Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Phyton (Buenos Aires)
versión On-line ISSN 1851-5657
Phyton (B. Aires) vol.82 no.2 Vicente López dic. 2013
ARTÍCULOS ORIGINALES
Pollen production in sunflower (Helianthus annuus L.) is affected by air temperature and relative humidity during early reproductive growth
La producción de polen en girasol (Helianthus annuus L.) es afectada por la temperatura y la humedad relativa del aire durante la fase reproductiva temprana
Astiz V1 & LF Hernández2,3
1 Laboratorio de Calidad Industrial de Granos. Chacra Experimental Integrada Barrow (convenio INTA-MAA), Argentina.
2 Laboratorio de Morfología Vegetal. Departamento de Agronomía, Universidad Nacional del Sur. Bahía Blanca, 8000.
3 Comisión de Investigaciones Científicas de la Pcia. de Buenos Aires (CIC). La Plata, 1900, Argentina.
Address Correspondence to: V. Astiz, e-mail: vastiz@correo.inta.gob.ar
Recibido / Received 10.VI.2013.
Aceptado / Accepted 4.VII.2013.
Abstract. During microsporogenesis, sunflower florets might be exposed to episodes of day- and night-time temperatures exceeding 30 and 20 °C, respectively, that can affect pollen availability and consequently plant's yield. The relationship between air temperature and relative humidity during the meiotic phase of microsporogenesis, and the quantity and quality of pollen produced by the flowers, were studied in two sunflower hybrids during two years. The hybrids were grown on irrigated plots in Bahía Blanca (38° 45' S; 62° 11' W) in three planting dates (PD) at a density of 5.6 plants/m2. Flowers were consecutively taken as anthesis progressed, sectorizing the capitulum in three regions (external, middle and internal), to determine pollen grain number per flower (PGF) and pollen viability (PV). Both average air temperature (AT, °C) and relative humidity (RH, %) from reproductive stage R1 to R2 [36 to 48 days from emergence (DFE), respectively] were calculated for each PD, hybrid and capitulum sectors. Pollen viability was high (> 90%) with no differences between PD, capitulum sectors and hybrids. The number of PGF, with respect to AT (19 to 31 °C) from R1 to R2, was adjusted to a second-order polynomial (R2 =0.53, n=24; p<0.0004), with a maximum of 45,000 PGF between 21 and 25 °C. Above and below these values, the number of PGF showed a reduction of approximately 20%. There also was a significant positive relationship between pollen production and air RH. A maximum of 45,679 PGF was observed at 57% RH adjusting a second-order polynomial (R2 =0.58, n=24; p<0.0001).Temperatures over 26 °C were supraoptimal for pollen production. It is concluded that pollen production in sunflower is determined by the cumulative effects of both air temperature and relative humidity above a critical value and not only by temperature effects at a specific developmental stage before flower opening. It remains unknown whether this observation is associated with a direct effect on the mitosis or indirectly with changes in the contribution of current photoassimilates to anthers during the meiotic phase of the microsporogenesis.
Keywords: Sunflower; Pollen production and viability; Temperature; Relative humidity; Phenology; Microsporogenesis.
