Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.82 no.1 Vicente López jun. 2013
ARTÍCULOS ORIGINALES
In vitro biocontrol of tomato pathogens using antagonists isolated from chicken-manure vermicompost
Biocontrol in vitro de fitopatógenos de tomate mediante antagonistas aislados de vermicomposta de gallinaza
Barocio-Ceja NB1, LF Ceja-Torres1*, JL Morales-García2, HV Silva-Rojas3, R Flores-Magallón1, S Ochoa-Estrada1
1 Instituto Politécnico Nacional, CIIDIR-U-MICHOACÁN, Justo Sierra No. 28, Col. Centro, C.P. 59510, Jiquilpan Michoacán, México.
2 Facultad de Agrobiología, "Presidente Juárez" U.M.S.N.H. Uruapan, Michoacán.
3 Colegio de Postgraduados. Producción de Semillas, Campus Montecillo, Texcoco, Edo. de México, C.P. 56230, México.
Address Correspondence to: Luis Fernando Ceja Torres, e-mail: lfceja@colpos.mx
Recibido / Received 13.XI.2012.
Aceptado / Accepted 26.I.2013.
Abstract. The objectives of this study were to (1) isolate and identify pathogenic fungi from vegetative material with wilt symptoms in tomato plantations belonging to the Cienega of Chapala, Michoacán, Mexico, and (2) determine the antagonistic capacity of Trichoderma sp. and Aspergillus spp. isolated from chicken-manure vermicompost. Pathogens were isolated by means of a completely randomized sampling in 6 locations; 9 plantations were inspected and 45 plants with symptoms of the disease were selected. Portions of root and stem were disinfected and placed on potato-dextroseagar acidified (PDA). Antagonists isolation was made from a dilution of chicken-manure vermicompost of 1 x 10-2 in PDA medium culture more streptomycin and tetracycline. The antagonistic activity was tested by the dual culture confrontation methods. Two pathogens were obtained on tomato in the study area, Fusarium spp. and Rhizoctonia sp., presenting an incidence of 92% and 5%, respectively. Morphological characteristics were determined in cultivation of PDA. Molecular analysis identifed F. oxysporum, F. solani, F. subglutinans and Rhizoctonia sp. Of 11 isolates of chicken manure vermicompost, only Trichoderma sp. and Aspergillus sp., had significant diferences (p≤0.05) with respect to the control. Inhibition of F. oxysporum growth ranged from 45% to 48%, and 24% to 27%, in presence of Trichoderma sp. and Aspergillus sp., respectively; these antagonistic species inhibited growth of Rhizoctonia sp. by 38% and 25% , respectively.
Keywords: Biocontrol; Antagonists; Solanum lycopersicum; Wilt.
Resumen. Los objetivos de este trabajo fueron (1) aislar e identificar hongos patógenos de plantas de tomate con síntomas de marchitez, en plantaciones de seis localidades de la Ciénega de Chapala, Michoacán, México, y (2) determinar la capacidad antagónica de Trichoderma sp. y Aspergillus sp. asociados a vermicomposta de gallinaza. Los patógenos se aislaron a partir de un muestreo al azar en nueve plantaciones de las cuales se seleccionaron 45 plantas de tomate con síntomas de la enfermedad. Porciones de raíz y tallo fueron desinfectados y colocados en medio papa-dextrosa-agar acidificado (PDA). El aislamiento de antagonistas se hizo a partir de una dilución de vermicomposta de gallinaza de 1 x 10-2 en medio de cultivo PDA más tetraciclina y estreptomicina. El antagonismo fue evaluado mediante confrontación por cultivos duales. Dos fitopatógenos fueron obtenidos del área de estudio; Fusarium spp. y Rhizoctonia sp. con una incidencia de aislamientos de 92% y 5%, respectivamente. Las características morfológicas se determinaron en cultivo de PDA, y mediante un análisis molecular se identificó a F. oxysporum, F. solani, F. subglutinas y Rhizoctonia sp. De 11 cepas aisladas de la vermicomposta de gallinaza, solo Trichoderma sp. y Aspergillus sp., tuvieron diferencias significativas (p≤0,05) con respecto al testigo. La inhibición del crecimiento "in vitro" de F. oxysporum por Trichoderma sp. y Aspergillus sp. varió de 45% a 48%, y de 24% a 27%, respectivamente; estos antagonistas inhibieron el crecimiento de Rhizoctonia sp. en un 38% y 25%, respectivamente.
