Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.81 no.2 Vicente López jul./dic. 2012
ARTÍCULO ORIGINAL
Biomass production and grain yield of three sorghum lines difering in drought resistance
Producción de biomasa y rendimiento de grano de tres líneas de sorgo que difieren en su resistencia a sequía
Castro-Nava S1, J Ortiz-Cereceres2, M del C Mendoza-Castillo2, AJ Huerta3
1 Universidad Autónoma de Tamaulipas. División de Estudios de Postgrado. Centro Universitario Victoria. Cd. Victoria Tamaulipas. C.P. 87149. México.
2 Colegio de Postgraduados. Campus Montecillo. Montecillo, Estado de México. C.P. 56230. Mexico.
3 Miami University. Department of Botany. Oxford, Ohio. 45056, USA.
Address Correspondence to: Sergio Castro-Nava. Phone/fax 52-834-318-1721, e-mail: scastro@uat.edu.mx
Recibido / Received 28.II.2012.
Aceptado / Accepted 14.III.2012.
Abstract. The aim of this study was to determine the eficiency of drought classification criteria that we previously reported for our grain sorghum genotypes. Two tolerant genotypes of grain sorghum [Sorghum bicolor (L.) Moench] (UAT-124 and UAT-152) and one susceptible (UAT-30) were subjected to drought under greenhouse conditions at either panicle initiation, flag leaf, or flowering. Results showed that the effects of drought depended on when drought stress occurred during development. Biomass of the three sorghum genotypes was significantly reduced at the flag leaf stage (48%) by drought stress, but the greatest reduction occurred in the susceptible genotype UAT-30 (71%). Results showed that biomass accumulation for UAT-124 and UAT-30 was strongly in accordance with our previous drought classification, but this was not true for UAT-152. Drought stress reduced grain yield significantly in all genotypes, when it was applied at fag leaf stage (24%) and at flowering (28%), but not at panicle initiation. Resistant and susceptible genotypes had the same response in terms of grain yield when stress was applied at any of the three phenological stages. The results indicate that genotype responses to drought treatment were inconsistent with their initial classification. The identification of tolerant and susceptible sorghum genotypes could be better accomplished by applying drought stress at the more susceptible stage of development, flag leaf, and selection must be based on biomass, grain yield and leaf area.
Keywords: Sorghum bicolor L. Moench; Water stress; Drought; Phenology; Biomass accumulation; Grain yield.
Resumen. El objetivo de este estudio fue determinar la eficiencia en los criterios de clasificación para sequía, reportados previamente en genotipos de sorgo para grano. Dos genotipos de sorgo [Sorghum bicolor (L.) Moench] tolerantes (UAT-124 y UAT-152) y uno susceptible (UAT-30) fueron sometidos a sequía bajo condiciones de invernadero cuando se encontraban en las etapas de iniciación de la panoja, hoja bandera y floración. Los resultados mostraron que el efecto de la sequía dependió de la etapa en la que ocurrió el estrés durante el desarrollo. La biomasa de los tres genotipos de sorgo fue significativamente reducida por la sequía en la etapa de hoja bandera (48%), aunque la reducción más significativa ocurrió en el genotipo susceptible UAT-30 (71%). Los resultados mostraron que la acumulación de biomasa para UAT-124 y UAT-30 coincidió fuertemente con la clasificación previa para la tolerancia a la sequía, aunque esto no fue congruente para UAT-152. El estrés por sequía redujo el rendimiento de grano significativamente en todos los genotipos, cuando éste fue aplicado en la etapa de hoja bandera (24%) y floración (28%), pero no en la iniciación de la panoja. Los genotipos resistentes y el susceptible tuvieron la misma respuesta en términos del rendimiento de grano, cuando el estrés fue aplicado en cualquiera de las tres etapas fenológicas. Los resultados indicaron que la respuesta de los genotipos fue inconsistente con su clasificación inicial. La identificación de genotipos de sorgo en tolerantes y susceptibles se debería hacer aplicando el estrés hídrico en la etapa más susceptible del desarrollo, la hoja bandera; y la selección debe estar basada en la biomasa, el rendimiento de grano y el área foliar.