Resumen. Durante la microsporogénesis las flores del girasol están frecuentemente expuestas a episodios de temperaturas diurnas y nocturnas superiores a 30/20 °C. Ello podría resultar en la reducción de la calidad y cantidad de polen producido y consecuentemente del rendimiento. En dos híbridos de girasol, durante 2 años, se estudió la relación entre la temperatura del aire en la fase meiótica de la microsporogénesis y la cantidad y calidad del polen producido. Los híbridos se sembraron en parcelas bajo riego en Bahía Blanca (38° 45' S; 62° 11' W) en tres fechas (FS) a una densidad de 5,6 plantas/m2. La temperatura del aire fue registrada en forma horaria. Para el recuento de granos de polen por flor (GPF) y su viabilidad (VP) se tomaron fores consecutivamente con el avance de la antesis, sectorizando el capítulo en tres regiones, externa, media e interna. Se calculó la temperatura media del aire (TMA) ocurrida entre R1 y R2 (36 días desde la emergencia (DDE) a 48 DDE según la FS) para cada FS, híbrido y sector del capítulo. La VP fue alta (> 90%) no habiendo diferencias entre FS, sectores del capítulo e híbridos. El número de GPF respecto a la TMA (entre 19 y 31 °C) ocurrida entre R1 y R2 ajustó a un polinomio de segundo orden (R2 =0.53, n=24; p<0,0004), con un máximo de 45.000 GPF observado entre 21 y 25 °C. Por debajo y por encima de estos valores, el número de GPF tuvo una disminución cercana al 20%. Hubo una relación positiva significativa (R2 =0.58, n=24; p<0,0001) entre la producción de polen y la humedad relativa del aire. Se observó un máximo de 45.679 GPF a 57% HR ajustando también a un polinomio de segundo orden. Las temperaturas superiores a 26 °C fueron supraóptimas para la producción de polen. Se concluye que la producción de polen en girasol está asociada a los efectos acumulativos de la temperatura del aire y la humedad relativa por encima de un valor crítico y no solamente por efecto de la temperatura en una etapa específica del desarrollo previa a la antesis. Se debería estudiar si esta observación está asociada a un efecto directo sobre la mitosis o indirectamente por cambios en el aporte de fotoasimilados actuales a las anteras durante la fase meiótica de la microsporogénesis.
Palabras clave: Fenología; Girasol; Humedad relativa del aire; Microsporogénesis; Temperatura del aire; Viabilidad y producción de polen.
INTRODUCTION
During plant reproductive development there are two phases of ontogeny, meiosis and flowering, which are particularly sensitive to environmental stresses (Lalonde et al., 1997; Saini & Westgate, 2000; Boyer & Westgate, 2004). Microspore development is significantly vulnerable to detrimental effects of high temperature stress (Abiko et al., 2005; Prasad et al., 2008).
The total production of pollen grains of any given plant is one of the most heat-sensitive developmental stages in cereals (Stone, 2001). Pollen grain mitosis is highly sensitive to heat stress in wheat (Triticum aestivum L.; Saini et al., 1984), sorghum (Sorghum vulgare L.; McLaren & Wehner, 1992) and barley (Hordeum vulgare L.; Sakata et al., 2000).
From an agronomical stand point, studies on the biology of pollen production in a crop plant can add relevant knowledge for breeding and management purposes (Faegri & Iversen, 1989). Even though sunfñower pollen nutritional quality is low (Praz et al., 2008), less amount of pollen implies less attraction to pollinators. Thus, the number of pollen grains could compensate for the low quality (Roulston et al., 2000; Wilcock & Neiland, 2002), and become an important variable for the attraction of pollinators (Pernal & Currie, 2001) and subsequent crop production.
The influence of environmental variables such as air temperature or air relative humidity upon sunfower growth has been studied (i.e. Ploschuck & Hall, 1995; DeGrandi-Hofman & Chambers, 2006). Nevertheless, very little is known on the relationships between sunflower pollen production and these environmental parameters.
Pollen maturation in the sunflower capitulum proceeds centripetally and is highly synchronized with differential exposure to current climatic variations (Vaknin et al., 2008). During microsporogenesis (reproductive stage R1, Schneiter & Miller, 1981; Lindström, 2012), sunflower florets are often exposed to episodes of day- or night-time temperatures exceeding 30 or 20 °C , respectively, that can affect pollen availability and consequently yield (Moriondo et al., 2011).
The objectives of this work were to study the relationships between mean air temperature and relative humidity and the quality (viability) and quantity (number of grains) of pollen grains produced by sunflower flowers of different regions of the capitulum. Studies were conducted at early stages of microsporogenesis.
