis gaditana. Revista Investigaciones Marinas 25: 241-244. [ Links ]
16. López-Elías, J.A. & D. Voltolina (1993). Semicontinuos cultures of four microalgae with a nonconventional medium. Ciencia y Mar 2: 169-180. [ Links ]
17. Lowry, O.H., Rosebrough, N.J. & A.L. Fair (1951). Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry 193: 265-275. [ Links ]
18. Martin, L., A. Arenal, J. Fajardo, E. Pimentel, L. Hidalgo, M. Pacheco, C. García & D. Santiesteban (2006). Complete and partial replacement of Artemia nauplii by Moina micrura during early postlarval culture of white shrimp (Litopenaeus schmitti). Aquaculture Nutrition 12: 89-96. [ Links ]
19. Martínez-Córdova, L.R., A. Campaña-Torres & M. Martínez- Porchas (2011). Effect of supplying four copepod densities (Acartia sp. and Calanus pacificus) on the productive response of Litopenaeus vannamei pregrown intensively at microcosm level. Ciencia y Mar 37: 415-423. [ Links ]
20. Pande, S.V., R.P. Khan & T.A. Venkitsubra (1963). Microdetermination of lipids and serum total fatty acids. Analytical Biochemistry 6: 415-423. [ Links ]
21. Paniagua-Michel, J., B.C. Farfán & L.F. Bückle-Ramírez (1987). Culture of marine microalgae with natural biodigested resources. Aquaculture 64: 249-256. [ Links ]
22. Ramírez-Sevilla, R., R.A. Rueda-Jasso, J.L. Ortíz-Galindo & B. González-Acosta (1991). Metodología para el cultivo experimental del rotífero Brachionus plicatilis. Investigaciones Marinas CICIMAR 6: 287-290. [ Links ]
23. Rebolloso-Fuentes, M.M., A. Navarro-Pérez, F. García-Camacho, J.J. Ramos-Miras & J.L. Guil-Guerrero (2001). Biomass nutrient profiles of the microalga Nannochloropsis. Journal of Agricultural and Food Chemistry 49: 2966-2972. [ Links ]
24. Renaud, S.M., D.L. Parry, L.V. Thinh, C. Kuo, A. Padovan & N. Sammy (1991). Effect of light intensity on the proximate biochemical composition and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. Journal of Applied Phycology. 3: 43-53. [ Links ]
25. Rippingale, R.J. & M.F. Payne (2005). Suitability of the copepod Gladioferens imparipes for intensive cultivation for aquaculture. Chapter 9. In: Lee, C.S., O´Bryen, P. & N.H. Marcus (eds). Copepods in Aquaculture. Blackwell Publishing. Ames, Iowa, USA. 209 pp. [ Links ]
26. Rueda-Jaso, R.A. (1996). Efecto nutricional de tres microalgas y una cianobacteria en el cultivo de rotíferos Brachionus plicatilis Müller: 1786. Ciencia y Mar 22: 313-328. [ Links ]
27. Sánchez-Saavedra, M.P. & D. Voltolina (2006). The growth rate, biomass production and composition of Chaetoceros sp. grown with different light sources. Aquacultural Engineering 35: 161-165. [ Links ]
28. Sánchez-Torres, H., J. Juscamaita-Morales, J. Vargas-Cárdenas & R. Oliveros-Ramos (2008). Producción de la microalga Nannochloropsis oculata (Droop) Hibberd, en medios enriquecidos con ensilado biológico de pescado. Applied Ecology 7: 149-158. [ Links ]
29. Simental-Trinidad, J.A., M.P. Sánchez-Saavedra & J.G. Correa- Reyes (2002). Biochemical composition of benthic manine diatoms using as culture medium a common agricultural fertilizer. Journal of Shellfish Research 20: 611-617. [ Links ]
30. Spolaore, P., C. Joannis-Cassan, E. Duran & A. Isambert (2006a). Commercial applications of microalgae. Journal of Bioscience and Bioengineering 101: 87-96. [ Links ]
31. Spolaore, P., Joannis-Cassan, C., Duran, E. & A. Isambert (2006b). Optimization of Nannochloropsis oculata growth using the response surface method. Journal of Chemical Technology and Biotechnology 81: 1049-1056. [ Links ]
32. Srivastava, A., K. Hamre, J. Stoss, R. Chakrabarti & K. Tonheim (2006). Protein content and amino acid composition of the live feed rotifer (Brachionus plicatilis): With emphasis on water soluble fraction. Aquaculture 254: 534-543. [ Links ]
33. Voltolina, D. & J.A. López-Elías (2002). Cultivos de apoyo: tendencias e innovaciones. In: Martínez-Córdova L.R. (ed). Camaronicultura, Avances y Tendencias. AGT Editores, México, D.F. 147 p. [ Links ]
34. Watanabe, T., C. Oowa, C. Kitajima & S. Fujita (1983). Nutritional values of live organisms used in Japan for mass propagation of fish: A review. Aquaculture 34: 115-143. [ Links ]
35. Wen, Z-Y. & F. Chen (2000). Heterotrophic production of eicosapentaenoic acid by the diatom Nitzchia laevis: effects of silicate and glucose. Journal of Industrial Microbiology and Biotechnology 25: 218-224. [ Links ]
36. Whyte, J.N.C. & W.D. Nagata (1990). Carbohydrate and fatty acid composition of the rotifer, Brachionus plicatilis, fed monospecific diets of yeast or phytoplankton. Aquaculture 89: 263-272. [ Links ]
37. Wiwattanapatapee, R., N, Padoongsombat, T. Choochom, S. Tang & A. Chaimongkol (2002). Water flea Moina macrocopa as a novel biocarrier of norfloxacin in aquaculture. Journal of Controlled Release 83: 23-28. [ Links ]
38. Yamamoto, T., K. Teruya, T. Hara, H. Hokazono, I. Kai, H. Hashimoto, H. Fuguita, H. Matsunari & K. Mushiake (2009). Nutritional evaluation of rotifers in rearing tanks without water exchange during seed production of amberjack Seriola dumeri. Fisheries Science 75: 697-705. [ Links ]
39. Yoshimura, K., K. Tanaka & T. Yoshimatsu (2003). A novel culture system for the ultra-high-density production of the rotifer, Brachionus rotundiformis - a preliminary report. Aquaculture 227: 165-172. [ Links ]
40. Yúfera, M. & N. Navarro (1995). Population dynamics on the rotifer Brachionus plicatilis cultured in non-limiting food consumption. Hydrobiologia 313-314: 399-405. [ Links ]