Palabras clave: Sorghum bicolor L. Moench; Estrés hídrico; Sequía; Fenología; Acumulación de biomasa; Rendimiento de grano.
INTRODUCTION
Drought is one of the major limiting factors to agriculture, and considered as the most important cause of yield reduction in crop plants (Bray et al., 2000; Mahajan & Tuteja, 2005). The aim of many studies has been to understand the adverse effects of drought on growth and metabolism of cultivated species. As a result of this type of work, the mean yield of crops in areas with high drought risk could be increased and stabilized using varieties that are tolerant to water stress (Bruce et al., 2002). Consequently, the development of drought-tolerant cultivars and water-use-eficient crops is a global concern (Barnabas et al., 2008). However, selection for drought tolerance has been a very dificult process because of inconsistency in testing environments and interaction between stages of plant growth and environment. Te genetic mechanisms that control the expression of drought tolerance traits in crop plants are poorly understood (Kebede et al., 2001). In plant stress research, drought tolerance is one of the most dificult traits to study and characterize. It has been demonstrated that biomass accumulation is a function of water use by plants (Balota et al., 2008), and it has been used in maize (Bolaños & Edmeades, 1993; Bolaños et al., 1993) and sorghum breeding programs (Castro et al., 2000) as an indicator of the response of plants to water stress. One of the main effects of drought on plants is the reduction of biomass accumulation (Lilley & Fukai, 1994; Salih et al., 1999, Rosales-Serna et al., 2000; Frederick et al., 2001, Tsuji et al., 2003). This is mainly caused by drought-induced inhibition of leaf and steam elongation, which difers among species (Pelleschi et al., 1997), and a reduction of relative growth and net CO2 assimilation rates (Younis et al., 2000). The result of drought stress in grasses is generally a reduction of grain yield (Sinclair et al., 1990; Xia, 1997). However, contrary to the observation mentioned above, Morgan (1991) found biomass accumulation and grain yield increases under water stress conditions in wheat. In grain sorghum, yield reduction under water stress is attributed mainly to variations in total biomass accumulation (Craufurd & Peacock, 1993). Te response of plants to drought depends to a large extent on the length and severity of the drought, as well as on the genotype (Kebede et al., 2001; Çakir, 2004). However, the total accumulated biomass depends mainly on the phenological stage in which drought occurs (Jamieson et al., 1995; Xia, 1997; Frederick et al., 2001). In addition, the biomass accumulation potential in sorghum has a high heritability (Sankarapandian et al., 1993). Therefore, total biomass accumulation is a trait which could be used as a criterion in the improvement of drought resistance with high probabilities of success (Lopez et al., 1996; Castro et al., 2000).
Using grain yield as well as total and root biomass accumulation as criteria, Castro et al. (2000) classified 29 grain sorghum genotypes based on their drought responses into resistant, intermediate and susceptible. Three of those geno-types with similar phenology and contrasting responses to drought (two resistant and one susceptible), were selected for this study. In order to determine the eficiency of the classification criteria applied, the main objectives of this study were to investigate the effects of water stress on phenology, biomass accumulation and grain yield to determine if the classifcation of the three genotypes was maintained when drought was applied at either of three different phenological stages.