MATERIALS AND METHODS
Plant material. Experiments were carried out during 2009 and 2010 at the Agronomy Department, Universidad Nacional del Sur, Argentina (38° 45' S; 62° 11' W). We used two high-oleic sunflower hybrids: Dekasol Oil Plus (DKOP) 3845, a short season, high-oleic hybrid, and DKOP3945, a medium season, high-oleic hybrid (Syngenta, Argentina).
Growing the hybrids at three planting dates (PD; October 16; November 17 and December 16, 2009, and October 25 and November 25, 2010) allowed its exposure to different levels of humidity and air temperature under natural conditions during their reproductive development. After seedling emergence, plant density was adjusted to 5.6 plants/m2 by manual thinning.
The experimental design consisted of completely randomized split plots, with hybrid assigned to main plots and planting dates to subplots, with three replicates per treatment. Each subplot had three rows 0.70 m apart and 6.0 m long.
During the growing season, soil moisture was kept near field capacity by drip irrigation. Qualitative definition of phenological stages of the crop was conducted according to the visual scale of Schneiter & Miller (1981). Weeds were controlled manually, and the crop was fertilized with 50.0 kg N/ha using NH4NO3 at V4.
During the two-year study, mean air temperatures (AT,°C) and relative humidity (RH, %) were daily recorded with one-hour interval at 1.5 m from the soil.
Qualitative and quantitative pollen analysis. Pollen production per flower and pollen viability were determined. Three plants per replicate were used for these measurements. Qualitative and quantitative pollen analysis were done in three capitulum sectors. Each sector was equal to 1/3 of the capitulum radius (external, middle and internal). Pollen grains per flower (PGF) were counted on 5 closed fowers from each sector using a haemocytometer (Godini, 1979).
To assess pollen viability, anthers were collected before dehiscence and split open on a glass slide. Pollen grains were stained using Alexander's stain (Alexander, 1969). Grains that stained dark blue were classified as viable, whereas those that remained transparent were classified as dead. The number of viable and non-viable grains was then counted.
One-way ANOVA (Di Rienzo et al., 2008; InfoStat version 2010) was used to analyze all data statistically.
The period in which the flowers in each sector under analysis of pollen production were in the reproductive stage interval R1-R2, was determined according to the phenology of the reproductive development in the crop. Recent studies (Lindström, 2012) have shown that in this phenological interval, microsporogenesis starts and ends in the anthers of the flowers. Average temperature of the air (°C) and relative humidity (%) were calculated for each time interval in each genotype, planting date and capitulum sector (Fig. 1).

Fig. 1. Schematic representation of the criterion used to calculate the average air temperature (°C) and the average air relative humidity (HR%) during three moments of the capitulum development. For each capitulum sector (external, middle and internal), these environmental variables were calculated when the florets (FL) where between the reproductive stages R1 and R2 (after Schneiter and Miller, 1981).
Fig. 1 . Representación esquemática del criterio utilizado para calcular la temperatura media del aire (°C) y la humedad relativa media del aire (HR%) durante tres momentos del desarrollo del capítulo. Para cada sector del capítulo (externo, medio e interno), se calcularon estas variables ambientales cuando las flores (FL) se encontraban entre los estados reproductivos R1 y R2 (según Schneiter y Miller, 1981).
RESULTS
Number of pollen grains. Table 1 shows the number of pollen grains per flower (PGF) for each sector of the capitulum in the different PD and for the two experimental years. Values obtained for both hybrids were similar. In the first year, the internal sector showed the highest number of PGF (30979 to 40448 PGF) and the external sector the lowest (21924 to 31625 PGF). The middle sector showed intermediate values (26063 to 35396 PGF) (Table 1). The differences between the external and the internal sector were significant (p<0.05) in both hybrids for the three PD (Table 1). The only exception was in the third PD where DK3945 did not show significant differences (p=0.25) among the sampled sectors with values ranging from 27167 to 30979 PGF (Table 1).
Table 1. Number of pollen grains per flower in each sector of the capitulum (external, middle and internal) at the three planting dates in the first year (2009, I to III) and at the two planting dates in the second year (2010, IV and V), for each hybrid: Dekasol Oilplus (DKOP) 3845 and DKOP 3945. Within each planting date and set of capitulum sectors, different letters within columns indicate values that are significantly different at p=0.05.