Palabras clave: Biocontrol; Antagonistas; Solanum lycopersicum; Marchitez.
INTRODUCTION
A wide range of production problems occurs in commercial tomato (Solanum lycopersicum). Wilt diseases are often the most important cause of these problems, and fungi are the usual causal agents. Fusarium spp. and Rhizoctonia spp. are the major genera involved in tomato wilt (Lugo & Sanabria, 2001; Michael-Aceves et al., 2008) causing significant reductions in crop yield (Ascencio-Álvarez et al., 2004).
An increasingly favored alternative to chemical control is the use of biological control organisms. Antagonistic microorganisms can suppress growth of many plant pathogens and promote growth of a range of beneficial microorganisms. This might result in improved crop development associated with increased levels of protection against a range of pests and diseases (Ezziyyani et al., 2006).
In recent years, there has been renewed interest in the use of organic manures in crop production, and also in the use of biological control agents for managing plant pathogens. Examples are Phytophthora nicotianae var. nicotianae in cabbage (Brassica oleracea L.), Fusarium oxysporum f. sp. lycopersici in tomato, and P. drechsleri, Rhizoctonia solani and F. oxysporum in gerbera (Gerbera jamesonii H. Bolus) (Villa-Briones et al., 2008; Holguín-Castaño & Mora-Delgado, 2009). Biological control agents can reduce the negative impacts of a number of adverse environmental factors, and provide commercially significant levels of crop protection (Rodriguez & Montilla, 2002).
Vermicompost is widely used as an organic substrate to enhance growth and development of crop plants and raise soil fertility (Castillo et al., 2000; De La Cruz-Lázaro et al., 2010). These composts contribute to a useful range of plant nutrients, and contain a large number of microorganisms including bacteria, fungi and actinomycetes. These microbes break down organic residues into simpler substances during vermicomposting. The increased presence of these substances increases the range and numbers of soil microorganisms, including those potentially antagonistic to phytopathogens (Holguín-Castaño & Mora-Delgado, 2009).
Microbial antagonists most used for biological control are fungi of the genus Trichoderma. This genus can reduce growth of P. infestans isolated from tomato by 16-85%, and of Alternaria solani by 39-81% (Michael-Aceves et al., 2008). These authors observed a 48% inhibition of F. oxysporum f. sp. lycopersici (FOL) by T. harzianum on the same crop (Srivastava et al., 2010). This antagonistic species inhibited growth of F. solani isolated from passion fruit by 70% (Suárez-Meza et al., 2008).
The mechanisms through which antagonistic species suppress phytopathogenic soil microorganisms are mainly through the production of toxic metabolites (antibiotics); these metabolites inhibit the development of many phytopathogenic fungi (Villa-Briones et al., 2008). Other mechanisms involved in the beneficial efects might be: (1) the production of enzymes that destroy pathogen cell walls; (2) mycoparasitism, and (3) the induction in the crop plant of an increase in its systemic resistance to pathogens (Fernandez & Vega, 2001; Ezziyyani et al., 2006).
However, there are few studies on the microbiota found in vermicomposted chicken manure, and even fewer that deal with their effectiveness in controlling plant pathogens in tomato. The objectives of this study were to (1) isolate and identify pathogens of the below- and aboveground systems of tomato plants growing in the Cienega de Chapala, Michoacan, Mexico, and (2) evaluate the in vitro activities of Trichoderma spp. and Aspergillus spp. on two important species of tomato-wilt pathogens (i.e., Fusarium and Rhizoctonia).
MATERIALS AND METHODS
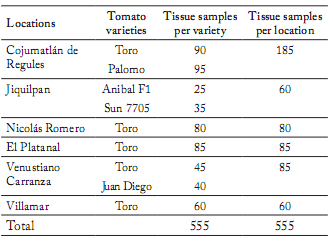
Sampling. The research was conducted in 2011 at six locations in the Cienega de Chapala, Michoacán, where nine saladette tomato plantations were investigated. Forty five plants with symptoms of wilting or root and stem rot were collected randomly for analysis. Of these, 25 samples were of the variety "Toro", and 5 samples of the varieties "Palomo", "F1 Anibal", "Sun 7705", and "Juan Diego". Tomato plants showing the stated symptoms were placed in labeled plastic bags and taken to the laboratory of the Plant Pathology Unit, CIIDIR, of Michoacán for analysis. A total of 555 samples were collected. The number of samples of each tomato variety depended on the availability of diseased tissue (Table 1).