MATERIALS AND METHODS
This study was conducted under greenhouse conditions at the Colegio de Posgraduados in Montecillo, Texcoco, Mexico (19° 31´ N, 98° 53´ W, 2242 m.a.s.l.). Three Mexican grain sorghum lines for their response to drought were compared in this study. Thwo of those genotypes were previously classified by Castro et al. (2000) as drought-resistant (UAT-124 and UAT-152), and one as drought- susceptible (UAT-30). Seeds from each of the three genotypes were sown in tall polyethylene bags containing 10 kg of a mixture of 50% of river soil and 50% sand. Two sorghum seeds were planted in each potting bag and thinned to one plant per bag after two weeks. From planting to panicle initiation (about 35 days), three irrigations with a nutrient solution were applied to all plants. After the initial period of growth without water stress, drought treatments were applied to plants from each of the three genotypes at either of three different phenological stages: a) panicle initiation (PI), when the apical meristem changed from vegetative to reproductive, b) flag leaf (FL), when the fag leaf was completely expanded, and c) flowering (FLW), when 50% of the spikelets on the panicle were shedding pollen. Grain maturity was judged as that time when the black layer of the grain appeared. Tho determine the timing of floral initiation, four plants from each genotype were dissected at two-day intervals and the apical meristem was observed under a stereoscopic microscope. In the well watered treatment, the soil was never allowed to dry, and it was maintained at 80% of field capacity throughout the study by watering as needed, typically every three to four days. For the drought treatments, irrigation was completely discontinued when each of the genotypes reached the developmental stage as described above (PI, FL, and FLW). After water stress was initiated for each treatment, plants were maintained without watering until the soil water potential reached the permanent wilting point (-1.5 MPa), as determined with a soil pressure bomb (16, 12 and 13 days, respectively). At this time, soil moisture was brought back to field capacity by re-watering each pot, effectively terminating drought stress. After re-watering, soil moisture was maintained similar to the well-watered treatment until the end of the experiment. In order to determine dry shoot biomass accumulation as afected by drought treatment, a subset of five plants was destructively measured before, during, and after each drought treatment, for each genotype. At grain maturity, grain yield per panicle, number of grains, and individual grain weight were determined. After the drought treatment and at grain maturity, leaf area was determined in a subset of five plants using a Portable Area Meter (LI-3000A, LI-COR, Inc. Lincoln, Nebraska, USA). The experimental factors (three genotypes and four watering treatments) were arranged in a randomized complete block design with a factorial arrangement containing a total of 12 treatments and four replicates. Data from all treatments were compared by analysis of variance, except for phenology, and the treatment means were compared using Tukey's test.
RESULTS AND DISCUSSION
Efect of drought on phenological development. Genotypes UAT-124 and UAT-30, when well watered, required almost the same number of days from planting to physiological maturity, panicle initiation to flowering, and flowering to physiological maturity (Table 1). However, the UAT-152 genotype, when well watered, required 6 d less than UAT-30 and 7 d less than UAT-124, to reach physiological maturity, due mainly to a shorter period between panicle initiation to flowering (7 d). The level of drought tolerance depends on the species and genotype (Rampino et al., 2006), the developmental stage of the crop when the stress occurs (Zhu et al., 2005) and the length and severity of water stress (Bartels & Souer, 2004). Very few studies have been done to determine the effects of drought on the process of floral induction in cereals per se, which is dificult to separate from post-induction floral development in many cases (Saini & Westgate, 2000). In this study, drought applied either at panicle initiation or flag leaf stage (Table 1), was found to reduce the time from panicle initiation to flowering (by 1 to 8 d), but delayed the time from flowering to physiological maturity (by 3 to 8 d), similar to the results of Craufurd & Peacock (1993). It is clear from our study that the UAT-30 genotype, previously classified as susceptible, was the most afected by drought during panicle initiation and flag leaf stages (development sped up by 7 to 8 d).
Table 1. Number of days required for sorghum genotypes from: (a) panicle initiation to flowering, (b) flowering to physiological maturity, and (c) planting to physiological maturity, in response to water stress applied during either panicle initiation, fag leaf, or flowering stages in three sorghum genotypes.
Tabla 1. Número de días requeridos para los genotipos de sorgo de: (a) iniciación de la panoja a floración, (b) floración a madurez fisiológica, y (c) siembra a madurez fisiológica, en respuesta al estrés hídrico aplicado durante las etapas de iniciación de la panoja, hoja bandera y floración en tres genotipos de sorgo.
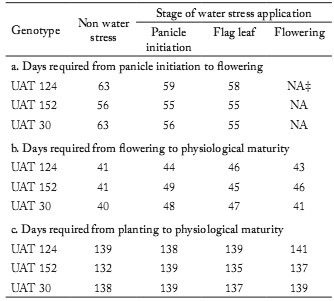
NA = Not applicable.
NA = No aplica.
