Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.81 no.1 Vicente López ene./jun. 2012
ORIGINAL ARTICLES
Histocytological examination on organogenesis and somatic embryogenesis of HBsAg-transgenic cherry tomato mutant
Evaluación histológica de la organogénesis y embriogénesis somática del tomate cereza mutante transgénico-HBsAg
Guan Z-J1,2*, B Guo1*, Y-L Huo3, J-K Dai4, Y-H Wei1
1 Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education Northwest University, Xi'an Shaanxi, 710069 P.R. China. e-mail: zhengjunguan@126.com
2 Department of Life Sciences, Yuncheng University, Yuncheng, Shanxi, 044000 P.R.China.
3 Centre of Biological and Chemical Exiperiment, Yuncheng University, Yuncheng, Shanxi, 044000 P.R. China.
4 Enzyme Engineering Institute of Shaanxi, Academy of Sciences, Xi'an Shaanxi, 710600 P.R. China.
* Guan Z-J and B Guo contributed equally to this paper.
Address Correspondence to: Yahui Wei, Ph. D. Key Laboratory of Resource Biology and Biotechnology in Western China, School of Life Science, Northwest University,
Xi'an 710069, P.R. China. e-mail: weiyahui@nwu.edu.cn ; guanzj722822@126.com
Recibido / Received 29.VII.2010.
Aceptado / Accepted 14.I.2012.
Abstract. The initiation and development of organogenic buds and somatic embryos in HBsAg-transgenic cherry tomato (Lycopersicum esculentum var. cerasiforme) mutant were studied histologically. The leaf explants of the mutant were cultured on Murashige & Skoog (MS) basal medium supplemented with 6-BA 1.0 mg/L and IAA 0.05 mg/L for callus induction. Histological studies on the leaf explants of the mutant at various developmental stages revealed that organogenic buds first appeared in the axillary position of explants on the 14th cultured day, and then somatic embryos formed in the same mutant explants after 35 days of culture. Transmission electron microscopy and scanning electron microscopy indicated that there were significant changes in morphology and quantity of some organelles in the mutant callus cells compared with the control. On the 7th day of culture, embryogenic callus cells of the control showed dense cytoplasm and abundant organelles; at the same time, there was little cytoplasm or less organelles, except for a great number of dense lipid bodies in the mutant callus cells. In the later stage, the chloroplasts, Golgi bodies and mitochondria in the mutant cells also had obvious differences. These findings demonstrated that the regeneration pathway in vitro of the HBsAg-transgenic cherry tomato mutant showed variation, which was useful for the regeneration and gene transfer protocol.
Keywords: Histocytological; Organogenesis; Somatic embryogenesis; HBsAg; Transgenic; Cherry tomato; Mutant.
Abbreviations: HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; MS: Murashige-Skoog; SEM: Scanning electron microscopy; TEM: Transmission electron microscopy.
Resumen. La iniciación y desarrollo de yemas organogénicas y embriones somáticos en el mutante Lycopersicon esculentum var. ceraciforme transgénico-HBsAg fueron estudiadas histológicamente. Los explantos foliares del mutante se cultivaron en medio basal Murashige y Skoog suplementado con 6-BA 1,0 mg/L y IAA 0,05 mg/L para inducir la formación de callos. Estudios histológicos en los explantos foliares del mutante en varios estados del desarrollo revelaron que las yemas organogénicas aparecieron primero en la posición axilar de los explantos después de 14 días de iniciado el estudio, y los embriones somáticos se formaron en los mismos explantos del mutante después de 35 días de cultivo. Microscopía electrónica de transmisión y de barrido indicaron que hubo cambios significativos en la morfología y cantidad de algunas organelas en las células del callo del mutante comparado con el control. Luego de 7 días de cultivo, las células del callo embriogénico del control mostraron un citoplasma denso y organelas abundantes; al mismo tiempo, hubo poco citoplasma o menos organelas, excepto por un gran número de cuerpos lípidos densos en las células del callo del mutante. En estados más tardíos, los cloroplastos, cuerpos de Golgi y mitocondria en las células mutantes también tuvieron diferencias obvias. Estos resultados demostraron que la vía de regeneración in vitro del mutante de tomate Lycopersicon esculentum var. ceraciforme transgénico-HBsAg mostró variabilidad, lo cual fue útil para la regeneración y el protocolo de transferencia de genes.