Table 1. Numbers of samples isolated from necrotic tissue obtained from the roots and stems of tomato plants (Solanum lycopersicum) showing symptoms of wilting in the Cienega de Chapala, Michoacán in 2011.
Tabla 1. Número de muestras aisladas de tejido necrótico mostrando síntomas de marchitez. Dicho tejido fue obtenido de las raíces y tallos de plantas de tomate (Solanum lycopersicum) en la Cienega de Chapala, Michoacán en 2011.
Isolation and identification of phytopathogens. Roots and stems showing necrotic symptoms were cut into sections about 1 cm long and surface-sterilized in 3% sodium hypochlorite solution for 2 min. Samples were thereafter rinsed three times with sterile distilled water, dried on sterile paper towels and placed in Petri dishes containing potato-dextrose agar (PDA, BIOXON®) medium. Samples were incubated for 5 days at 28 °C. When isolates grew, microscopic preparations were made using glass slides with lactophenol-cotton blue, and observed under a compound microscope (Zeiss® Ser. Nr. 993718). They were identified using the morphological taxonomic keys of Barnett & Hunter (1978) and of Nelson et al. (1983). Each isolate was purified by the hyphal-tip technique, and was also sent to the Colegio de Postgraduados for molecular identification.
Isolation of Trichoderma and Aspergillus spp. from chicken-manure vermicompost. The isolation of antagonists was made by mixing 10 g of chicken-manure vermicompost (VG) in 90 mL of sterile distilled water, from which serial dilutions were made to 10-2. Aliquots (0.3 mL) of the 10-2 dilution were uniformly distributed with a glass rod on Petri dishes containing PDA + TS medium. Four replicates of each sample were plated and incubated at 28 °C for five days. The fungi that developed were subcultured to acidified PDA medium to obtain pure cultures. Colonies having obviously different cultural features were selected for morphological and molecular identification.
Genomic DNA extraction. Each isolate was grown on PDA medium at room temperature for 10 days. The mycelium was then scraped from the surface of the plate and crushed with a mortar in 1 mL of lysis solution (2% Triton-X 100, 1% SDS, 100 mM NaCl, 10 mM Tris-HCl, pH 8.0) and 10 μL of protease K (10 mg/mL). Next, 600 μL of the sample were transferred to a 2 mL Eppendorf tube for DNA extraction following the protocol of Bainbridge et al. (1990) with slight modifications.
The DNA pellet from each tube was suspended in 50 μL of TE buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA). The quality of the DNA was confirmed by electrophoresis in a 1% agarose gel in 1x TAE buffer (Tris Acetate-EDTA) run at 87 V/cm for 1 h. The gel was stained with ethidium bromide (3 mg/L), and the bands were visualized using a Gel Doc 2000 UV transilluminator (Bio-Rad). The DNA concentration was quantified using a Perkin Elmer spectrophotometer (Lambda BIO10), and the samples were diluted to 20 ng/μL for PCR (Polimerase Chain Reaction).
PCR amplification of ribosomal RNA genes. The primers ITS5 (5'-GGAAGTAAAAGTCGTAACAAGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') (White et al., 1990) were used to amplify the ITS region of the 18S rDNA gene (partial sequence); the 5.8S rDNA gene, internal transcribed spacers 1 and 2 (complete sequence); and the conserved domain in 28S rDNA gene (partial sequence). A fragment of 580 bp was expected.
A PCR master mix was prepared in a final volume of 25 μL containing 1x Taq DNA polymerase buffer, 2 mM MgCl2, 0.8 mM deoxynucleoside triphosphates (0.2 mM of each), 100 ng DNA, 20 pmol of each primer, and two units of GoTaq DNA (Promega). PCR amplifications were performed with an initial denaturation at 95 oC for 2 min; 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 2 min; followed by a single fnal extension cycle at 72 °C for 10 min.
All PCR reactions were carried out in a Peltier Termal Cycler PTC-200 (Bio-Rad), and the PCR products were verified by loading 5 μL in a 1.2% agarose electrophoresis gel, which was stained as described above. The remaining amplified PCR products were purified using a purification kit (Promega) following the manufacturer's instructions. To ensure that there was no misreading, the PCR-products were sequenced in both directions using an Applied Biosystems model 3130 automated DNA sequencing system (Applied BioSystems).