Furthermore, the time required between panicle initiation to flowering and flowering to physiological maturity was reflected in time required from planting to reach physiological maturity; but the degree of the effect, according to Rampino et al. (2006), depended on the genotype tested. In general, our results confirmed the suggestions of Prasad et al. (2008) and the results reported in some cereals by Winkel et al. (1997), Wopereis et al. (1996), and Barnabás et al. (2008); but differ from those obtained by Boonjung & Fukai (1996).
Application of drought at flowering seemed to have less effect on increasing the number of days required for development than from flowering to physiological maturity (1 to 5 d), but again the effect depended on the genotype. The total number of days required for development from planting to physiological maturity in response to drought applied at flowering increased by 2 d in UAT 124, by 1 d in UAT 30, and by 5 d in UAT-152 (Table 1). Our results generally demonstrated that diferent genotypes showed different developmental responses to drought, and that the response of each genotype depended on when the drought occurred during development in agreement with other studies (Zhu et al., 2005). This differential phenological response between genotypes could be useful for breeding programs selecting drought resistant varieties (Lazar et al., 1995).
Effect of drought on biomass accumulation. Highly significant variation for genotypes and soil moisture was found for biomass accumulated (Table 2) when drought stress was applied during the flag leaf stage. These results were similar to those found for genotypes when drought stress was applied on panicle initiation. The most important variation that we found was in the flag leaf stage, where the magnitude of variation attributable to the genotypes and soil moisture, estimated as a percentage of the total sum of squares, was 24% and 39%, respectively. Highly significant interaction in genotype x soil moisture was observed in drought stress applied during flag leaf and flowering stage, with a percentage of variation of 23% and 30%, respectively. During the drought recovery period, there was significant variation between genotypes for biomass accumulated in the flowering stage, and significant variation between soil moisture in the flag leaf stage (33%). Significant interaction in genotype x soil moisture was observed when drought stress was applied in the three phenological stages.
Table 2. Effect of genotype and stress (soil moisture) on sorghum biomass accumulated under three phenological stages during: (a) the drought period and (b) the recovery period (from end of drought to physiological maturity).
Tabla 2. Efecto del genotipo y el estrés (humedad del suelo) sobre la acumulación de biomasa en sorgo en tres etapas fenológicas durante: (a) el período de estrés y (b) el período de recuperación (desde el final del estrés a la madurez fisiológica).
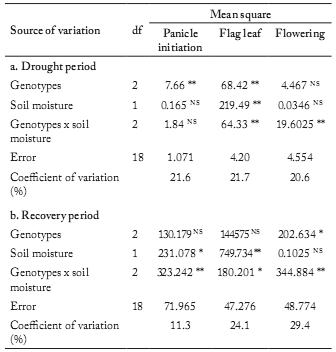
*, ** Indicate signifcance at the 0.05 and 0.01 probability levels, respectively; NS Not signifcant.
*, ** Indica signifcancia a los niveles de probabilidad de 0,05 y 0,01, respectivamente; NS No signifcativo.
In general, the effect of drought was more important when it was applied at the flag leaf stage, as shown by the significant reduction of biomass accumulated (48%) when compared to the well-watered treatment (Table 3a). A similar reduction was observed by Borrell et al. (2000) and Tsuji et al. (2003) in sorghum genotypes under drought stress. All genotypes that we tested accumulated similar amounts of biomass during the drought period when it was applied at panicle initiation and flowering. When drought stress was applied at the fag leaf stage, biomass accumulation was reduced during the stress period, when compared to the non-stress treatment. The UAT-30 genotype (susceptible) showed the largest reduction (71%). This could be attributed to reduced growth and net assimilation rates, in agreement with the report of Younis et al. (2000).
Table 3. Shoot biomass (g/plant) accumulated during (a) the drought period, and (b) the recovery period (end of drought to physiological maturity) for three sorghum genotypes when water stress was applied at either of three phenological stages.
Tabla 3. Biomasa aérea acumulada (g planta) durante (a) el período de estrés, y (b) el período de recuperación (desde el final del estrés a la madurez fisiológica) para tres genotipos de sorgo cuando el estrés hídrico fue aplicado en tres etapas fenológicas.