Palabras clave: Histocitológica; Organogénesis; Embriogénesis somática; HBsAg; Transgénico; Tomate cherry; Mutante.
Abreviaturas: HBsAg: Antígeno de superficie de Hepatitis B; HBV: Virus de Hepatitis B; MS: Murashige-Skoog; SEM: Microscopía electrónica de barrido; TEM: Microscopía electrónica de transmisión.
INTRODUCTION
Cherry tomato (Lycopersicon esculentum var. cerasiforme) is a subvariety of common tomato, an annual or perennial herb of the nightshade (Solanaceae), tomato genus (Lycopersicon). Being a delicious, nutritious food, it is one of the most extensive and in-depth materials used in research fields such as genetics, breeding, physiology and molecular biology. In our previous work, a transgenic plant expression vector containing HBsAg gene was constructed and an HBsAg-genetically-modified cherry tomato mutant was obtained by Agrobacterium mediation (Hao et al., 2007; Guan et al., 2010). The transgenic mutant of cherry tomato had a good expression of hepatitis B surface antigen. The transfer of HBsAg gene caused a series of complex changes of genetic basis, and a profound influence on biochemical and physiological growth and development of the transformed mutant. The mutant exhibited robust growth, dark green leaves, less obvious leaf margin notch, hypertrophic leaves, short and thick stems, more adventitious roots, round fruits, and abortive seeds (Guan et al., 2011a ).
In recent years, the follow-up reports of transgenic tomato focused on some physiological changes (Luo et al., 2000), post-harvest physiological characteristics (Tian et al., 2003; Huang et al., 2006), endogenous hormone levels, fruit cell structures, resistance (Delannay et al., 1989; McGurl et al., 1994) and nutritive qualities (Romer et al., 2000; Fraser et al., 2002). There were no detailed reports on morphogenesis during in vitro regeneration of transgenic tomato plants.
Somatic embryogenesis and organogenesis are two ways that can lead to the formation of plantlets in vitro. There were some reports on cherry tomatoes cultured in vitro (Sun, 2000; Lu, 2001; Chen et al., 2002; Zhou et al., 2002; Bartley & Ishida, 2007).The leaves of cherry tomato as explants were cultured on MS medium added with 6-BA and IAA to form embryogenetic callus. Some of the mesophyll cells started to form embryonic cells at the cultured 5th day and could continue to produce somatic embryos (Guan et al., 2011b). However, the research on in vitro culture of transgenic cherry tomato has not been reported. Also, no detailed microstructural and ultrastructural studies have been carried out in order to clarify the morphogenesis variation of genetically transformed plants from callus culture.
Further information on developmental stages of embryogenesis and organogenesis are needed to study the developmental pattern of in vitro morphogenesis in transgenic mutant. This could lead to a better understanding of in vitro development of cherry tomato, and consequently result in higher regeneration rates that should benefit clonal propagation and transformation works. Leaf explants of HBsAg-transgenic cherry tomato mutant were induced in vitro and the changes of morphogenesis were processed for TEM and SEM during its callus development to characterize the embryonic development in the transgenic plants. This paper described the developmental pattern of the mutant, embryogenesis and organogenesis, which were induced from the leaf explant source at a cellular level scale. This research might have important theoretical significance on the use of transgenic plant mutants, and help to understand further the role of the HBsAg gene.
MATERIALS AND METHODS
The control (wild) plant. Seeds of cherry tomato (Lycopersicon esculentum var. cerasiforme Cherry No.1) were kindly provided by DENG J J (Shannxi, xi'an, Vegetable Research Institute). After immersed in water for two hours, the seeds were sterilized with 70% alcohol for one minute, and were washed with sterile distilled water several times. Then, they were sterilized with 10% NaClO for five minutes and were rinsed several times. Finally, the seeds were placed to germinate on 1/2 half strength Murashige-Skoog (MS) (Murashige & Skoog, 1962) medium without any hormone. Seedlings of non-transformed cherry tomato plants were used as controls NK.