Phylogenetic analysis of ITS. Sequences corresponding to both regions were assembled and edited using Bioedit software version 7.1.3 (Hall, 1999) and a consensus sequence of each isolate was created and submitted to BLASTN 2.2.19 (Zhang et al., 2000).
For evolutionary analyses, all consensus sequences were compiled into a single file (FASTA format) and aligned using the profile mode, Clustal W 1.81 (Tompson et al., 1994) included in the MEGA 5 software (Tamura et al., 2011).
Phylogenetic reconstructions were performed for the ITS dataset using the maximum parsimony method. This analysis was conducted using the Close Neighbour Interchange (CNI) search option (level = 1) with initial tree by random addition (10 reps), and gaps ⁄ missing data were considered as complete deletions.
To determine the confidence values for clades within the resulting tree, a bootstrap analysis was assessed with 1000 replicates (Felsenstein, 1985). The accession numbers of F. oxysporum, F. subglutinans, and F. solani deposited in the NCBI Gen Bank database were downloaded and included as reference species along with the sequences obtained in this study. Phoma herbarum (accession number EU082106) was designated as out group for construction of the evolutionary tree (Fig. 2).
Nucleotide sequences. The new sequences obtained in this study were deposited in the Gen Bank at the NCBI. For cases in which multiple isolates had an identical sequence, only one accession number was deposited, this representing the common sequence of those isolates for each region.
Microbial antagonism assay. To observe the effects between antagonistic fungi and the isolated pathogens, they were confronted by the dual cultures method (Larralde et al., 2008). One 5 mm-PDA disc of Fusarium spp. and/or of Rhizoctonia sp. were taken from a 10-day-old culture and placed at one extreme margin of a Petri dish containing acidifed PDA medium. Two days later a 5 mm-PDA disc of Trichoderma sp. and / or of Aspergillus spp. from a 7-day-old culture was put at the other extreme. Petri dishes were then incubated for 8 days at 28 °C.
Visual and microscopic observations were carried out every 24 h to determine the action of Aspergillus sp. using the scale proposed by Aquino-Martínez et al. (2008), where the three possibilities were: a) invasion of the pathogen colony by the antagonist, b) cessation of the pathogen colony, and c) mutual antagonism.
The evaluation of Trichoderma sp. was based on the scale of Bell et al. (1982), where: 1 = Trichoderma completely covers the surface of the medium where the phytopathogen is growing, 2 = Trichoderma covers two thirds of the surface of the medium where the phytopathogen is growing, 3 = Trichoderma and the phytopathogen each colonize about half of the surface and none of them seems to dominate, 4 = the phytopathogen colonizes two-thirds of the surface and seems to resist the invasion of Trichoderma, and 5 = the phytopathogen occupies the whole plate area. This experiment involved four repeats per treatment and the percentage of inhibition was calculated using the formula described by Quiroz-Sarmiento et al. (2008):
Inhibition % = (D1-D2) / D1 x 100
where, D1 = radial growth of the pathogen without antagonist (control) and D2 = radial growth of the pathogen with antagonist.
Statistical analysis. Data were analyzed using ANOVA. When F tests were significant, differences between treatment means were compared using Tukey (p=0.05). Significance levels are reported as *=p<0.05, **=p<0.01, ***=p<0.001, and NS=not significant. The data presented are mean values ± SE (SAS, 2002).
RESULTS AND DISCUSSION
Fungi isolated from roots and stems of tomato plants with wilt symptoms. The localities of Jiquilpan, Cojumatlán of Regules and Nicolas Romero had the highest frequency of fungal isolates with 80%, 69% and 66% respectively. The variability in incidence between localities could be due to any or all of: the sample site, the climate, the crop management and the tomato variety (Quiroga-Madrigal et al., 2007).
In most locations, the incidence of fungi was higher in the root (50% to 74%) than in the stem (24% to 56%) of tomato plants (Fig. 1). This may be because fungi of the genus Fusarium mainly penetrate the root at transplanting or when secondary roots emerge. The process of stem colonization causes severe damage to the plant (Rodriguez & Montilla, 2002).

Fig. 1. Incidence of fungi in roots and stems of tomato plants (Solanum lycopersicum), in different localities.
Fig. 1. Incidencia de hongos en raíz y tallo de plantas de tomate (Solanum lycopersicum), en diferentes localidades.