Means within columns followed by the same letter are not signifcantly different at the p<0.05 level by Tukey´s multiple range test.
Promedios dentro de columnas, seguidos con la misma letra no son signifcativamente diferentes a una p<0,05 según la prueba de rango múltiple de Tukey.
After the drought treatment was suspended (recovery period), all three genotypes showed a significant biomass reduction when drought stress was applied at the flag leaf stage (33%). When drought stress was applied at the stage of panicle initiation, UAT-124 (tolerant) produced significantly more biomass than the non-stressed treatment (32%). This was likely due to variation in crop growth rate (Borrell et al., 2000). Furthermore, it is interesting to note that during this period only the drought-treated UAT-124 plants accumulated about the same amount of biomass as the non-stressed UAT-124 plants during the flag leaf stage (Table 3b).
When drought stress was applied at the flowering stage, the differences in biomass production during the stress period between the stress and non-stressed treatments were very small and inconsistent in all three genotypes. A similar situation of inconsistency was observed in the case of biomass accumulation during the recovery period. The inconsistency of the genotype response to drought stress could be attributed to differences in drought patterns or extent used during the study (Salih et al., 1999).
These results allow us to conclude that biomass accumulation for UAT 124 and UAT 30 genotypes was strongly in accordance with the susceptibility to drought classification of Castro et al. (2000), but not for UAT 152. Consequently, our results showed that biomass accumulation and grain yield under drought stress are useful traits to study for the classification of genotypes in resistant and susceptible to drought, although it will depend on the variability within the genotype. Also, we can conclude that the flag leaf stage was the most susceptible to drought, in agreement with Saini & Westgate (2000), Boyer & Westgate (2004) and Barnabás et al. (2008), This is because stress applied during this developmental stage caused the greatest reduction in biomass in both stress and recovery periods. This response is likely due to the flact that this phenological stage coincides with the onset of meiosis and early grain initiation (Saini, 1997).
Effect of drought on grain yield and its components. The differences among genotype as well as the genotype x soil moisture interaction were not significant for all water stress treatments, and for all traits (data not shown). Meanwhile, compared with non-stressed (control) plants, drought stress reduced grain yield significantly (Table 4) when it was applied at the flag leaf stage (24%) and at flowering (28%), but not when drought occurred at panicle initiation.
Table 4. Grain yield (a) and its components (b and c), for three sorghum genotypes when water stress was applied during either of three phenological stages. Means were compared between non-water stress and each phenological stage of water stress application.
Tabla 4. Rendimiento de grano (a) y sus componentes (b y c), para tres genotipos de sorgo cuando el estrés hídrico fue aplicado durante tres etapas fenológicas. Los promedios fueron comparados entre riego y cada etapa fenológica donde se aplicó el estrés hídrico.
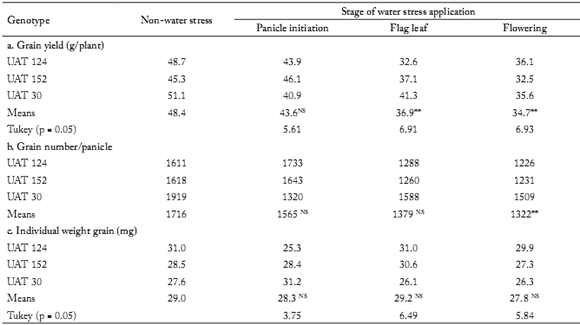
NS, ** indicate signifcance between non-water stress and each water stress application at the 0.05 and 0.01 probability level, respectively.
NS, ** Indica signifcancia entre riego y cada tratamiento de estrés aplicado, a una probabilidad del 0,05 y 0,01, respectivamente.
Grain number is an important component of grain yield in sorghum (van Oosterom & Hammer, 2008). In this investigation, the reduction of grain yield at the flowering stage was attributable to a significant reduction of this grain component (23%).
Grain yield reduction caused by water stress has been observed in sorghum by Sankarapandian et al. (1993), Craufurd & Peacock (1993) and Borrell et al. (2000). In cereals, yield losses are caused mainly by a reduction in starch accumulation during flowering or grain development (Barnabás et al., 2008). It is also clear that grain yield reduction depends on the phenological stage in which the stress occurs and in some cases by the intensity and length of the drought (Rosales-Serna et al., 2000; Frederick et al., 2001).