The HBsAg-transgenic mutant. The transgenic mutant was obtained after co-cultivating leaf explants with Agrobacterium tumefaciens, strain LBA4404, harbouring the vector pCAMBIA1301/HB. The T-DNA of pCAMBIA1301/HB contains the HBsAg gene (approx. 0.7 kb). Transformation procedures used to obtain the mutant were described in Hao et al. (2007). To allow sterile seeds of the existing transgenic mutant, the transformed plants were subcultured for follow-up research.
Callus induction and culture conditions. Leaves were excised from 3-week-old seedlings of the transgenic mutant and the control for callus induction. The explants were grown on MS medium, supplemented with 6-BA 1.0 mg/L and IAA 0.05 mg/L, under a 14-h photoperiod, irradiance of 2500 lx, and temperature of 25 ± 2 °C.
Cytological observations. Light microscopy: Leaf explants were harvested at 0, 7, 14, 21, 28 and 35 days from culture on both the control and the mutant cultured on medium. Samples were immediately fixed in formalin/glacial acetic acid (FAA) and 50% ethanol mixture (5:5:90: v/v/v) in 24 h, followed by dehydration in a graded ethanol series (30%, 50%, 70%, 85%, 95%, 100%) and embedded in paraffin wax. An 8-mm thick section was cut from each paraffin block containing the corresponding sample with a rotary Microtome (Laica, Germany) and then stained with 1% safranine, and counterstained with 0.2% fast green, for general histological examinations. The sections were observed and digitally recorded using a Leica microscope (DMLB) equipped with a video camera (Leica, DC 300F; Leica Microscopy and Systems GmbH, Wetzlar, Germany).
Transmission electron microscopy: Leaf explants at various development stages were pre-fixed at 4 °C overnight in 2.5% glutaraldehyde (v/v) in 0.1M PBS (Phosphate Buffered Saline) at pH 7.2, and then they were washed three times with the same buffer. The samples were post-fixed in 2% OsO4 in 0.1M PBS for 2 hour, then washed three times in 0.1M PBS, with 10 minutes intervals between each washing. Then they were dehydrated through a graded ethanol series from 50% to 100%, and then infiltrated and embedded in Spurr's resin. Ultrathin sections (40 nm), obtained by a Reichert OM-U3 ultramicrotome, were stained with uranyl-acetate (Gibbons & Grimstone, 1960) and lead citrate (Reynolds, 1963), and observed under a transmission electron microscope (JEOL TEM-1230EX).
Scanning Electron Microscopy: Calli at different developmental stages were fixed in 2.5% buffered glutaraldehyde, washed in 0.05 mol/L potassium phosphate buffer (pH 6.8), dehydrated in ethanol series from 30% to 100%, critical point dried in liquid CO2 and coated with gold-palladium. The mounted samples were observed with a JEOL6100 Scanning Electron Microscope.
RESULTS AND DISCUSSION
We analyzed the callus of both the mutant and the control using safranine/fast green as stains. Using a light microscope, histological examination showed that the mutant had a different regeneration pattern from the control during the callus formation and differentiation. To study more closely the morphogenesis process of the HBsAg-transformed mutant tissues, we performed microscopic analysis at different developmental stages. Meristematic areas were seen on the surface of the leaf explants as early as 7 days after culture (Fig. 1).