A total of 28 strains of fungi were obtained from the tissue samples taken from the diseased tomato plants. From the morphological identification methods, 92% of these were Fusarium spp. and 5% were Rhizoctonia spp. Some strains (3%) could not be identified because sporulation did not occur. Similar results on the incidence of F. oxysporum (77% to 100%) were reported in fields of tomatoes in Sinaloa, México (Apodaca-Sánchez et al., 2002).
The isolates of Fusarium spp. developed colonies that were, variously: pale pink, purple, orange, red or white-grayish and cottony-looking. They presented microconidia, curved hyaline macroconidia and simple and branched conidiophores (Barnett & Hunter, 1978).
According to the morphological features for each species, Fusarium oxysporum microconidia presented ovoid, fusiform macroconidia with 3-5 septa, forming monophyalides, short conidiophores and chlamydospores developed alone or in pairs and usually thick-walled and globose (Nelson et al., 1983; Lugo & Sanabria, 2001).
Fusarium solani formed abundant unicellular microconidia and macroconidia that were cylindrical with rounded basal cells with monophyalides, long conidiophores and with single or paired chlamydospores. Fusarium subglutinans was characterized by the presence of poliphyalides with unicellular or septate oval microconidia and abundant macroconidia.
Morphological identification was confirmed at the molecular level by amplifying the Internal Transcribed Space (ITS) of rDNA in the Laboratory of Biotechnology and Seed Pathology at the Colegio de Postgraduados. Tree clades were observed, grouped according to reference sequence. Clade I comprised isolates of the F. oxysporum species complex, clade II isolates were of F. subglutinans and are considered within the Giberella fujikuroi species complex, and the species of clade III fell within the F. solani species complex due to the variability of the isolates within each species (Fig. 2).

Fig. 2. Phylogenetic tree constructed with sequences corresponding to the Internal Transcribed Space (ITS) of rDNA of Fusarium species isolated from the roots and stems of tomato (Solanum lycopersicum) cv Saladette, growing in the Cienega de Chapala, Michoacán in 2011.
Fig. 2. Árbol filogenético construido con secuencias correspondientes al Espacio Transcrito Interno (ITS) del rDNA de especies de Fusarium aislados de las raíces y tallos de tomate (Solanum lycopersicum) tipo Saladette cultivado en la Ciénega de Chapala, Michoacán en 2011..
The strain of Rhizoctonia sp. presented a septate mycelium with white pigmentation in young cultures and brown in mature cultures. Branch angle between the main hyphae was 90° (Barnett & Hunter, 1978; Agrios, 1988). The sequences of two isolates of Rhizoctonia were deposited in the NCBI Genbank.
Isolation and identification of antagonists. The total number of fungi (6.5x104 to 1.8x105 conidia/g) quantified here in vermicomposted chicken manure was significantly higher than that reported (Nagavallemma et al., 2006) in vermicomposted cow manure (8x104 conidia/g). The difference in population density of these organisms depends on both the substrate (the sort of manure) and on the earthworm species (Eisenia foetida, Eisenia eugeniae, Lampito mauritii or Perionyx excavatus) used for vermicomposting (Parthasarathi et al., 2007). Each worm species has different nutritional requirements while the ingested microorganisms vary with the nature of the organic matter. Therefore, the microorganisms which develop depend on the nutritional makeup of the source (Guedez et al., 2009).
In all, eleven different fungi were obtained from the chicken-manure vermicompost suspensions and ten of these were identified as: Trichoderma sp. "VC 1", Cladosporium sp. "VC 3", Curvularia sp. "CV 4", Fusarium spp. "VC 5", "VC 6" and "VC 7", Penicillium sp. "VC 8", Mucor sp. "VC 10" and Aspergillus spp. "VC 9" and "VC 11". Only two of these showed antagonism against the phytopathogens examined here.
In a PDA medium, Trichoderma sp. "VC1" presented a white pigment in the early stages of growth and a green one in more mature colonies. It also developed branched conidiophores, simple and grouped phialides, and ovoid conidia cells presented in small terminal clusters. This fungus is very common as a saprophyte and is widely distributed in soils due to its high reproductive capacity and its ability to survive adverse conditions (Barnett & Hunter, 1978; Benitez et al., 2004). Aspergillus sp. "VC11" is characterized by developing a green pigmentation on PDA medium with extensive sporulation; morphologically simple, vertically-presented conidiophores, the conidia are formed of a single cell and the surrounding vesicle is globose or claviform (Barnett & Hunter, 1978). Saprophytic fungi such as Aspergillus spp. are usually supported by substrates exuded by the roots (Guedez et al., 2009).