All varieties (UAT-124, UAT 30 and UAT-152) had the same grain yield behavior when stress was applied at three different phenological stages. These results indicate that genotype responses to drought were inconsistent with their initial classification (Castro et al., 2000), as previously mentioned (UAT-124 and UAT-152 as tolerant, and UAT-30 as susceptible). This inconsistency with respect to their classification was observed when water stress was applied at the flag leaf stage since the drought tolerant UAT 124, had a greater reduction in grain yield than the susceptible genotype Furthermore, at the flowering stage, UAT 30 (susceptible) showed a greater reduction in response to water stress compared with UAT 124 (tolerant).
Drought stress significantly reduced leaf area (Table 5a) of the three sorghum genotypes when water stress was applied at the panicle initiation (25%) and flag leaf (33%) stages. A similar response was reported by Tsuji et al. (2003). The reduction of leaf area was most pronounced when water stress was applied at the flag leaf stage, and was dependent on genotype. The susceptible genotype UAT 30 showed the greatest reduction of leaf area (46%).
Table 5. Leaf area (cm2) at the end of the drought (a) and at the physiological maturity (b) for three sorghum genotypes when the water
stress was applied at either of three phenological stages.
Tabla 5. Área foliar (cm2) al final del estrés hídrico (a) y a la madurez fisiológica (b) para tres genotipos de sorgo cuando el estrés hídrico fue
aplicado en tres etapas fenológicas.

Means were compared between well watered and water stress conditions at each phenological stage of water stress application.
Los promedios fueron comparados entre riego y la condición de estrés en cada etapa fenológica donde se aplicó el estrés hídrico.
However, the genotype resistant UAT-124 showed a significant recovery capacity (Table 3) after water stress was eliminated. We found that when drought stress occurred at a time close to panicle initiation and flowering; the recovery response was due to the capacity of the plant to retain physiologically active leaves (Table 5b), allowing the plant to reach physiological maturity, similar to the results reported by Lilley & Fukai (1994) and Fukai & Cooper (1995). A quick drought recovery allows a plant to produce photoassimilates for a longer period of time during the recovery period, in addition to maintaining the capacity to mobilize these photoassimilates to the grain during grain filling (Yang et al., 2001 a, b; Yang et al., 2002).
The recovery capacity shown by the resistant genotype UAT-124 was not reflected in the grain yield (Table 4). This could be due to the flact that the classification of the genotypes with respect to their water stress response was performed in an experiment where drought was applied during panicle initiation, while in this study it was applied at different phenological stages. Similar results were obtained by Sankarapandian et al. (1993) and Craufurd & Peacock (1993). This study demonstrated that the sensitivity to drought stress was greater at the reproductive stage, similar to the results reported by Younis et al. (2000).
CONCLUSIONS
Water stress reduced biomass accumulation, leaf area and consequently grain yield, depending on the characteristics of the genotype and the phenological stage in which it occurred. The most susceptible developmental stage was the flag leaf expansion. The identification of tolerant and susceptible genotypes could be accomplished by applying drought stress during the more susceptible stage or stages of development, and using the accumulation of biomass, leaf area and grain yield in both the drought stress and recovery periods as a criterion for drought tolerance.