Tabla 1. Número de granos de polen por flor en cada sector del capítulo (externo, medio e interno) en las tres fechas de siembra del primer año (2009, I a III) y en las dos fechas de siembra del segundo año (2010, IV y V), para cada híbrido: Dekasol Oilplus (DKOP) 3845 y DKOP 3945. Para cada fecha de siembra y entre el conjunto de los tres sectores del capítulo, diferentes letras en las columnas indican valores que son significativamente diferentes (p = 0,05).

In the second year, the number of PGF showed highly significant differences (p<0.01) among sectors of the capitulum (43542 to 50250 PGF) with the lowest number (27007 and 32000 PGF) for the external sector (Table 1).
During the two experimental years, a similar variation in the number of PGF among capitulum sectors was observed in both hybrids and in all PD. Thus, PGF was the highest in the internal sector, followed by the middle sector, and with the lowest production observed in the external sector (Table 1).
Pollen viability was high (96.6% to 98.4%; Astiz et al., 2012) in both genotypes during the two-year study in the different planting dates, with no significant differences among capitulum sectors (p=0.70).
Relationship between the number of pollen grains per flower versus mean air temperature and relative humidity. To isolate the positional effect of the number of pollen grains observed in the sunflower`s capitulum (Astiz et al., 2012), a correction factor was obtained. It was done by relating the number of pollen grains in each capitulum sector, discriminating data from the two years under study, and gathering the results of the two hybrids studied. It was applied to the total number of PGF in each of the capitulum sectors (Table 1).
Pollen grain number data presented in Table 1 was plotted against average air temperature or air relative humidity that occurred +/− three days after the attainment of the phenological interval R1-R2 (Fig. 2).
Fig. 2. Relationship between the capitulum sectors (spatial location) in both sunflower hybrids and pollen grains produced per flower. The regression equations obtained during the first year: y=22,486.0 + 4,643.5 x, R2 = 0.784, and during the second year: y=21,666.1+ 48,916.7 x, R2 = 0.959 were used as a correction tool to isolate the positional effect of the total number of grains per flower produced each year and planting date. First year: ●: DK3845; ○: DK3945; second year: ☐: DK3845; ■: DK3945.
Fig. 2. Relación entre los sectores del capítulo (localización espacial) en los dos híbridos de girasol y los granos de polen producidos por flor. Las ecuaciones de regresión obtenidas durante el primer año: y = 22.486,0 + 4.643,5 x, R2 = 0,784, y durante el segundo año: y = 21.666,1 48.916,7 + x, R2 = 0,959 fueron utilizadas como una herramienta de corrección para aislar el efecto posicional del número total de granos por flor producidos cada año y para cada fecha de siembra. Primer año: ●: DK3845; ○: DK3945; segundo año: ☐: DK3845; ■: DK3945.
The number of PGF with respect to the AT (9 to 31 °C) fit a second-order polynomial (R2=0.53, n=24, p=0.0004; Fig. 3), with a maximum of 45000 PGF between 21 and 24 °C. Below and above these values, the number of PGF showed a reduction of approximately 20%.
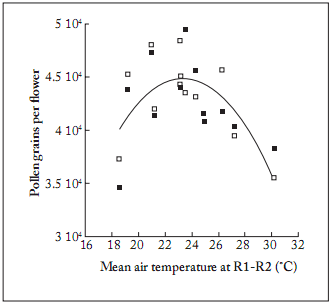
Fig. 3. Pollen grains per flower vs mean air temperature (°C) for both hybrids used in the present work (☐: DK3845; ■: DK3945) during the two-year study.
Pollen grain number = 44,182.4 + 36.6 x - 175.7 x2, R2 = 0.53, Sy,x = 2, 833.5; n=24, p=0.0004; where: x = air temperature (°C).