Microbial antagonism tests. Of the eleven isolates obtained from chicken-manure vermicompost, only two showed antagonism, Trichoderma sp. "VC1" and Aspergillus sp. "VC11". Both difered significantly from the control (without antagonist fungus pathogen activity). Inhibition obtained with VC1 was between 45% (against F. oxysporum) and 48% (against F. subglutinans) (Fig. 3). Similar results obtained Michel-Aceves (2001), were Trichoderma spp. showed inhibition of 25% to 69% on F. oxysporum, and 30% to 73% on F. subglutinans. Also, T. harzianum showed a 48% inhibition of F. oxysporum f. sp. lycopersici (Srivastava et al., 2010). VC1 strain inhibited a 38% to Rhizoctonia sp. Based on the scale proposed by Bell et al. (1982), the fungus Trichoderma sp. developed a class 2 antagonism on F. oxysporum, F. subglutinans and Rhizoctonia sp., with overgrowth on two-thirds of the surface of the medium (Fig. 3).

Fig. 3. Trichoderma sp. "VC 1" (right) isolated from chicken-manure vermicompost inhibits the growth of (A) F. oxysporum, (B) F. subglutinans and (C) Rhizoctonia sp. (left).
Fig. 3. Trichoderma sp. "VC 1" (derecha) aislado de vermicomposta de gallinaza, inhibe el crecimiento de (A) F. oxysporum, (B) F. subglutinans y (C) Rhizoctonia sp. (izquierda).
Aquino-Martínez (2008) observed inhibition of mycelium growth in F. oxysporum f. sp. dianthi (FOD) by Trichodermalignorum (Tl) attributing this to mycoparasitism. In general, it is well known that the antagonistic effect of Trichoderma spp. on pathogenic microorganisms is attributable to lytic enzymes produced by the fungus that degrade the cell walls of the host (mycoparasitism). Competition for nutrients also exerts a suppressing efect on phytopathogenic activity with these factors together contributing to biological control. Trichoderma spp. are also widely distributed in soils all around the world (Aquino-Martínez, 2008; Michael-Aceves et al., 2008).
Meanwhile, Aspergillus sp. "VC11" inhibited the growth of F. oxysporum by 27% and of F. subglutinans by 24%; for Rhizoctonia sp. the inhibition was 25% (Fig. 4). Responses of Aspergillus and Trichoderma as antagonists of Fusarium and Rhizoctonia were different than those reported in other studies. This is due to the selectivity that the species have, and the origin of evaluated strains (Quiroz-Sarmiento et al., 2008; Guédez et al., 2009).

Fig. 4. Aspergillus sp. "VC 11" (right) isolated from chicken-manure vermicompost inhibits the growth of (A) F. oxysporum, (B) F. subglutinans and (C) Rhizoctonia sp. (left).
Fig. 4. Aspergillus sp. "VC 11" (derecha) aislado de vermicomposta de gallinaza, inhibe el crecimiento de (A) F. oxysporum, (B) F. subglutinans y (C) Rhizoctonia sp. (izquierda).
Due to this antagonistic action, application of chickenmanure vermicompost has considerable potential as a management tool to control root diseases in vegetable crops such as tomatoes. Because it contains Trichoderma and Aspergillus, and other potentially benefcial microorganisms, it has the potential to suppress the growth of agriculturally important phytopathogens. Moreover, such substrates can also promote growth of natural populations of microbial antagonists by improving soil conditions, thus reducing competition between similar soil microbiota, further enhancing the biological control (Rodríguez-Dimas et al., 2007).
CONCLUSIONS
The fungi Fusarium (incidence 92%) and Rhizoctonia (incidence 5%) were isolated and identified from tomato plants showing symptoms of wilting at Cienega de Chapala, Michoacán. Under in vitro conditions, Trichoderma sp. inhibited the development of F. oxysporum (by 45%), F. subglutinans (by 48%) and Rhizoctonia sp. (by 38%) with antagonism class 2. Aspergillus sp. also inhibited the same phytopathogens but less efectively (24% to 25%). Soil applications of chicken-manure vermicompost can significantly increase the soil microbe populations (6.5 x 104 to 1.8 x 105 conidia/g), thus diversifying the microbiota, and promoting the populations of these phytopathogenic antagonists.