REFERENCES
1. Balota, M., W.A. Payne, W. Rooney & D. Rosenow (2008). Gas exchange and transpiration ratio in sorghum. Crop Science 48: 2361-2371. [ Links ]
2. Bartels, D. & E. Souer (2004). Molecular responses of higher plants to dehydration. In: H. Hirt and K. Shinozaki (eds.). Plant responses to abiotic stress. pp. 9-38. Springer-Verlag, Berlin and Heidelberg, Germany. [ Links ]
3. Barnabás, B., K. Jäger & A. Fehér (2008). The effect of drought and heat stress on reproductive processes in cereals. Plant Cell and Environment 31: 11-38. [ Links ]
4. Bolaños, J. & G.O. Edmeades (1993). Eight cycles of selection for drought tolerance in lowland tropical maize. I. Responses in grain yield, biomass and radiation utilization. Field Crops Research 31: 233-252. [ Links ]
5. Bolaños, J., G.O. Edmeades & L. Martínez (1993). Eight cycles of selection for drought tolerance in lowland tropical maize. III. Responses in drought-adaptive physiological and morphological traits. Field Crops Research 31: 269-286. [ Links ]
6. Boonjung, H. & S. Fukai (1996). Effects of soil water deficit at different growth stages on rice growth and yield under upland conditions. 2. Phenology, biomass production and yield. Field Crops Research 48: 47-55. [ Links ]
7. Borrell, A.K., G.L. Hammer & R.G. Henzell (2000). Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Science 40: 1037-1048. [ Links ]
8. Boyer, J.S. & M.E. Westgate (2004). Grain yields with limited water. Journal of Experimental Botany 55: 2385-2394. [ Links ]
9. Bray, E.A., J. Bailey-Serres & E. Weretilnyk (2000). Responses to abiotic stresses. In: W. Gruissem, B. Buchannan, R. Jones (eds.). Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp. 1158-1249. [ Links ]
10. Bruce, W.B., G.O. Edmeades & T.C. Barker (2002). Molecular and physiological approaches to maize improvement for drought tolerance. Journal Experimental Botany 53: 13-25. [ Links ]
11. Çakir, R. (2004). Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crop Research 89: 1-16. [ Links ]
12. Castro N., S., J. Ortiz C., M. del C. Mendoza C. & F. Zavala G. (2000). Producción de biomasa en líneas de sorgo como respuesta al estrés hídrico. Revista Fitotecnia Mexicana 23: 321-334. [ Links ]
13. Craufurd, P.Q. & J.M. Peacock (1993). Effect of heat and drought stress on sorghum (Sorghum bicolor). II. Grain yield. Experimental Agriculture 29: 77-86. [ Links ]
14. De Roo, H.C. (1969). Leaf water potentials of sorghum and corn estimated with the pressure bomb. Agronomy Journal 61: 969-970. [ Links ]
15. Frederick, J.R., C.R. Camp & P.J. Bauer (2001). Drought-stress effects on branch and mainstem seed yield and yield components of determinate soybean. Crop Science 41: 759-763. [ Links ]
16. Fukai, S. & M. Cooper (1995). Development of drought-resistant cultivars using physiological traits in rice. Field Crops Research 40: 67-86. [ Links ]
17. Jamieson, P.D., R.J. Martin, G.S. Francis & D.R. Wilson (1995). Drought effects on biomass production and radiation-use efficiency in barley. Field Crops Research 43: 77-86. [ Links ]
18. Kebede, H., P.K. Subudhi & D.T. Rosenow (2001). Quantitative trait loci influencing drought tolerance in grain sorghum (Sorghum bicolor L. Moench). Theoretical and Applied Genetics 103: 266-276. [ Links ]
19. Lazar, M.D., C.D. Salisbury & W.D. Worrall (1995). Variation in drought susceptibility among closely related wheat lines. Field Crops Research 41: 147-153. [ Links ]
20. Lilley, J.M. & S. Fukai (1994). Effect of timing and severity of water deficit on four diverse rice cultivars. III. - Phenological development, crop growth and grain yield. Field Crops Research 37: 225-234. [ Links ]
21. López, F.B., C. Johansen & Y.S. Chauhan (1996). Effects of timing of drought stress on phenology, yield and yield components of short-duration pigeon pea. Journal Agronomy Crop Science 177: 311-320. [ Links ]