Fig. 3. Granos de polen por flor vs la temperatura media del aire (°C) para los híbridos utilizados en el presente trabajo (☐: DK3845; ■: DK3945) durante los dos años de estudio.
Número de granos de polen = 44.182,4 + 36,6 x - 175,7 x2, R2 = 0,53, Sy, x = 2.833,5, n = 24, p = 0,0004; donde: x = temperatura del aire (°C).
There was a strong and significant positive relationship between pollen production and air RH. The response also fit a second-order polynomial (R2=0.58, n=24, p<0.0001) with a maximum of 45679 PGF at 57% RH (Fig. 4).

Fig. 4. Pollen grains per flower vs air relative humidity (RH%) for both sunflower hybrids used in the present work (●: DK3845; ¡: DK3945) during the two-year study.
Pollen grain number = 29,063.3 + 307.5 x - 12.9 x2, R2 = 0.58, Sy,x = 2, 660.8; n=24, p<0.0001; where: x = air relative humidity (%).
Fig. 4. Granos de polen por flor vs la humedad relativa del aire (RH%) para los híbridos de girasol utilizados en el presente trabajo (●: DK3845; ¡: DK3945) durante los dos años de estudio.
Número de granos de polen = 29.063,3 + 307,5 x - 12,9 x2, R2 = 0,58, Sy,x = 2.660,8, n = 24, p<0,0001; donde: x = humedad relativa del aire (%).
The number of PGF adjusted significantly to the model of surface response with AT and air RH as independent variables (R2=0.72; Fig. 5).

Fig. 5. Regression surface of corrected number of pollen grains per floret vs air temperature (°C) and air relative humidity (%) measured at early microsporogenesis (see text). The software Statistica v. 10 (Statsoft Inc., Tulsa, USA) was used to analyze these results. Equation for the ftted surface is:
Pollen grain number= -69,202.1 + 6,447.5 x + 1,419.5 y - 115.7 x2 - 22.3 xy - 7.2 y2
where: x = air temperature (°C) and y = air relative humidity (%), R2 = 0.72; SAirTemp, HR% = 0.23, and n=48.
Fig. 5. Superficie del respuesta del número corregido de granos de polen por flor vs la temperatura del aire (°C) y la humedad relativa del aire (%) medidas durante la microsporogénesis (véase el texto). Para analizar los resultados, se utilizó el software Statistica v. 10 (Statsoft Inc., Tulsa, EE.UU.). La ecuación para la superficie ajustada es:
Número de granos de polen = -69.202,1 + 6,447,5 x + 1.419,5 y - 115,7 x2 - 22,3 xy - 7,2 y2
donde: x = temperatura del aire (°C) e y = humedad relativa del aire (%),R2 = 0,72; SAirTemp , HR%= 0,23, y n = 48.
DISCUSSION
Temperatures during early microsporogenesis below 21 °C and over 26 °C reduced mean total pollen grain number per fower up to 25% as measured when air temperatures dropped to 17-18 °C (Fig. 3). Air moisture on the other hand showed a positive correlation up to a maximum optimum level of 57% RH. Above this value the number of pollen grains did not show significant increases (Fig. 4). Then the ceiling day temperature and relative humidity flor pollen production was 23°C and 57%, respectively (Fig. 5).
The large variability shown in the pollen grain number vs. air temperature indicates that this variable was not a particularly a robust indicator of the degree of temperature stress on pollen grains per floret; on the other hand, RH was close to better explain changes of magnitude observed in that parameter. Not unexpectedly, the analysis using both variables improved the regression fit compared with using only air temperatures (Fig. 5).
De Beer (1963) reported that the number of pollen grains of groundnut (Arachis hypogaea L.) was reduced by 71%, from 3388 to 987 GPF, when the constant day and night temperature was increased from 24 to 33 °C, and no viable pollen was produced at 33 °C.
Similarly, studies in tomato (Licopersicon esculentum L.) have shown that both fruit weight and number, and fruit set, decreased as daily mean temperature increased from 25 to 29°C (Peet et al., 1998). This was associated with the effects of heat stress on pollen development and pollen release (Peet et al., 1998).