REFERENCES
1. Agrios, G.N. (1988). Plant Pathology; 3rd ed. Academic, San Diego, CA, USA; 803 p. [ Links ]
2. Apodaca-Sánchez, M.A., E. Zavaleta-Mejia, R. García-Espinoza, S. Osada-Kawasoe & J.G. Valenzuela-Ureta (2002). Frecuencia de campos infestados con Fusarium oxysporum f. sp. radicis-lycopersici en Sinaloa, México, y su control. Revista Mexicana de Fitopatología 20: 1-7. [ Links ]
3. Aquino-Martínez, J.G. (2008). Biocontrol in vitro e in vivo de Fusarium oxysporum Schlecht. f. sp. dianthi (Prill. y Delacr.) Snyder y Hans. con hongos antagonistas nativos de la zona florícola de Villa Guerrero, Estado de México. Revista Mexicana de Fitopatología 26: 127-137. [ Links ]
4. Ascencio-Álvarez, A., A. López-Benítez, F. Borrego-Escalante, S.A. Rodríguez-Herrera, A. Flores-Oliva, F. Jiménez-Díaz & A.J. Gámez-Vázquez (2008). Marchitez Vascular del Tomate: I. Presencia de razas de Fusarium oxysporum f. sp. lycopersisi (Sacc.) Snyder y Hansen en Culiacán, Sinaloa, México. Revista Mexicana de Fitopatología 26: 114-120. [ Links ]
5. Bainbridge, B.W., C.L. Spreadbury, F.G. Scalise & J. Cohen (1990). Improved methods for the preparation of high molecular weight DNA from large and small scale cultures of filamentous fungi. Microbiology Letters 66: 113-118. [ Links ]
6. Barnett, H.L. & B.B. Hunter (1978). Illustrated Genera of Imperfect Fungi. 4th. ed. MacMillan Publishing Co. New York, USA 217 p. [ Links ]
7. Benítez, T., A.M. Rincón, M.C. Limón & A.C. Codón (2004). Biocontrol mechanisms of Trichoderma strains. International Microbiology 7: 249-260. [ Links ]
8. Bell, D.K., H.D. Wells & C.R. Markham (1982). In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 72: 379-382. [ Links ]
9. Castillo, A.E., S.H. Quarín & M.C. Iglesias (2000). Caracterización química y física de compost de lombrices elaboradas a partir de residuos orgánicos puros y combinados. Agricultura Técnica (Chile) 60: 7-79. [ Links ]
10. De La Cruz-Lázaro, E., R. Osorio-Osorio, E. Martínez-Moreno, A.J. Lozano del Río, A. Gómez-Vázquez & R. Sánchez-Hernández (2010). Uso de compostas y vermicompostas para la producción de tomate orgánico en invernadero. Interciencia (Venezuela) 35: 363-368. [ Links ]
11. Ezziyyani, M., A.A. Sid, S.C. Pérez, M.E. Requena & M.E. Candela (2006). Control biológico por microorganismos antagonistas. Revista Horticultura 191: 8-15. [ Links ]
12. Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783-791. [ Links ]
13. Fernández, O. & L. Vega (2001). Microorganismos antagonistas para el control fitosanitario. Manejo Integrado de Plagas 62: 96-100. [ Links ]
14. Guédez, C., L.M. Cañizalez, C. Castillo & R. Olivar (2009). Micoflora asociada a dos sustratos orgánicos y su efecto en el control de Rhizoctonia solani Kühn. Agronomía Colombiana 27: 395-399. [ Links ]
15. Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Ser. 41: 95-98. [ Links ]
16. Holguin-Castaño, V.A. & J. Mora-Delgado (2009). Dinámica microbiana en vermicompostas comerciales con y sin inoculación del hongo Trichoderma spp. Revista Luna Azul 29: 18-24. [ Links ]
17. Larralde, C.C., M.R. Santiago, R.A. Sifuentes, L.I. Rodríguez, P.M. Rodríguez, K. Shirai & Z.J. Narváez (2008). Biocontrol potential and polyphasic characterization of novel native Trichoderma strains against Macrophomina phaseolina isolated from sorghum and common bean. Applied Microbiology and Biotechnology 80: 167-177. [ Links ]
18. Lugo, Z.C. & N.H. Sanabria (2001). Características culturales y patogénicas en aislamientos de Fusarium oxysporum f. sp. lycopersici procedentes de plantaciones comerciales de tomate. Agronomía Tropical 51: 519-130. [ Links ]