22. Mahajan, S. & N. Tuteja (2005). Cold, salinity and drought stresses: An overview. Archives of Biochemistry and Biophysics 444: 139-158. [ Links ]
23. Morgan, J.M. (1991). A gene controlling differences in osmorregulation in wheat. Australian Journal Plant Physiology 18: 249-257. [ Links ]
24. Pelleschi, S., J.P. Rocher & J.L. Prioul (1997). Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environment 20: 493-503. [ Links ]
25. Prasad, P.V.V., S.A. Staggenborg & Z. Ristic (2008). Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In: Advances in agricultural systems modeling. Ser. 1. ASA, CSSA, and SSSA, Madison, WI. pp. 1-55. [ Links ]
26. Rampino, P., S. Pataleo, C. Gererdi, G. Mita & C. Perrotta (2006). Drought response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell and Environment 29: 2143-2152. [ Links ]
27. Rosales-Serna, R., P. Ramírez-Vallejo, J.A. Acosta-Gallegos, F. Castillo-González & J.D. Nelly (2000). Rendimiento de grano y tolerancia a la sequía del frijol común en condiciones de campo. Agrociencia 34: 153-165. [ Links ]
28. Saini, H.S. (1997). Effect of water stress on male gametophyte development in plants. Sexual Plant Reproduction 10: 67-73. [ Links ]
29. Saini, H.S. & M.E. Westgate (2000). Reproductive development in grain crops during drought. In: D.L. Spartes (ed.). Advances in Agronomy. Vol. 68. pp. 59-96. Academic Press. San Diego, CA. USA. [ Links ]
30. Salih, A.A., I.A. Ali, A. Lux, M. Luxov, Y. Cohen, Y. Sigimoto & S. Inanaga (1999). Root water uptake of two sorghum cultivars. Crop Science 39: 168-173. [ Links ]
31. Sankarapandian, R., D. Krishnadoss, N. Muppidathi & S. Chidambaram (1993). Variability studies in grain sorghum for certain physiological characters under water stress conditions. Crop Improvement 20: 45-50. [ Links ]
32. Sinclair, T.R., J.M. Bennett & R.C. Muchow (1990). Relative sensitivity of grain yield and biomass accumulation to drought in field-grown maize. Crop Science 30: 690-693. [ Links ]
33. Tsuji, W., M.E.K. Ali, S. Inanaga & Y. Sugimoto (2003). Growth and gas exchange of three sorghum cultivars under drought stress. Biologia Plantarum 46: 583-587. [ Links ]
34. Van Oosterom, E.J. & G.L. Hammer (2008). Determination of grain number in sorghum. Field Crops Research 108: 259-268. [ Links ]
35. Winkel, T., J.F. Renno & W.A. Payne (1997). Effect of the timing of water deficit on growth, phenology and yield of pearl millet [Pennisetum glaucum (L.) R. br.] grown in sahelian conditions. Journal of Experimental Botany 48: 1001-1009. [ Links ]
36. Wopereis, M.C.S., M.J. Kropf, A.R. Maligaya & T.P. Tuong (1996). Drought-stress responses of two lowland rice cultivars to soil water status. Field Crops Research 46: 21-39. [ Links ]
37. Xia, M.Z. (1997). Effects of soil drought during the generative development phase on seed yield and nutrient uptake of faba bean (Vicia faba). Australian Journal Agricultural Research 48: 447-451. [ Links ]
38. Yang, J., J. Zhang, Z. Wang, Q. Zhu & L. Liu (2001a). Water deficitinduced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agronomy Journal 93: 196-206. [ Links ]
39. Yang, J., J. Zhang, Z. Wang, Q. Zhu & W. Wang (2001b). Hormonal changes in the grain of rice subjected to water stress during filling. Plant Physiology 127: 315-323. [ Links ]
40. Yang, J., J. Zhang, L. Liu, Z. Wang & Q. Zhu (2002). Carbon remobilization and grain filling in japonica/indica hybrid rice subjected to post anthesis water deficits. Agronomy Journal 94: 102-109. [ Links ]
41. Younis, M.E., O.A. El-Shhaby, S.A. Abo-Hamed & A.H. Ibrahim (2000). Effects of water stress on growth, pigments and 14CO2 assimilation in three sorghum cultivars. Journal Agronomy Crop Science 185: 73-82. [ Links ]
42. Zhu, X., H. Gong, G. Chen, S. Wang & C. Zhang (2005). Different solute levels in two spring wheat cultivars induced by progressive field water stress at different developmental stages. Journal of Arid Environments 62: 1-14. [ Links ]