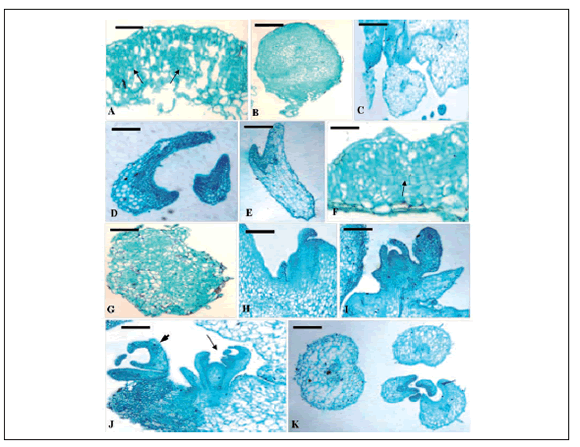
Fig. 1 Cytological analysis of the leaf explant development of both HBsAg-transgenic mutant and the control. A~F. Histological observation of somatic embryo development of the control. A. Initiation of rapid cell division (arrows) in the leaf surface after 7 d on MS medium (bar = 400 µm); B. Initiation of many globular embryos with a suspensor after 14 d on MS medium (bar = 400 µm); C. The heart-shaped globular embryo further developed (bar = 400 µm), torpedo-shaped embryoid (D, bar = 400 µm) and finally formed cotyledon embryoid (E, bar = 400 µm) after 35 days of culture (F, bar = 400 µm). F~K. Histological observations of organogenetic and somatic embryo development of the transgenic mutant. F. Cells generally started to divide strong in periclinal or anticlinal way in the local area, or cortex, epidermis outside the vascular tissue of the mutant leaf explants after 7 d on MS medium (bar = 400 µm); G. General view of an independent callus produced at the proximal part of the 14-day-old explant (bar = 250 µm); H. Emergence of adventitious shoot primordia in the axillary position (arrows) (bar = 250 µm); I. Well-developed bud originating from the peripheral cell layers of a shoot primordium (Bar = 400 µm); J. Shoot organogenesis (arrows) and somatic embryo ( arrowheads) happened simultaneously after 35 days on MS medium (bar = 250 µm); K. Various embryo appeared after 35 days of culture (bar = 250 µm).
Fig. 1. Análisis citológico del desarrollo de explantos foliares tanto para el mutante transgénico-HBsAg como para el control. A~F. Observación histológica del desarrollo de embriones somáticos del control. A. Iniciación de división celular rápida (flechas) en la superficie foliar después de 7 días en medio MS (barra = 400 µm); B. Iniciación de muchos embriones globulares con un suspensor después de 14 días en medio MS (barra = 400 µm); C. El embrión más desarrollado globular, con forma de corazón (barra = 400 µm), embrioide con forma de torpedo (D, barra = 400 µm) y finalmente la formación del cotiledón embrioide (E, barra = 400 µm) después de 35 días de cultivo (F, barra = 400 µm). F~K. Observaciones histológicas del desarrollo del embrión somático y organogenético del mutante transgénico. F. Las células comenzaron a dividirse fuertemente en forma anticlinal o periclinal en el área local, o corteza, epidermis fuera de los tejidos vasculares de los explantos foliares del mutante después de 7 días en medio MS (barra = 400 µm); G. Vista general de un callo independiente producido en la parte proximal del explanto de 14 días de edad (barra = 250 µm); H. Emergencia de primordios de tallos adventicios en la posición axilar (flechas) (barra = 250 µm); I. Yema bien desarrollada originada de las capas de células periféricas de un primardio de tallo (barra = 400 µm). J. La organogénesis del tallo (flechas) y del embrión somático (punta de flechas) sucedieron simultáneamente después de 35 días en medio MS (barra = 250 µm); K. Varios embriones aparecieron luego de 35 días de cultivo (barra = 250 µm).
In the 7-day-old control callus, many spherical cell clusters appeared, which had clear boundaries with the surrounding parenchyma cells. Some cells of large-nuclear and small in size, especially multinucleated cells could be observed (Fig. 1A). The subsequent developmental process showed that these peculiar cells in the control callus, what were called embryonic cells, were the original source of somatic embryogenesis (Fig. 1B). Then their development progressed through typical globular, heart and torpedo-shapes at cotyledonary stages (Fig. 1C~E). However, the formation periods of the majority of the embryos did not possess a clearly defined boundary. Various types of embryos all appeared simultaneously after 35 days of cultivation. These results indicated that regeneration of the control occurred via embryogenesis on MS medium containing 6-BA and IAA. The presence of somatic embryos at different developmental stages at the same time showed the asynchrony of this process, which has been related to the strong trend of scutellum of somatic embryos to secondary proliferation, so that two or more embryoid generations could appear simultaneously (Wang & Vasil, 1982).