In our findings, air temperatures ranging from 20 to 24 °C appeared to be optimal, with significantly different means only found in the lowest (18 °C) or the highest (26 °C) temperature treatment. On the other hand, values of air RH ranging from 56 to 65% did not show any effect on increasing the number of PGF (Fig. 4).
The deleterious effect of sub and supraoptimal temperatures on the production of pollen grains in crop plants can be explained by the increased levels of reactive oxygen species (ROS; Barnabás et al., 2008; Frank et al., 2009) that can trigger transcriptional changes, and by hormone production changes (Kotak et al., 2007). Also, heat stress during the period of microspore meiosis in wheat can induce tapetum degradation (Saini et al., 1984; Sakata et al., 2000) that leads to reduced microsporogenesis and pollen sterility.
The results showed in the present work show that temperatures over 26 °C are supraoptimal for pollen production in sunflower, even under well-watered conditions.
We conclude that pollen production in sunflower is determined by the cumulative effects of air temperature and air relative humidity above a critical value, not just by temperature effects at a specific developmental stage before flower opening.
Microsporogenesis was observed to be significantly more vulnerable to air temperature than fertilization, which presents a tolerance limit of 30 °C, and a RH of 60% (Barnabás et al., 2008). It still remains unknown whether this observation is associated with a direct effect on mitosis or indirectly associated with variations in the contribution of current photoassimilates to anthers during the meiotic phase.
REFERENCES
1. Abiko, M., K. Akibayashi, T. Sakata, M. Kimura, M. Kihara, K. Itoh, E. Asamizu, S. Sato, H. Takahashi & A. Higashitani (2005). High-temperature induction of male sterility during barley (Hordeum vulgare L.) anther development is mediated by transcriptional inhibition. Sexual Plant Reproduction 18: 91-100. [ Links ]
2. Alexander, M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technology 44: 117-22. [ Links ]
3. Astiz, V., A. Flemmer & L.F. Hernández (2012). Can the intraplant pollen offer be related with fruit set failures in sunflower (Helianthus annuus L.)? Studies in modern hybrids and cross-pollinated varieties. Actas de la XVII International Sunfower Conf., Mar del Plata, Argentina, 02-VC-3. [ Links ]
4. Barnabás, B. K. Jäger & A. Fehër (2008). The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell and Environment 31: 11-38. [ Links ]
5. Boyer, J.S & M.E. Westgate (2004). Grain yields with limited water. Journal of Experimental Botany 58: 267-277. [ Links ]
6. De Beer, J.R. (1963). Influence of temperature on Arachis hypogaea L. with special reference to its pollen viability. PhD Thesis, State Agricultural University, Wageningen, The Netherlands. [ Links ]
7. DeGrandi-Hoffman, G. & M. Chambers (2006). Effects of Honey Bee (Hymenoptera: Apidae) Foraging on Seed Set in Self-Fertile Sunflowers (Helianthus annuus L.). Enviromental Entomology 35: 1103-1108. [ Links ]
8. Di Rienzo, J.A., F. Casanoves, M.G. Balzarini, I. González, M. Tablada & C.W. Robledo (2008). InfoStat, versión 2008, Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. [ Links ]
9. Faegri, K. & J. Iversen (1989). Textbook of Pollen Analysis. 4th ed. K. Faegri, P.E. Kalland & K. Krzywinski. J. Wiley & Sons, Chichester. [ Links ]
10. Frank, G., E. Pressman, R. Ophir, L. Althan, R. Shaked, M. Freedman, S. Shen & N. Firon (2009). Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. Journal of Experimental Botany 60: 3891-3908. [ Links ]
11. Godini, A. (1979). Counting pollen grains of some Almond cultivars by means of an haemocytometer. CIHEAM- Options Mediterraneennes. Instituto di Coltivazioni Arboree, Università di Bari. [ Links ]