19. Michel-Aceves, A.C., M.A. Otero-Sánchez, R.D. Martínez-Rojero, R. Ariza-Flores, A. Barrios-Ayala & A. Rebolledo-Martínez. (2008). Control biológico in vitro de enfermedades fungosas en tomate Lycopersicum esculentum Mill. Avances en Investigación Agropecuaria. Universidad de Colima, México 12: 55-68. [ Links ]
20. Nagavallemma, K.P., S.P. Wani, L. Stephane, V.V. Padmajan, C. Vineela, M. BabuRao & K.L. Sahrawat (2006). Vermicomposting: Recycling wastes into valuable organic fertilizer. India: International Crops Research Institute for the Semi-Arid Tropics. SAT eJournal 2: 16. [ Links ]
21. Nelson, P.E., T.A. Toussoun & W.F.O. Marasas (1983). Fusarium Species: An Ilustrated Manual for Identification. The Pennsylvania State University Press. University Park and London 193 p. [ Links ]
22. Parthasarathi, K., L.S. Ranganathan, V. Anandi & J. Zeyer (2007). Diversity of microflora in the gut and casts of tropical composting earthworms reared on diferent substrates. Journal of Environmental Biology 28: 87-97. [ Links ]
23. Quiroga-Madrigal, R., M. Rosales-Esquinca, P. Rincón-Espinosa & E. Hernández-Gómez. 2007. Enfermedades Causadas por Hongos y Nematodos en el Cultivo de Tomate (Lycopersicon esculentum Mill.) en el Municipio de Villaflores, Chiapas, México. Revista Mexicana de Fitopatología 25: 114-119. [ Links ]
24. Quiroz-Sarmiento, V.F., R. Ferrera-Cerrato, A. Alarcón & M.E. Lara-Hernández (2008). Antagonismo in vitro de cepas de Aspergillus y Trichoderma hacia hongos filamentosos que afectan al cultivo del ajo. Revista Mexicana de Micología 26: 27-34. [ Links ]
25. Rodríguez, D.A. & J.O. Montilla (2002). Disminución de la marchitez causada por Fusarium en tomate con extracto de Citrus paradisi. Manejo Integrado de Plagas 63: 46-50. [ Links ]
26. Rodríguez-Dimas, N., P. Cano-Ríos, E. Favela-Chávez, U. Figueroa-Viramontes, V. De Paul-Álvarez, A. Palomo-Gil, C. Márquez-Hernández & A. Moreno-Reséndez. (2007). Vermicomposta como alternativa orgánica en la producción de tomate en invernadero. Revista Chapingo Serie horticultura 13: 185-192. [ Links ]
27. Srivastava, R., A.K. Sharma & A. Khalid (2010). Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderm aharzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biological Control 53: 24-31. [ Links ]
28. Statistical Analysis System SAS (2002). Statistical Analysis System for Windows Version 9. SAS Institute Inc., Carry, NC, USA. [ Links ]
29. Suárez-Meza, C.L., R.J. Fernández-Barbosa, N.O. Valero, R.M. Gámez-Carrillo & A.R. Páez-Redondo (2008). Antagonismo in vitro de Trichoderma harzianum Rifai sobre Fusarium solani (Mart.) Sacc., asociado a la marchitez en maracuyá. Revista Colombiana de Biotecnología 5: 35-43. [ Links ]
30. Tamura, K., D. Peterson, N. Peterson, G. Stecher, M. Nei & S. Kumar (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731-2739. [ Links ]
31. Tompson, J.D., D.G. Higgins & T.J. Gibson (1994). Clustal-W improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673-4680. [ Links ]
32. Villa-Briones, A., E. Zavaleta-Mejía, M. Vargas-Hernández, O. Gómez-Rodríguez & A. Ramírez-Alarcón (2008). Incorporación de vermicomposta para el manejo de Nacobbus aberrans en jitomate (Lycopersicon esculentum Mill.). Revista Chapingo Serie horticultura 14: 249-255. [ Links ]
33. White, T.J., T. Bruns, S. Lee & J. Taylor (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., D. H. Gelfand, J.J. Sninsky, & T.J. White (eds), pp. 315-322. PCR protocols: A guide to methods and amplifications. Academic Press, San Diego, California, USA. [ Links ]
34. Zhang, Z., S. Schwartz, L. Wagner & W. Miller (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7: 203-14. [ Links ]