At the cortex, epidermis outside the vascular tissue of 7-day-old mutant leaf explants, cells generally started to divide strong in periclinal or anticlinal ways (Fig. 1F), and further developed into an independent callus so as to get the whole explant swelling, and the surface falling apart at 14 days of culture (Fig. 1G). Subsequently, adventitious shoot primordia emerged in the axillary position of callus mass (Fig. 1H) and developed into a complete bud (Fig. 1I). Cultured after 35 days, many embryomatic cells formed near the buds of N244 callus (Fig. 1J~K).
We demonstrated that shoot organogenesis and somatic embryogenesis occurred together during the in vitro regeneration of transgenic cherry tomato mutant leaf explants treated by 6-BA combined with IAA. However, only somatic embryogenesis pathway was observed during the regeneration of non-transformed cherry tomato plants under the same culture condition. In addition, after dedifferentiation, the cells of the control explants directly differentiate into embryonic cells and further formed somatic embryos; nevertheless, the complete callus was firstly produced on the surface of the mutant leaf explants, and then indirectly formed organ primordia and somatic embryos.
To study further the variation of callus development in the transgenic mutant, we performed an observation at the electron microscopy scale. The 7-day-old control callus was mostly composed of well-developed meristematic tissues containing (1) small, uniform, actively dividing meristem-like cells with large nucleus, (2) prominent stained nucleoli, (3) lack of intercellular spaces, (4) few small vacuoles, (5) dense cytoplasm, (6) abundant organelles, and (7) a small number of lipid bodies (Fig. 2A~C). These cellular masses could be easily fragmentated resembling young globular somatic embryos (Fig. 2D). This indicated that these somatic cells changed to embryonic cells. In general, in these structures and in the embryogenic calli, a large number of amyloplasts were present in almost all cells. Moreover, normal chloroplasts were never found (Fig. 2E). With the further development of embryonic cells, nuclear shifting to the end, cell wall thickening (Fig. 2F), and lipid bodies increased significantly; to a later stage, cells were very rich in organelles (e.g., Golgi bodies) (Fig. 2G), enlarged lipid bodies, particularly with respect to an increased number of active mitochondria (Fig. 2H). At the stage of cotyledonary embryo, cells with large central vacuole appeared, the nucleus was close to the cell wall, numerous chloroplasts began to form and were affixed to the cell wall, and other organelles decreased (Fig. 2I).

Fig. 2 The somatic embryogenetic ultrastructure of the control plant. A. SEM observation of callus surface after 7 days of culture (bar = 20 µm). Its traits included prominent nucleoli (B, bar = 2 µm), few small vacuoles, abundant organelles, a small number of the lipid bodies (LB) and dense cytoplasm (C, bar = 2 µm). D. SEM evidence of young globular somatic embryos (bar = 10 µm). E. A great quantity of amyloplasts were present in the somatic cells (bar = 2 µm). With the further development of embryonic cells, nuclear shifting to the end, cell wall thickening (F, bar = 2 µm); to a later stage, cells were very rich in Golgi bodies (G, bar = 0.5 µm), particularly in the increased number of active mitochondria (H, bar = 0.5 µm). At the stage of cotyledonary embryo, cells with large central vacuoles appeared, the nucleus was close to the cell wall, numerous chloroplast began to form and were affixed to the cell wall (I, bar = 1 µm).
Fig. 2. Ultraestructura embriogenética somática de la planta control. A. Observación SEM de la superficie del callo después de 7 días de cultivo (barra = 20 µm). Sus características incluyeron nucleolo prominente (B, barra = 2 µm), unas pocas vacuolas pequeñas, organelas abundantes, un pequeño número de los cuerpos lipídicos (LB) y citoplasma denso (C, barra = 2 µm). D. Evidencia SEM de embriones somáticos globulares jóvenes (barra = 10 µm). E. Un gran número de amiloplastos estuvieron presentes en las células somáticas (barra = 2 µm). Con el desarrollo adicional de células embriónicas, cambio nuclear hacia el final, espesamiento de la pared celular (F, barra = 2 µm); en un estado más tardío, las células contuvieron un gran número de cuerpos de Golgi (G, barra = 0,5 µm), particularmente en un mayor número de mitocondrias activas (H, barra = 0,5 µm). En el estado de embrión cotiledonar, aparecieron células con grandes vacuolas centrales, el núcleo estuvo cerca de la pared celular, se comenzaron a formar numerosos cloroplastos y se fijaron a la pared celular (I, barra = 1 µm).