12. Kotak, S., J. Larkindale, U. Lee, P. von Koskull-Doring, E. Vierling & K.D. Scharf (2007). Complexity of the heat stress response in plants. Current Opinion in Plant Biology 10: 310-316. [ Links ]
13. Lalonde, S., D.U. Beebe & H.S. Saini (1997). Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sexual Plant Reproduction 10: 40-48. [ Links ]
14. Lindstrom, L.I. (2012). Histogénesis del fruto de girasol (Helianthus annuus L.): su aplicación al análisis del efecto de la radiación incidente sobre el peso y la aptitud al descascarado de los frutos y sus variables subyacentes. Doctoral dissertation. Departamento de Agronomía Universidad Nacional del Sur, Argentina, 189 p. [ Links ]
15. McLaren, N.W. & F.C. Wehner (1992). Pre-flowering low temperature predisposition of sorghum to sugary disease (Claviceps africana). Journal of Phytopathology 135: 328-334. [ Links ]
16. Moriondo, M., C. Giannakopoulos & M. Bindi (2011). Climate change impact assessment: the role of climate extremes in crop yield simulation. Climatic Change 104: 679-701. [ Links ]
17. Peet, M.M., S. Sato & R.G. Gardner (1998). Comparing heat stress effects on male-fertile and male-sterile tomatoes. Plant, Cell and Environment 21: 225-231. [ Links ]
18. Pernal, S.F. & R.W. Currie (2001). The influence of pollen quality on foraging behavior in honeybees (Apis mellifera L.). Behavioral Ecology and Sociobiology 51: 53-68. [ Links ]
19. Ploschuck , E.L. & A.J. Hall (1995). Capitulum position in sunflower affects grain temperature and duration of grain filling. Field Crops Research 44: 111-117. [ Links ]
20. Prasad, P.V.V., S.R. Pisipati, R.N. Mutava & M.R. Tuinstra (2008). Sensitivity of grain sorghum to high temperature stress during reproductive development. Journal of Crop Science 48: 1911-1917. [ Links ]
21. Praz, C.J., A.Müller & S. Dorn (2008). Specialized bees fail to develop on non-host pollen: do plants chemically protect their pollen? Ecology 89: 795-804. [ Links ]
22. Roulston, T. H., J. Cane & S. L. Buchmann (2000). What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecological Monographs 70: 617-643. [ Links ]
23. Saini, H.S. & M. E. Westgate (2000). Reproductive development in grain crops during drought. Advances in Agronomy 68: 59-96. [ Links ]
24. Saini, H.S., M. Sedgley & D. Aspinall (1984). Developmental anatomy in wheat of male sterility induced by heat stress, water deficit of abscisic acid. Australian Journal of Plant Physiology 11: 243-253. [ Links ]
25. Sakata, T., H. Takahashi, I. Nishiyama & A. Higashitani (2000). Effects of high temperature on the development of pollen mother cells and microspores in barley (Hordeum vulgare L.). Journal of Plant Research 113: 395-402. [ Links ]
26. Schneiter, A.A. & J.F. Miller (1981). Description of sunflower growth stages. Crop Science 21: 901-903. [ Links ]
27. Solomon, A.M. & H.D. Hayes (1972). Desert pollen production I. Qualitative influence of moisture. Journal of the Arizona Academy of Science 7: 52-74. [ Links ]
28. Stone, P. (2001). The effects of heat stress on cereal yield and quality. In: Crop Responses and Adaptations to Temperature Stress (A.S. Basra Ed.), pp 2443-291. Food Products Press, Binghamton, N.Y., USA. [ Links ]
29. Vaknin, Y. N. Barr & Y. Saranga (2008). Preliminary investigations into the significance of floral applications of calcium, boron and polyphenols for increased seed set in confection sunflowers (Helianthus annuus L.). Field Crops Research 107: 155-160. [ Links ]
30. Wilcock, C. & R. Neiland (2002). Pollination failure in plants: why it happens and when it matters. Trends in Plant Science 7: 270-277. [ Links ]