Compared with the control, there were dramatic differences in the cellular ultrastructure of the mutant callus (Fig. 3). On the 7th day of culture, the most outer mesophyll cells of the incision died, the disrupted layer appeared, and some elongated and disorganized cells formed on the surface of the mutant callus (Fig. 3A, B). There were little cytoplasm or other organelles, except a great number of dense lipid bodies.

Fig. 3 The organogenetic and embryonic ultrastructure of the transgenic mutant. A. After 7 days of culture, cut edges of leaf explants showed dead cellular layer (bar = 20 µm). B. Elongated and disorganized cells in the mutant callus (bar = 10 µm). C. A great number of dense plastids appeared, and some small vacuoles of different size were around the central vacuole (bar = 2 µm). D. After 13 days of culture, the meristematic cell clusters near the surface of the callus differentiated into normal bud primordia (arrow) (bar = 20 µm). E. After 18 days of culture, intracellular organelles significantly increased (e.g., mitochondria and rough endoplasmic reticulum), and the double-membrane stucture of chloroplast was visible (bar = 1 µm). F~G. After 26 days of culture, the plastids with some starch grains of varying sizes changed obviously (bar = 1 µm). In addition, a mass of digestive bubble was present within the central vacuole (H, bar = 1 µm). After the formation of organs began, intracellular starch grains gradually disappeared (I, bar = 2 µm), plastids gradually changed to chloroplasts (J, bar = 1 µm), chloroplast lamellar system was reduced (K, bar = 1 µm), until it finally disappeared completely (L, bar = 1 µm). Ch: chloroplast; ER: endoplasmic reticulum; G: Golgi body; M: mitochondria; N: Nuclear; Nu: nucleolus; P: plastid; S: starch grains; rER: rough endoplasmic reticulum; V: vacuole.
Fig. 3. La ultraestructura embriónica y organogenética del mutante transgénico. A. Después de 7 días de cultivo, cortes de explantos foliares, mostraron capas de células muertas (barra = 20 µm). B. Células desorganizadas y alongadas en el callo mutante (barra = 10 µm). C. Aparece un gran número de plástidos densos, y algunas vacuolas pequeñas de diferente tamaño estuvieron alrededor de la vacuola central (barra = 2 µm). D. Después de 13 días de cultivo, los grupos de células meristemáticas cerca de la superficie del callus se diferenciaron en primordios de yemas normales (flecha) (barra = 20 µm). E. Después de 18 días de cultivo, las organelas intracelulares se incrementaron significativamente (ej. mitocondria y retículo endoplasmático en etapas iniciales), y fue visible la estructura de doble membrana de los cloroplastos (barra = 1 µm). F-G. Después de 26 días de cultivo, los plástidos con algunos granos de almidón de tamaño variado cambiaron obviamente (barra = 1 µm). Después que comenzó la formación de órganos, los granos de almidón intracelulares desaparecieron gradualmente (I, barra = 2 µm), los plástidos cambiaron gradualmente a cloroplastos (J, barra = 1 µm), el sistema lamelar de los cloroplastos fue reducido (K, barra = 1 µm) hasta que desapareció completamente (L, barra = 1 µm). Ch: Cloroplasto; ER: Retículo endoplasmático; G: Cuerpos de Golgi; M: Mitocondria; N: Núcleo; Nu: Nucleolo; P: Plástido; S: granos de almidón; rER: retículo endoplasmático en sus inicios; V: vacuola.
Some small vacuoles of different size were around the central vacuole (Fig. 3C). After 21 days from culture, the meristematic cell clusters near the surface of the mutant callus differentiated into normal bud primordia (Fig. 3D). At this time, intracellular organelles increased significantly, especially mitochondria and rough endoplasmic reticulum. The lipid bodies obviously changed, and chloroplast grana lamellae formed interspersed with some starch grains of varying sizes. Also the double-membrane structure of the chloroplast, and other organelles such as mitochondria and plastids were clearly visible (Fig. 3E~G). In addition, a mass of digestive bubbles were presented within the central vacuole (Fig. 3H). After the formation of organs began, intracellular starch grains gradually disappeared, and the chloroplast lamellar system also became smaller until it disappeared completely (Fig. 3J~L). These differences in cellular ultra-microstructure probably determined the distinct morphogenesis pathway of the mutant, and that the shoot organogenesis and somatic embryogenesis occurred together.
This paper mainly described organogenesis and somatic embryogenesis in vitro in transgenic mutant, especially in transformed cherry tomato (Lycopersicon esculentum var. cerasiforme). The organogenesis of transgenic cherry tomato developed indirectly via callus formation and further differentiation to adventitious buds. That was to say, for introduction of foreign gene, the regeneration in vitro of the wild cherry tomato added to new pattern - organogenesis. And adventitious shoots and somatic embryos were simultaneously induced on the same explant for the transgenic mutant.
Somatic embryogenesis and organogenesis in vitro are a direct result of hereditary, developmental, and environmental conditions, as well as growth regulators, and other physical and chemical components of the culture media (Finstad et al., 1993). Initial genetic dissections of the organogenesis process in plant tissue culture were performed through the characterization of the mutants in terms of their morphogenic responses (Ozawa et al., 1998; Yancheva et al., 2003). The present study examined shoot organogenesis and somatic embryos from the mutant leaf explants. It was done from a developmental point of view on the basis of the current understanding of the concept of tissue competence, determination and differentiation. Considerable attention was given to the development of the regeneration process using detailed histology, SEM and TEM.
In conclusion, the present work elucidated the temporal framework and certain aspects of the developmental states of cell determination and morphological differentiation that are associated with shoots and somatic embryos development in transgenic cherry tomato mutant leaf explants. Histological and ultrastructural observations of the early events in the induction and development of shoot meristems in transgenic mutant could be useful for the regeneration and gene transfer protocol. This study contributed to the basis for a further molecular analysis of the genes involved in the in vitro cherry tomato explant regeneration. Further research on the physiological, biochemistry variation mechanisms of the regeneration pathway of the in vitro transformed mutant is ongoing.
ACKNOWLEDGEMENTS
This work was financially supported by the National Natural Science Foundation of China (No. 31000144); Opening Foundation of Key Laboratory of Resource Biology and Biotechnology in Western China (Northwest University) (08JZ72); Specialized Foundation of the Department of Education of Shaanxi Province, China (09JK746); Northwestern University graduate self-innovation project (10YZZ36); Yuncheng University Doctor Scientific research project (YQ- 2011042). We are grateful to Deng Jun-jun for providing the seeds of cherry tomato, CAMBIA Institute of Australia for providing plasmid pCAMBIA1301, and all members of our laboratory for numerous valuable discussions and advice about this paper.
REFERENCES
1. Bartley, G.E. & B.K. Ishida (2007). Ethylene-sensitive and insensitive regulation of transcription factor expression during in vitro tomato sepal ripening. Journal of Experimental Botany 58: 2043- 2051. [ Links ]
2. Chen, Q., R.Z. Hu, H.D. Li, L. Jiang & L.H. Wang (2002). Studies on breeding technology of cherry tomato. Plant Physiology Communications 38: 118-120. [ Links ]
3. Delannay, X., B.J. LaVallee, R.K. Proksch, R.L. Fuchs, S.R. Sims, J.T. Greenplate, P.G. Marrone, R.B. Dodson, J.J. Augustine, J.G. Layton & D.A. Fischhoff (1989). Field performance of transgenic tomato plants expressing the Bacillus Thuringiensis var. Kurstaki insect control protein. Nature Biotechnology 7: 1265-1269. [ Links ]
4. Finstad, K., D.C.W. Brown & K. Joy (1993). Characterization of competence during induction of somatic embryogenesis in alfalfa tissue culture. Plant Cell, Tissue and Organ Culture 34: 125-132. [ Links ]
5. Fraser, P.D., S. Romer, C.A. Shipton, P.B. Mills, J.W. Kiano, N. Misawa, R.G. Drake, W. Schuch & P.M. Bramley (2002) Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proceedings of the National Academy of Sciences USA 99: 1092-1097. [ Links ]
6. Guan, Z.J., B. Guo, Y.L. Huo, Z.J. Guan & Y.H. Wei (2010). Overview of expression of hepatitis B surface antigen in transgenic plants. Vaccine 28: 7351-7362. [ Links ]
7. Guan, Z.J., B. Guo, H.Y. Hao, Y.L. Huo, Z.J. Guan & Y.H. Wei (2011a). Morphological and Physiological Characteristics of Transgenic Cherry Tomato Mutant with HBsAg Gene. Russian Journal of Genetics 47: 923-930. [ Links ]
8. Guan, Z.J., B. Guo & Y.H. Wei (2011b). Histocytological observation on somatic embryogenesis derived from leaves of cherry tomato. Journal of Chinese Electron Microscopy Society 30: 158-165. [ Links ]
9. Gibbons, I.R. & A.V. Grimstone (1960). On the flagellate structure in certain flagellates. Journal of Biophysical and Biochemical Cytology 7: 697-716. [ Links ]
10. Hao, H.Y., J.G. Zhu & Y.H. Wei (2007). Expression of Oral Hepatitis B Vaccine in Transgenic Tomato. Food science 28: 201-204. [ Links ]
11. Huang, J.X., X.J. Song, J.K. Li, S.Y. Li, J.H. Yang, Y.J. Liu & Z. Zhao (2006). Relation of fruit ripeness and ethylene content in transgenic tomato. Southwest Horticulture 34: 8-9. [ Links ]
12. Lu, Q.N. (2001). Studies on tissue culture and rapid propagation of cherry tomatoes. Journal of Yichun University (Natural science) 23: 51-53. [ Links ]
13. Luo, Y.B., S.P. Hao & J.P. Sheng (2000). Characters of postharvest physiology of antisense ACS transgenic tomato fruits. Journal of China Agricultural University 5: 13-17. [ Links ]
14. McGurl, B., M. Orozco-Cardenas, G. Pearce & C.A. Ryan (1994). Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. PNAS 91: 9799-9802. [ Links ]
15. Murashige, T. & F. Skoog (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497. [ Links ]
16. Ozawa, S., I. Yasutani, H. Fukuda, A. Komamine & M. Sugiyama (1998). Organogenic responses in tissue culture of srd mutants of Arabidopsis thaliana. Development 125: 135-142. [ Links ]
17. Reynolds, E.S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology 17: 208-212. [ Links ]
18. Romer, S., P.D. Fraser, J.W. Kiano, C. A. Shipton, N. Misawa, W. Schuch & P.M. Bramley (2000). Elevation of the provitamin A content of transgenic tomato plants. Nature Biotechnology 18: 666-669. [ Links ]
19. Sun, L.N. (2000). Tissue culture and rapid propagation of cherry tomatoes. Plant Physiology Communications 36: 135. [ Links ]
20. Tian, J.L., Y.A. Yang & Y.K. He (2003). Salt tolerance of transgenic tomato with HAL1gene. Journal of Plant Physiology and Molecular Biology 29: 409-414. [ Links ]
21. Wang, D. & I.K. Vasil (1982). Somatic embryogenesis and plant regeneration from inflorescence segments of Pennisetum purpureum Schum. (Napier of Elephant Grass). Plant Science Letters 25: 147- 154. [ Links ]
22. Yancheva, S.D., S. Golubowicz, E. Fisher, S. Lev-Yadun & M.A. Flaishman (2003). Auxin type and timing of application determine the activation of the developmental program during in vitro organogenesis in apple. Plant Science 165: 299-309. [ Links ]
23. Zhou, L.J., W. Wei, J.X. Zhou & Y.L. Cao (2002). Tissue Culture and Plantlet Regeneration of Lycopersicum esculentum var. cerasiforme. Plant Physiology Communications 38: 356. [ Links ]














